Abstract
Heavy metal in the physical environment may alter immune function and predispose to develop asthma in human. Our study was aimed to investigate associations between urinary heavy metals and asthma in adults. A retrospective cross-sectional study was conducted with 3425 subjects aged 20 years and older in the US National Health and Nutrition Examination Survey (NHANES) 2011–2014. Binary logistic regression was applied to analyze associations between cobalt (Co), tungsten (W), and uranium (U) and asthma. We found positive associations between U and asthma (OR = 1.74, 95%CI: 1.25, 2.44, P for trend < 0.01). U was positively associated with asthma in 20–59 years group (OR = 1.65, 95%CI: 1.11, 2.46), while W and Co were related with asthma among in above 60 years group (OR = 2.39, 95%CI: 1.24, 4.58, P for trend = 0.02; OR = 1.88, 95%CI: 1.02, 3.47, respectively). U was linked with asthma in both males and females (OR = 1.93, 95%CI: 1.16, 3.20; OR = 1.59, 95%CI: 1.01, 2.51, respectively). Positive associations between U and asthma were discovered among adults with family history of asthma or not (OR = 2.15, 95%CI: 1.17, 3.95, P for trend = 0.03; OR = 1.62, 95%CI: 1.08, 2.43, P for trend = 0.03, respectively). Remarkable association was observed between U and asthma in adults without hay fever (OR = 1.79, 95%CI: 1.24, 2.60, P for trend = 0.02). Our findings provide epidemiological evidence to highlight a need to prioritize heavy metals exposure with asthma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a common chronic disease, asthma is characterized by chronic airway inflammation, increasing susceptibility to respiratory viral infection, and altering airway microbiology (or microenvironment) (Gibson et al. 2017). Globally, approximately 300 million people of all ethnic groups throughout all ages had asthma, and 250,000 deaths were directly caused by asthma every year (Croisant 2014). Prevalence of asthma symptoms in both children and adults is still globally continuing especially in low- to middle-income countries, although some reports suggest that it has subsided in some high-income countries (Dharmage et al. 2019, Ferrante & La Grutta 2018). However, there were nearly 26 million patients diagnosed as asthma by physicians, and its prevalence was up to 7.7% in the USA in 2018 (CDC 2018, Yaghoubi et al. 2019). During 2008–2013, the US treatment and mortality costs on asthma have been estimated at $81 billion (Nurmagambetov et al. 2018). Risk factors for asthma included genetic, host, and environmental factors, such as allergens, smoking, and genetic susceptibility (Croisant 2014, Tan et al. 2015). Some studies showed the differences of asthma incidences stratified by sex and age (Dratva et al. 2018, Mohammad et al. 2016). Previous research have observed associations between heavy metals and asthma in the USA and China (Wu et al. 2018, Zeng et al. 2016). For example, lead and cadmium were found to be positively associated with asthma (Choi et al. 2017).
Heavy metals are ubiquitously dispersed in the environment, including soil, water, air, dust, diet, and manufacturing products (Wang et al. 2018, Zeng et al. 2015). The general population is commonly exposed to low concentration of metals via ingestion of contaminated water and food or indrawing of ambient air pollution (Meeker et al. 2008, Menke et al. 2016, Wang et al. 2016). Heavy metals exposed to environment may adhere to fine particles in the air and cause asthma as an environmental allergen (Zeng et al. 2016). Several studies observed that heavy metal–induced asthma was triggered by the immune system, suggesting that heavy metals have enormous inflammatory potential and immunomodulatory effects on human (Lehmann 2017). Many studies reported the correlations between common heavy metals (lead, cadmium, mercury and manganese) and asthma (Huang et al. 2016, Jung et al. 2015, Miyake et al. 2011, Park et al. 2016, Rosa et al. 2016, Urushidate et al. 2010). However, few reports were conducted on the association of Co, W, and U and asthma.
Co is an important and widely used raw material for the production of alloys in industry. It is usually mixed with other metals, and its compounds are commonly used as trace element additives in medicine and agriculture (Liao et al. 2019). From household appliances to high-end technology products, the industrial and military uses of W-based products are increasing (Oburger et al. 2018). As a heavy metal and radionuclide, U is widespread in the environment, not only because of the leaching of natural sediments but also from the exploration and utilization of nuclear energy, which leads to the release of plant tailings and emissions from the nuclear industry. Occupational exposure to Co, W, and U dust has been found to be associated with asthma (Cirla 1994, Gheysens et al. 1985, Stefaniak et al. 2009), allergic contact dermatitis (Fischer and Rystedt, 1983), and hard metal disease (Davison et al. 1983, Shumate et al. 2017). Workers exposed to Co, W, and U are reported to have an increasing incidence of asthma, which may involve both immune and non-immune mechanisms (Nemery 1990, Sauni et al. 2010). However, little is known to the contribution of Co, W, and U on the risk of asthma in general population.
In our retrospective cross-sectional study, we investigated the associations between the heavy metals (Co, W and U) and asthma using a nationally representative sample of adults, who participated in the 2011–2014 (two survey cycles) of the NHANES in the USA. We also comprehensively explored whether the relationships were different in sex, age, family history of asthma, and hay fever.
Material and methods
Study design
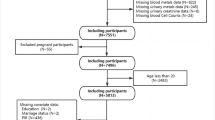
The National Health and Nutrition Examination Survey is designed to assess the health and nutritional status among adults and children in the USA. A unique feature of this survey is the collection of health examination data for a nationally representative sample of the resident, civilian noninstitutionalized US population. The survey consists of questionnaires administered in the home, followed by a standardized health examination in specially equipped mobile examination centers (MECs) (Johnson et al. 2014). Data collection for NHANES consists of a household screener, an interview, and an examination. Demographic, socioeconomic, and dietary data were collected by interview. Medical data, dental data, and physiological data were measured by examination and lab portions. For this retrospective cross-sectional study, survey data used to analyze was from 2011–2012 and 2013–2014 cycles. Ultimately, the age of selective study population was 20 years and older. Participants with both urinary heavy metals and asthma data were included in the analysis (n = 3425). The details are shown in Fig. 1. For missing data from the covariates, we used a median fill method.
Urinary metals measurement
The urine samples of this study were collected from participants aged 20 years and older. Heavy metals were measured in the mobile examination center (MEC). Urine specimens are processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis. Heavy metals were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS), a multi-element analytical technique. Liquid samples entered the ICP through a nebulizer and spray chamber carried by a flowing argon stream. By coupling radiofrequency power into flowing argon, plasma is generated in which the main compositions were positive argon ions and electrons. The sample traverses a region of the plasma with a temperature of 6000–8000 K. The thermal energy atomizes the sample and then ionizes the atoms. The ions, together with the argon, enter the mass spectrometer through an interface separated by the ICP and the mass spectrometer. The mass spectrometer can detect ions of each mass at the fastest speed and can determine the individual isotope of an element. Electrical signals generated by the detection of the ions are processed into digital information that is used to indicate first the intensity of the ions and then the concentration of the element. This study chose the following three heavy metals to analyze: Co, W, and U.
Asthma assessment
The assessment of asthma was on the basis of the information from questionnaire section of the US National Health Interview Survey. In order to assess asthma, participants were asked “Has a doctor or other health professional ever told you that you have asthma?” If the participant responds “yes,” he or she was regarded as asthma patient.
Covariates
Considering potential confounders associated with heavy metals and asthma, we controlled the following factors (from existing literature): age, gender, race, education, ratio of family income to poverty (PIR), body mass index (BMI), urinary creatinine, smoking status, alcohols, family history of asthma, and hay fever. Age was divided into two groups: 20–59 years and 60 years and older. Race was categorized as Mexican American, Other Hispanic, Non-Hispanic white, Non-Hispanic black, and Other Race—including multiracial. PIR is a measure of the extent of family poverty and is divided into two categories by the cut-off of 1. We used BMI to measure the state of obesity. The formula for BMI is BMI = weight (kg)/height (m2). Our evaluation of BMI was based on the standard of World Health Organization (WHO). Education level was divided into three layers: below high school, high school graduate//GED or equivalent, and higher than high school. Smoking status and alcohols were analyzed from questionnaire information. Given that family history of asthma might have an impact on the outcome of the analysis (Mejias and Ramphul, 2018), we analyzed asthma family history as a covariate. Family history of asthma was defined as whether any of your close relatives including living and deceased, such as father, mother, sisters or brothers, ever been told by a health professional that they had asthma. Considering that history of hay fever is also associated with asthma, we included it as a covariate (Rzehak et al. 2008, Wu et al. 2018). The participants will be asked have they had an episode of hay fever during the past 12 months. Evidence from previous studies demonstrated creatinine concentrations were used to determine whether the urinary validity and the excretion rate were relatively constant, and creatinine adjustment was recommended to normalize analyte concentrations in assessing the relationship between urine-based environmental exposure monitoring and health outcome (Barr et al. 2005, O'Brien et al. 2017).
Statistical analysis
Data were performed with the Solutions Statistical Package for the Social Sciences (IBM SPSS Statistics Premium V25.0). Descriptive analyses were presented to provide information on the general characteristics of the study population. The concentrations of Co, W, and U in the urine were divided into quartiles. Since urine creatinine was skewed, we used log-transformed data to improve normality in our analysis. Continuous variables were shown as mean ± standard error (SE), while categorical variables were shown as numbers and percentage (%). We compared baseline characteristics by Student’s t test for normally distributed data among population, while we calculated Chi-square test for classification data. We used logistic regression models to evaluate the relationships between single heavy metals and asthma. Two models (crude model and adjusted model) were applied to explore the association between heavy metals (Co, W, and U) and asthma in our analysis. We adjusted only Co, W, and U in the crude model. We further adjusted for age, race, education, PIR, BMI, urinary creatinine, family history of asthma, smoking status, alcohols, and hay fever in the adjusted model. We developed stratified analyses by age (20–59 years and ≥ 60 years), sex (male and female), family history of asthma (yes and no), and hay fever (yes and no). Age was used as continuous variables in the analysis except the stratification analysis.
Several studies proved that the incidence of asthma was different in male and female, which may be related to age (Cadeddu et al. 2016). The incidence of asthma is higher in boys than in girls in childhood, while it becomes more prevalent and severe in women after puberty (Pignataro et al. 2017). Twins studies proved that asthma could be transmitted by genetic factors (Harris et al. 1997, Koppelman et al. 1999), which might induce the difference of prevalence among population with a positive history of asthma or not. Notably, aeroallergens were strong risk factors inducing asthma (Sullivan et al. 2019). Previous study convinced that tree pollen was the risk factors for children and adults (Lee et al. 2019). Therefore, we applied stratified analyses by aforesaid factors to study the relationship between heavy metals and asthma controlling these confounding factors, respectively.
Sample weights were aimed to diminish the selection bias among subgroups for age, sex, and race in the NHANES survey. Therefore, unweighted analysis was recommended because variables used to calculate sample weights had already been contained in the adjusted model (Graubard and Korn, 1999, Kim et al. 2017, Zhang et al. 2019).
All significances were analyzed using a two-tailed test, and a P value < 0.05 was considered to show statistical significance in this study.
Results
Demographic characteristics of study subjects
Table 1 presents the baseline characteristics among the 3425 participants, including age, creatinine, gender, race, education, PIR, BMI, smoking status and alcohol, family history of asthma, and hay fever. Significant differences in age, gender, race, PIR, BMI, smoking status, family history of asthma, and hay fever were found in study population stratified with asthma or not. Totally, 6.16% males and 8.79% females suffered from asthma. Among the study population, 8.91% participants without family history of asthma had developed asthma, and 11.56% of participants without hay fever had asthma.
Distribution of urinary heavy metals concentrations (Co, W, and U)
The distributions of Co, W, and U in the urine in the study population are shown in Table S1. Among these participants, the concentration of urinary Co was the highest with the geometric mean concentrations of 0.342 μg/L, while the concentration of urinary U was the lowest with the geometric mean concentrations of 0.006 μg/L.
Logistic regression model to assess the association between urinary heavy metals (Co, W, and U) with asthma
We used logistic regression model to assess the individual effect of each heavy metals on asthma. As shown in Table 2, in the crude model, Co showed significant association with asthma in the upper quartiles (Q4: cOR = 1.38, 95%CI: 1.05, 1.80). We observed that W was positively associated with asthma in the upper quartiles (Q4: cOR = 1.67, 95%CI: 1.28, 2.17, P for trend < 0.01). Meanwhile, U was shown obviously significant relationship with asthma in all quartiles compared with the reference (Q2: cOR = 1.41, 95%CI: 1.07, 1.86; Q3: cOR = 1.47, 95%CI: 1.12, 1.94; Q4: cOR = 1.61, 95%CI: 1.23, 2.11, P for trend = 0.01). After adjusted for all the covariates, U was also found to be significantly linked to asthma in all quartiles as well as the results in crude model (Q2: aOR = 1.42, 95%CI: 1.05, 1.93; Q3: aOR = 1.45, 95%CI: 1.05, 2.00; Q4: aOR = 1.74, 95%CI: 1.25, 2.44, P for trend = 0.01). There was no evidence shown in significant associations between other heavy metals (Co and W) and asthma.
Logistic regression model to assess the association between urinary heavy metals (Co, W, and U) with asthma stratified by age
According to the results of Table 1, we found several factors vary widely between asthmatic and non-asthmatic populations. In order to assess the impact of these factors in our findings, we stratified these factors separately for further analyses. The relationships between the three metals and asthma stratified by age are shown in Table 3. The association between Co and asthma was strongly significant among 20–59 years old group in the crude model (Q4: cOR = 1.42, 95%CI: 1.05, 1.93, P for trend = 0.04). We discovered that Co was associated with asthma in 60 years and older group after adjusted for covariates (Q3: aOR = 1.88, 95%CI: 1.02, 3.47). W was associated with asthma among population aged 60 years and older in the crude model (Q3: cOR = 1.76, 95%CI: 1.05, 2.94; Q4: cOR = 1.84, 95%CI: 1.07, 3.17, P for trend = 0.02). After adjusted for covariates, the relationship described above were also significant (Q3: aOR = 2.19, 95%CI: 1.21, 3.97; Q4: aOR = 2.39, 95%CI: 1.24, 4.58). W was a risk factor among 20–59 years in the crude model (Q4: cOR = 1.58, 95%CI: 1.17, 2.14, P for trend < 0.01); similar trend existed after adjusted all the covariates (Q4: aOR = 2.39, 95%CI: 1.24, 4.58, P for trend = 0.02). We observed that U was linked to asthma among population aged 20–59 years in the crude model (Q3: cOR = 1.63, 95%CI: 1.19, 2.23; Q4: cOR = 1.67, 95%CI: 1.23, 2.30, P for trend < 0.01). The relationship was also observed among 20–59 years in the adjusted model (Q3: aOR = 1.47, 95%CI: 1.01, 2.15; Q4: aOR = 1.65, 95%CI: 1.11, 2.46). The correlation between U and asthma was found in the second quartile in population aged 60 years and older in the adjusted model (Q2: aOR = 1.80, 95%CI: 1.01, 3.21).
Logistic regression model to assess the association between urinary heavy metals (Co, W, and U) with asthma stratified by gender
Next, we evaluated the influence of gender on the relationships between heavy metals and asthma (Table 4). In the crude model, Co and W were found significantly associated with asthma in female (Co, Q4: cOR = 1.44, 95%CI: 1.02, 2.04; W, Q4: cOR = 2.05, 95%CI: 1.44, 2.92). We discovered a significant relationship between U and asthma in females before adjusting for all covariates (Q2: cOR = 1.53, 95%CI: 1.07, 2.19; Q3: cOR = 1.70, 95%CI: 1.19, 2.42; Q4: cOR = 1.70, 95%CI: 1.19, 2.44, P for trend = 0.01). After adjusted for covariates, only U showed a positive relationship with asthma in the upper quartiles in females (Q4: aOR = 1.59, 95%CI: 1.01, 2.51). U showed a strong association with asthma in male regardless of adjustment for covariates (Q4: cOR = 1.60, 95%CI: 1.06, 2.43; Q4: aOR = 1.93, 95%CI: 1.16, 3.20, respectively).
Logistic regression model to assess the association between urinary heavy metals (Co, W, and U) with asthma stratified by family history of asthma
We also examined whether the association of these asthma risk factors differed by family history of asthma. As shown in Table 5, U was positively associated with asthma among population with family history of asthma in both crude model and adjusted model (Q2: cOR = 1.97, 95%CI: 1.23, 3.17; Q3: cOR = 1.73, 95%CI: 1.07, 2.79; Q4: cOR = 1.66, 95%CI: 1.03, 2.70, P for trend = 0.03; Q2: aOR = 2.37, 95%CI: 1.38, 4.06; Q3: aOR = 2.04, 95%CI: 1.15, 3.65; Q4: aOR = 2.15, 95%CI: 1.17, 3.95, P for trend = 0.02; respectively). Similar trends were observed in the participants without family history of asthma (Q4: cOR = 1.61, 95%CI: 1.15, 2.24, P for trend = 0.03; Q4: aOR = 1.62, 95%CI: 1.08, 2.43; respectively). W showed a significant association in population without family history of asthma in the crude model (Q4: cOR = 1.69, 95%CI: 1.20, 2.36, P for trend = 0.01).
Logistic regression model to assess the association between urinary heavy metals (Co, W, and U) with asthma stratified by hay fever
We further explored whether hay fever modified the association between heavy metals (Co, W, and U) and asthma (Table 6). Co was associated with asthma in the upper quartiles among populations without hay fever in the crude model (Q4: cOR = 1.57, 95%CI: 1.16, 2.12, P for trend = 0.02). The relationship between W and asthma was significant among population without hay fever in the crude model (Q3: cOR = 1.37, 95%CI: 1.02, 1.83; Q4: cOR = 1.85, 95%CI: 1.37, 2.49, P for trend < 0.01), while U was found significantly in all quartiles compared with reference among populations without hay fever in both the crude model and adjusted model (Q2: cOR = 1.50, 95%CI: 1.09, 2.07; Q3: cOR = 1.62, 95%CI: 1.18, 2.23; Q4: cOR = 1.86, 95%CI: 1.37, 2.53, P for trend < 0.01; Q2: aOR = 1.48, 95%CI: 1.05, 2.09; Q3: aOR = 1.49, 95%CI: 1.04, 2.14; Q4: aOR = 1.79, 95%CI: 1.24, 2.60, P for trend = 0.02; respectively).
Discussion
This is a cross-sectional analysis to evaluate the associations between asthma and heavy metals (Co, W, and U) among 3425 participants aged 20 years and older in the USA. Our results demonstrated that W and U were significantly associated with asthma. U was associated with asthma among population aged 20 years and older, and W was associated with asthma among population aged 60 years and older. In addition, U showed a positive relationship with asthma in the upper quartiles in male and female. Furthermore, a remarkable association was discovered between U and asthma in all quartiles compared with reference among population without hay fever.
Although few studies have investigated the association between Co and asthma, no definitive evidence has been discovered in general population (Christensen and Poulsen, 1994, Lauwerys and Lison, 1994, Stefaniak et al. 2007, Swennen et al. 1993). In this study, no evidence was found between Co and asthma, which was conformed with the study of Huang et al. conducted in a general hospital in China (Huang et al. 2016). Co distributed in the body once being absorbed and mainly excreted in the urine with a half-life of several hours to hebdomad (Mendy et al. 2012). Urinary Co reflects recent exposure levels, but urinary levels in occupational exposure groups are often higher than in the general population. Therefore, the representativeness of the general population was better to explain the relationship between Co exposure and asthma in the general population.
In line with our findings, previous researches have shown that W exposure was positively associated with asthma (Huang et al. 2016, Mendy et al. 2012, Stefaniak et al. 2007). We performed this study to reveal the association between urinary heavy metals and asthma and conducted a detailed stratified analysis to explore the effects of various risk factors on the association of urinary heavy metals and asthma. W exposure might cause a marked inflammatory response in lung tissue, and leukocyte exudation might invade the alveolar area of the lung (Huang et al. 2016). W also caused lung diseases (“hard metal diseases”) proposed for decades (Mendy et al. 2012). Intriguingly, a mixed exposure of W and Co induced occupational asthma (Stefaniak et al. 2009). However, the underlying mechanism of W and asthma is not mentioned.
Previous studies have focused on the adverse effects of U exposure on the respiratory system (Huang et al. 2016, Mendy et al. 2012). Our findings showed that U was positively related with asthma, which was consistent with the research from Wuhan, China (Huang et al. 2016). Inhaled U exists for several years in lungs; therefore, lung tissue was one of its target organs. U particles < 5 μm in size can penetrate deep into the alveoli, causing toxicological effects at the site of contact (Monleau et al. 2006). Studies have found that the immune system is one of the most sensitive systems for damage caused by chronic U poisoning (Hao et al. 2013). Abnormalities in the immunoglobulin Ig E pathway are often considered to be a major feature of allergic asthma and are related not only to asthma attacks but also to their acute exacerbations (Gong et al. 2018).
The etiology of asthma is multifactorial: heredity, epigenetic, developmental, and environmental factors all play roles in biological process. Airway inflammation and oxidative stress are also considered as the primary pathways through which metals induce and influence the development of asthma (Huang et al. 2016). Heavy metals may affect immunocompetent cells, which can induce immunocompetent cells releasing a variety of cytokines. These cytokines may act on a variety of cells in the proinflammatory response (Cohn et al. 2004). Activated immune cells experience respiratory burst and produce oxidants, such as reactive oxygen species. It was reported that they were sensitive to acetylcholine-induced contraction of airway muscle, induced airway hyper responsiveness (AHR), and increased mucus secretion and epithelial shedding (Huang et al. 2016, Katsumata et al. 1990, Nabe et al. 2012, Schneider et al. 2012, Zuo and Clanton, 2005). The link between inflammation and oxidative stress may create a positive feedback loop that aggravates asthma (Huang et al. 2016).
To the best of our knowledge, our study was one of the few studies investigating the relationships between urinary heavy metals and asthma in the USA, which provided more epidemiological evidence to the subject. However, our study had several limitations. First, since this was a cross-sectional design, our research prevented us from explicitly identifying the causal relationships observed. Second, although we adjusted for potential confounding factors, the lack of documentation of confounding factors in the analysis also affected the findings and residual confounding remained. For example, outdoor air pollutants ozone have been associated with increased risk of asthma, such as nitrogen dioxide and particulate matter (Bowatte et al. 2017, Yang et al. 2018). Furthermore, exposure to high concentrations of fungus is also linked to asthma exacerbation and mortality (O'Hollaren et al. 1991). As the primary route of metal excretion, urine is the preferred noninvasive matrix for metal biomonitoring especially in surveys involving a large number of participants (Smolders et al. 2014). The absorbed heavy metals accumulate in tissues and organs with a half-life of several years or decades, which increases proportionally with urine output and can reflect long-term or short-term exposure of heavy metals (Mendy et al. 2012). Thus, our study used the single measured urinary heavy metal concentrations to reflect the long-term exposure to heavy metals. However, the use of single urinary sample could influence the association between heavy metals and asthma because of short-term variability of metal excretion and urine dilution. In future, we will consider to collect urinary or serum samples at multiple time points for dynamic analysis based on a large cohort.
Conclusions
In summary, our study revealed significant associations between urinary heavy metals (W and U) and asthma among the study group aged 20 years and older. Although the exact mechanism of action of heavy metals on asthma has not been fully elucidated, our findings will help better to understand asthma from an epidemiological perspective.
Data availability
The datasets analyzed during the current study are available in the NHANES repository (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Surveys
- MECs:
-
Mobile examination centers
- ICP-MS:
-
Inductively coupled plasma-mass spectrometry
- Co:
-
Cobalt
- W:
-
Tungsten
- U:
-
Uranium
- BMI:
-
Body mass index
- PIR:
-
Ratio of family income to poverty
- SE:
-
Standard error
- CI:
-
Confidence intervals
- OR:
-
Odd ratio
- cOR:
-
Crude odd ratio
- aOR:
-
Adjusted odd ratio
- GM:
-
Geometric mean
- Ref:
-
Reference
References
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113:192–200
Bowatte G, Erbas B, Lodge CJ, Knibbs LD, Gurrin LC, Marks GB, Thomas PS, Johns DP, Giles GG, Hui J, Dennekamp M, Perret JL, Abramson MJ, Walters EH, Matheson MC, Dharmage SC (2017) Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. Eur Respir J 50
Cadeddu C, Capizzi S, Colombo D, Nica M, De Belvis AG (2016) Literature review of gender differences in respiratory conditions: a focus on asthma and chronic obstructive pulmonary disease (COPD). Ig Sanita Pubbl 72:481–504
CDC (2018) Centers for Disease Control and Prevention. Data, statistics, and surveillance: asthma surveillance data. Asthma Prevalence and Health Care Resource Utilization Estimates, United States, 2001-2017
Choi JH, Lee B, Han KD, Hwang SH, Cho JH (2017) The impact of parity and age at first and last childbirth on the prevalence of delayed-onset asthma in women: The Korean National Health and Nutrition Examination Survey. Maturitas 97:22–27
Christensen JM, Poulsen OM (1994) A 1982-1992 surveillance programme on Danish pottery painters. Biological levels and health effects following exposure to soluble or insoluble cobalt compounds in cobalt blue dyes. Sci Total Environ 150:95–104
Cirla AM (1994) Cobalt-related asthma: clinical and immunological aspects. Sci Total Environ 150:85–94
Cohn L, Elias JA, Chupp GL (2004) Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 22:789–815
Croisant S (2014) Epidemiology of asthma: prevalence and burden of disease. Advances in experimental medicine and biology 795:17–29
Davison AG, Haslam PL, Corrin B, Coutts II, Dewar A, Riding WD, Studdy PR, Newman-Taylor AJ (1983) Interstitial lung disease and asthma in hard-metal workers: bronchoalveolar lavage, ultrastructural, and analytical findings and results of bronchial provocation tests. Thorax 38:119–128
Dharmage SC, Perret JL, Custovic A (2019) Epidemiology of asthma in children and adults. Front Pediatr 7:246
Dratva J, Caviezel S, Schaffner E, Stolz D, Rothe T, Kuenzli N, Schmidt-Trucksass A, Zemp E, Probst-Hensch N (2018) Is there a gender-specific association between asthma and carotid intima media thickness in Swiss adolescents? European journal of pediatrics 177:699–707
Ferrante G, La Grutta S (2018) The burden of pediatric asthma. Front Pediatr 6:186
Fischer T, Rystedt I (1983) Cobalt allergy in hard metal workers. Contact Dermatitis 9:115–121
Gheysens B, Auwerx J, Van den Eeckhout A, Demedts M (1985) Cobalt-induced bronchial asthma in diamond polishers. Chest 88:740–744
Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Marks GB, Baraket M, Powell H, Taylor SL, Leong LEX, Rogers GB, Simpson JL (2017) Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. The Lancet 390:659–668
Gong F, Zhu HY, Zhu J, Dong QJ, Huang X, Jiang DJ (2018) Circulating CXCR5(+)CD4(+) T cells participate in the IgE accumulation in allergic asthma. Immunol Lett 197:9–14
Graubard BI, Korn EL (1999) Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst 91:1005–1016
Hao Y, Ren J, Liu J, Yang Z, Liu C, Li R, Su Y (2013) Immunological changes of chronic oral exposure to depleted uranium in mice. Toxicology 309:81–90
Harris JR, Magnus P, Samuelsen SO, Tambs K (1997) No evidence for effects of family environment on asthma. A retrospective study of Norwegian twins. Am J Respir Crit Care Med 156:43–49
Huang X, Xie J, Cui X, Zhou Y, Wu X, Lu W, Shen Y, Yuan J, Chen W (2016) Association between concentrations of metals in urine and adult asthma: a case-control study in Wuhan, China. PLoS One 11:e0155818
Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK (2014) National health and nutrition examination survey: sample design, 2011-2014. Vital Health Stat 2:1–33
Jung JY, Leem AY, Kim SK, Chang J, Kang YA, Kim YS, Park MS, Kim SY, Kim EY, Chung KS (2015) Relationship between blood levels of heavy metals and lung function based on the Korean National Health and Nutrition Examination Survey IV–V. International Journal of Chronic Obstructive Pulmonary Disease:1559
Katsumata U, Miura M, Ichinose M, Kimura K, Takahashi T, Inoue H, Takishima T (1990) Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. Am Rev Respir Dis 141:1158–1161
Kim S, Kim S, Won S, Choi K (2017) Considering common sources of exposure in association studies - urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone balance in the NHANES 2007-2008. Environ Int 107:25–32
Koppelman GH, Los H, Postma DS (1999) Genetic and environment in asthma: the answer of twin studies. Eur Respir J 13:2–4
Lauwerys R, Lison D (1994) Health risks associated with cobalt exposure--an overview. Sci Total Environ 150:1–6
Lee SW, Yon DK, James CC, Lee S, Koh HY, Sheen YH, Oh JW, Han MY, Sugihara G (2019) Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: age-stratified time-series analysis. J Allergy Clin Immunol 144:1542–1550.e1
Lehmann I (2017) Environmental pollutants as adjuvant factors of immune system derived diseases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 60:592–596
Liao KW, Pan WH, Liou SH, Sun CW, Huang PC, Wang SL (2019) Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ Sci Pollut Res Int
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116:1473–1479
Mejias SG, Ramphul K (2018) Prevalence and associated risk factors of bronchial asthma in children in Santo Domingo, Dominican Republic. Cureus 10:e2211
Mendy A, Gasana J, Vieira ER (2012) Urinary heavy metals and associated medical conditions in the US adult population. Int J Environ Health Res 22:105–118
Menke A, Guallar E, Cowie CC (2016) Metals in urine and diabetes in U.S. adults. Diabetes 65:164–171
Miyake Y, Tanaka K, Yasutake A, Sasaki S, Hirota Y (2011) Lack of association of mercury with risk of wheeze and eczema in Japanese children: the Osaka Maternal and Child Health Study. Environ Res 111:1180–1184
Mohammad HR, Belgrave D, Kopec Harding K, Murray CS, Simpson A, Custovic A (2016) Age, sex and the association between skin test responses and IgE titres with asthma. Pediatr Allergy Immunol 27:313–319
Monleau M, De Meo M, Paquet F, Chazel V, Dumenil G, Donnadieu-Claraz M (2006) Genotoxic and inflammatory effects of depleted uranium particles inhaled by rats. Toxicol Sci 89:287–295
Nabe T, Ikedo A, Hosokawa F, Kishima M, Fujii M, Mizutani N, Yoshino S, Ishihara K, Akiba S, Chaplin DD (2012) Regulatory role of antigen-induced interleukin-10, produced by CD4(+) T cells, in airway neutrophilia in a murine model for asthma. Eur J Pharmacol 677:154–162
Nemery B (1990) Metal toxicity and the respiratory tract. Eur Respir J 3:202–219
Nurmagambetov T, Kuwahara R, Garbe P (2018) The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc 15:348–356
O'Brien KM, Upson K, Buckley JP (2017) Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Curr Environ Health Rep 4:44–50
Oburger E, Vergara Cid C, Preiner J, Hu J, Hann S, Wanek W, Richter A (2018) pH-dependent bioavailability, speciation, and phytotoxicity of tungsten (W) in soil affect growth and molybdoenzyme activity of nodulated soybeans. Environ Sci Technol 52:6146–6156
O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, Sachs MI (1991) Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med 324:359–363
Park S, Lee EH, Kho Y (2016) The association of asthma, total IgE, and blood lead and cadmium levels. J Allergy Clin Immunol 138:1701–1703.e6
Pignataro FS, Bonini M, Forgione A, Melandri S, Usmani OS (2017) Asthma and gender: the female lung. Pharmacol Res 119:384–390
Rosa MJ, Benedetti C, Peli M, Donna F, Nazzaro M, Fedrighi C, Zoni S, Marcon A, Zimmerman N, Wright R, Lucchini R (2016) Association between personal exposure to ambient metals and respiratory disease in Italian adolescents: a cross-sectional study. BMC Pulm Med 16:6
Rzehak P, Schoefer Y, Wichmann HE, Heinrich J (2008) A prospective study on the association between hay fever among children and incidence of asthma in East Germany. Eur J Epidemiol 23:17–22
Sauni R, Linna A, Oksa P, Nordman H, Tuppurainen M, Uitti J (2010) Cobalt asthma--a case series from a cobalt plant. Occup Med (Lond) 60:301–306
Schneider BC, Constant SL, Patierno SR, Jurjus RA, Ceryak SM (2012) Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol 259:38–44
Shumate AM, Yeoman K, Victoroff T, Evans K, Karr R, Sanchez T, Sood A, Laney AS (2017) Morbidity and health risk factors among New Mexico miners: a comparison across mining sectors. J Occup Environ Med 59:789–794
Smolders R, Koch HM, Moos RK, Cocker J, Jones K, Warren N, Levy L, Bevan R, Hays SM, Aylward LL (2014) Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 1: metals. Toxicol Lett 231:249–260
Stefaniak AB, Day GA, Harvey CJ, Leonard SS, Schwegler-Berry DE, Chipera SJ, Sahakian NM, Chisholm WP (2007) Characteristics of dusts encountered during the production of cemented tungsten carbides. Ind Health 45:793–803
Stefaniak AB, Virji MA, Day GA (2009) Characterization of exposures among cemented tungsten carbide workers. Part I: size-fractionated exposures to airborne cobalt and tungsten particles. Journal of exposure science & environmental epidemiology 19:475–491
Sullivan PW, Kavati A, Ghushchyan VH, Lanz MJ, Ortiz B, Maselli DJ, LeCocq J (2019) Impact of allergies on health-related quality of life in patients with asthma. J Asthma:1–10
Swennen B, Buchet JP, Stanescu D, Lison D, Lauwerys R (1993) Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Br J Ind Med 50:835–842
Tan DJ, Walters EH, Perret JL, Lodge CJ, Lowe AJ, Matheson MC, Dharmage SC (2015) Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature. Expert review of respiratory medicine 9:109–123
Urushidate S, Matsuzaka M, Okubo N, Iwasaki H, Hasebe T, Tsuya R, Iwane K, Inoue R, Yamai K, Danjo K, Takahashi I, Umeda T, Ando S, Itai K, Nakaji S (2010) Association between concentration of trace elements in serum and bronchial asthma among Japanese general population. J Trace Elem Med Biol 24:236–242
Wang YX, Sun Y, Huang Z, Wang P, Feng W, Li J, Yang P, Wang M, Sun L, Chen YJ, Liu C, Yue J, Gu LJ, Zeng Q, Lu WQ (2016) Associations of urinary metal levels with serum hormones, spermatozoa apoptosis and sperm DNA damage in a Chinese population. Environ Int 94:177–188
Wang X, Mukherjee B, Park SK (2018) Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003-2014. Environ Int 121:683–694
Wu KG, Chang CY, Yen CY, Lai CC (2018) Associations between environmental heavy metal exposure and childhood asthma: a population-based study. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi
Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M (2019) The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med 200:1102–1112
Yang M, Chu C, Bloom MS, Li S, Chen G, Heinrich J, Markevych I, Knibbs LD, Bowatte G, Dharmage SC, Komppula M, Leskinen A, Hirvonen MR, Roponen M, Jalava P, Wang SQ, Lin S, Zeng XW, Hu LW, Liu KK, Yang BY, Chen W, Guo Y, Dong GH (2018) Is smaller worse? New insights about associations of PM1 and respiratory health in children and adolescents. Environ Int 120:516–524
Zeng Q, Feng W, Zhou B, Wang YX, He XS, Yang P, You L, Yue J, Li YF, Lu WQ (2015) Urinary metal concentrations in relation to semen quality: a cross-sectional study in China. Environ Sci Technol 49:5052–5059
Zeng X, Xu X, Zheng X, Reponen T, Chen A, Huo X (2016) Heavy metals in PM2.5 and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environmental pollution 210:346–353
Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X, Xia Y (2019) Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int 123:325–336
Zuo L, Clanton TL (2005) Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol 289:C207–C216
Acknowledgments
We thank all the research staff and students who took part in this work.
Funding
Funding is provided by grants from the National Key Research and Development Program of China (2017YFC0211605 and 2017YFC0211606), the National Natural Science Foundation of China (81671461 and 81502832), and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine) and the Scientific Research Development Fund of Kangda College, Nanjing Medical University (KD2018KYJJZD003).
Author information
Authors and Affiliations
Contributions
XL and YF designed this research and performed overall project management. XL drafted the initial manuscript. Statistical analysis was performed by XL with YZ, XH, ZH, MY, QX, and XH. CL and XW conceived of the study and participated in its design. CL and XW directed the study and had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
NHANES received approval from the National Center for Health Statistics Research Ethics Review Board (Protocol #2011-17), and informed consent was obtained for all enrolled participants.
Consent for publication
Not applicable.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Li, X., Fan, Y., Zhang, Y. et al. Association between selected urinary heavy metals and asthma in adults: a retrospective cross-sectional study of the US National Health and Nutrition Examination Survey. Environ Sci Pollut Res 28, 5833–5844 (2021). https://doi.org/10.1007/s11356-020-10906-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10906-w