Abstract
Nanocomposite materials can enhance the capabilities of water treatment processes such as photocatalysis. In this work, novel light-driven nanocatalysts were synthesized by using nickel ferrite (NiFe2O4) to nanoenable graphene oxide (GO) substrates. GO is an emerging 2D nanomaterial with high conductivity and adsorption properties. Moreover, the electric properties of GO improve photocatalytic performance by promoting charge carrier separation. Results of the characterization of the nickel ferrite nanoenabled graphene oxide (NiFe2O4@GO) nanocomposites demonstrate that homogeneous and stable photocatalysts were produced. The as-synthesized nanocatalysts enabled complete decolorization of the colored water matrix in short irradiation times of 150 min using minimal catalyst loading at 0.5 g L−1. The selective hook and destroy mechanism reduced the competitive effect of co-existing ions in solution. Furthermore, the use of specific scavengers helped to elucidate the degradation mechanisms of organic dye methylene blue by NiFe2O4@GO nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile manufacturing is a water-intensive process that consumes over 200 kg of water per kg of fabric produced. In particular, the dying process for fabrics requires large volumes of water which result in the release of colored effluents (Brillas and Martínez-Huitle 2015). Green manufacturing processes should consider approaches that reduce the production of wastewater effluents while enabling water reuse strategies (Mansour et al. 2018). Idiosyncrasies of manufacturing countries must be considered when evaluating water reuse technologies to be adopted. Mid-sized textile industries in developing countries are required to implement fit-for-purpose technologies that eliminate color and reuse water from dye bath effluents (Soares et al. 2016). However, these technologies should be low-cost processes with small physical footprint.
Light-driven processes are emerging approaches that have been explored for environmental applications in different contexts (Fagan et al. 2016; Marcelino and Amorim 2019; Tugaoen et al. 2017). In photocatalysis, for example, the electrons from the valence band of a semiconductor material are photo-excited to the conduction band by photons from an irradiation source (Loeb et al. 2019; de Luna et al. 2018). Only photons of higher energy than the semiconductor bandgap (Eg) enable photoexcitation. The photoexcitation reaction (1) results in the generation of two charge carriers: reductive electrons (ecb−) and oxidant holes (hvb+) (Dominguez et al. 2017; Khedr et al. 2017). Photogenerated hvb+ are highly oxidative species that can yield hydroxyl radical (•OH) from water oxidation reaction (2) (Lin et al. 2018; Villaluz et al. 2019). Organic pollutants in water can then be degraded by both oxidant species (i.e., hvb+ and •OH), which can result in fast decolorization of recalcitrant dyes from textile industry dyebaths (Batista et al. 2017; de Luna et al. 2016). However, process efficiency might be diminished by the recombination of charge carriers following reaction (3) (Fagan et al. 2016; Tugaoen et al. 2018).
Nanocomposite materials can increase photocatalytic treatment efficiencies by employing engineering design strategies that exploit the unique properties of nanomaterials (Perreault et al. 2015). Graphene oxide (GO) is a two-dimensional nanomaterial formed by a single layer of carbon atoms in a patterned hexagonal lattice. GO has excellent electrical properties and when combined with a semiconductor catalyst can act as electron sink, which contributes to reduce the extent of recombination reaction (3) by enhancing charge carrier separation (Lin et al. 2016a; Moreira et al. 2018). In addition, the planar structure of GO has proven to favor selective adsorption of dye molecules through π stacking interaction with aromatic rings (Apul et al. 2012; Ersan et al. 2019). This can enhance the pollutant degradation capability of heterogeneous catalysts, usually limited by mass transfer across increasing concentration of organics on the catalyst surface, through a hook and destroy approach (Lee et al. 2018).
This work explores the properties of earth-abundant materials based on nickel ferrite (NiFe2O4) semiconductor as photocatalyst (AlSalka et al. 2019; Chirik and Morris 2015). This earth-abundant and inexpensive metal oxide was used to nanoenable GO. The method described herein allowed the formation of homogeneous and stable nanocomposites with excellent photocatalytic properties that were evaluated in terms of removal of methylene blue (MB), a typical aromatic dye molecule. Material characterization was compared against pristine GO used as substrate of NiFe2O4. Effects of experimental variables were evaluated to define optimum operational conditions. Moreover, experiments to explore the impact of co-existing ions in more complex water matrices were conducted.
Experimental
Materials and chemicals
All chemicals were of analytical grade except when specified. Ultrapure deionized water (> 18 MΩ cm) from a Barnstead compact ultrapure water system (USA) was used in all the experiments. Methylene blue dye (C16H18ClN3S) purchased from Acros Organics was used as the model compound. Sodium nitrate (NaNO3), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), and hydrochloric acid (HCl) that were used during GO synthesis were all purchased from Showa Chemicals, Inc. and Merck. Nanoenabling was done using nickel (II) nitrate hexahydrate (N2NiO6•6H2O) from Alfa Aesar and iron (III) nitrate nonahydrate (Fe(NO3)2·9H2O) from Merck.
Synthesis of graphene and graphene–metal ferrite catalysts
GO was prepared from natural graphene powder following Hummers’ method (Hou et al. 2020; Muzyka et al. 2017). Graphene powder (5 g) was added to 115 mL of concentrated H2SO4 with 0.25 M Na2SO4 and kept under stirring for 10 min in an ice bath. Then, 15 g of KMnO4 was slowly added to the graphene suspension and stirred for 30 min at 35 °C. After this step, 230 mL of ultrapure water was added to the mixture, and the resulting mixture was heated to 98 °C. The mixture was kept under vigorous stirring at this temperature for 15 min. Aqueous solution of H2O2 at 3% v/v was added, which turned the color of the solution from dark brown to bright yellow. The supernatant was collected and centrifuged. Recovered GO powders were washed first with 5% HCl and then ultrapure water to eliminate the metal ion. Obtained GO was dispersed in water.
Photocatalytic nanocomposites of NiFe2O4@GO were prepared following a solution combustion synthesis route (Kumar et al. 2017). Briefly, GO (0.150 g) was dispersed in a solution containing 1.48 mL of ultrapure water and 18 mL ethanol. Subsequently, 20 mL of solution containing 0.05 mM Ni2+ and 0.1 mM Fe3+ was added to the GO dispersion and kept under stirring for 1 h. After adding urea (8 mM), the solution was heated to 100 °C until the formation of a gel-like precursor that was subsequently incinerated at 400 °C for 30 min. This procedure resulted in a black foam-type NiFe2O4@GO nanocomposite.
Analytical methods
Scanning electron microscopy (SEM) images of the nanoenabled GO were obtained using a FEI Quanta 200 SEM. Crystallographic characterization was conducted with a Bruker D8 X-ray diffractometer (XRD). Textural properties of the nanocomposites were determined using the Brunauer–Emmett–Teller (BET) method for specific surface area estimation and Barett–Joyner–Halenda (BJH) to determine pore size distribution. These calculations were based on N2 adsorption–desorption at 77 K using Micromeritics 2020. Further characterization analyses of nanocomposites were conducted using a Fourier-transform infrared spectrophotometer DR 6000 from HACH and X-ray photoelectron spectrometer ESCA model PHI 500 from VersaProbe. Decolorization kinetics was evaluated from the abatement of solution absorbance of filtered samples using an UV-vis spectrophotometer (Thermo Spectronic Genesys 20) at the characteristic maximum absorption MB wavelength of 665 nm. The point-of-zero charge pH (pHPZC) was determined by using the drift method (Dos Santos et al. 2019). NiFe2O4@GO nanocomposite (0.05 g) was added to solutions of 50 mL of 0.01 M NaCl pre-adjusted at different pH (1.0–11.0). These solutions were kept under constant stirring at 25 °C for 48 h, then the final pH was measured. The pHPZC was identified as the intersection of the representation of pHfinal = pHinitial with the plot of final pH measured for each solution pH initial. The reactive oxygen species involved in the photocatalytic MB degradation were identified with the use of selective scavengers. Ammonium oxalate, p-benzoquinone, and tert-butyl alcohol were used as scavengers for holes, superoxide radicals, and hydroxyl radicals, respectively (de Luna et al. 2019b; Sun et al. 2017). In each run, 5 mM of scavenger species was added to the mixture containing 0.04-mM MB dye and 0.5 g L−1 NiFe2O4@GO.
Photocatalytic degradation runs
The photocatalytic activity of NiFe2O4@GO was evaluated from the decolorization of 500-mL solutions of MB containing different doses of photocatalyst in a slurry. Experiments were carried out under comparable conditions in a cylindrical batch photoreactor (114-mm diameter and 143-mm height) under magnetic stirring to ensure transport of reagents towards/from the photocatalytic surface as it is commonly considered for heterogeneous catalytic processes. Photocatalyst NiFe2O4@GO was added to the solution and kept in the dark under continuous stirring for 30 min to ensure solution homogenization and to attain adsorption equilibria. Thereafter, the solution was irradiated and the photocatalytic reactions proceeded for 120 min. Hence, the reported total treatment time of 150 min includes both stages: dark adsorption and photocatalysis. Blank experiments were performed for 120 min to compare (i) adsorption in the dark, (ii) photodegradation, and (iii) photocatalysis. Solution pH did not change during treatment. Four monochromatic 10-W UV lamps (λ = 254 nm) were used as the irradiation source. The reaction was maintained at constant temperature using a cooling water jacket around the reactor.
Results and discussion
Characterization of photocatalysts
Figure 1a illustrates the characteristic layered sheet morphology of GO, whereas micrography of Fig. 1b highlights the notable difference with respect to nanoenabled NiFe2O4@GO. Nanoparticles of NiFe2O4 were homogenously distributed on a GO thin film, displaying attachment between the components of the composite nanomaterial (Liu et al. 2013).
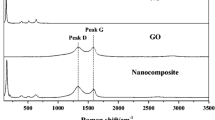
The XRD analysis of pristine GO depicts a characteristic strong peak at 2θ of 10° associated with the interlayer spacing induced by the presence of the oxygen functional group (Thema et al. 2013). The wide peak observed is associated with plane (002) of graphite. The nanocomposite NiFe2O4@GO photocatalyst depicts a differentiated XRD pattern from pristine GO, which allows inferring a homogeneous dispersion of NiFe2O4 on the GO sheets as observed in the SEM images (see Fig. 1). The peaks observed in NiFe2O4@GO correspond to a single-phase spinel-type structure (JCPDS 54-0964) with characteristic crystal planes identified in Fig. 2a (Sivakumar et al. 2012). The crystalline structure of NiFe2O4 with atomic occupancy is described as (Fe3+)A[Ni2+ Fe3+]B\( {\mathrm{O}}_4^{2-} \), where A and B denote tetrahedral and octahedral sites in the spinel structure (Liu et al. 2013), respectively. The adsorption–desorption study allowed to determine a specific surface area (SBET) of 76.7 m2 g−1, average pore diameter of 7.76 nm, and pore volume of 0.15 cm3 g−1.
Fourier-transform infrared (FT-IR) was used to investigate the surface functional groups of GO and NiFe2O4@GO. Figure 2b depicts the existence of oxygen containing functional groups on the graphene surface, which demonstrates successful oxidation treatment. The strong peak at 3420 cm−1 can be associated with the stretching vibration of OH groups. Characteristic peaks associated with C=O stretching vibration at 1727 cm−1, C=C stretching at 1633 cm−1, O–H deformation at 1400 cm−1, C–O (epoxy) stretching vibration at 1200 cm−1, and C–O (alcoxy) at 1054 cm−1 were clearly observed (He et al. 2015; Zhang et al. 2017). The FT-IR spectra of the nanocomposite NiFe2O4@GO have bands assigned to the vibration of ions in crystal lattices, which indicate the presence of homogeneously distributed ferrite on the surface. The peak observed at 400 cm−1 is assigned to the octahedral–metal stretching Ni–O, while the bands at 582 cm−1 and 687 cm−1 are associated with the Fe–O vibrations (Gautam et al. 2016; Saire-Saire et al. 2019). The bandgap of NiFe2O4@GO nanocomposites was evaluated by UV-diffuse reflectance spectroscopy. The Tauc plot denotes an energy bandgap (Eg) of 2.9 eV. The nanocomposite point-of-zero charge (PZC) pHPZC = 7.2 was determined by using the pH drift method.
The elemental compositions of GO and NiFe2O4@GO were investigated by using XPS (Fig. 2c). The XPS spectrum of pristine GO showed only the peaks of carbon C1s at 280 eV and oxygen O1s at 526 eV, which are characteristic of graphene layers functionalized with oxygen groups. The XPS spectrum changed when GO was nanoenabled by NiFe2O4. While the signals of carbon and oxygen remained, the intensity of O1s increased due to the higher content of oxygen associated with the metal oxide nanoparticles. Moreover, small signals of nickel and ferrite were found at 849 eV (Ni2p3) and 703 eV (Fe2p3), respectively. The elemental composition of the NiFe2O4@GO nanocomposite included carbon (53%), oxygen (32%), ferrite (8.2%), and nickel (6.7%).
Benchmarking photocatalytic activity of nanocomposite against pristine graphene oxide
The photocatalytic activity of the nanocomposite was evaluated from the comparative treatment of 0.04-mM MB synthetic effluents. Figure 3 compares the decolorization of dye-containing solutions under different experimental conditions. As shown, UV irradiation of solutions in the absence of a catalyst did not result in appreciable decolorization, which can be justified by the high photostability of organic dyes. Addition of 0.5 g L−1 of pristine GO in the dark (no UV irradiation) resulted in a slight decolorization of ~ 6.2% that can be attributed to minimal dye adsorption on the GO layers (Ersan et al. 2019). Experiments conducted with GO under UV irradiation reported similar degree of decolorization, from which can be inferred the null photocatalytic activity of pristine GO under the stated experimental conditions. However, nanoenabling of GO by NiFe2O4 enhanced the adsorption capabilities of the resulting material as depicted in Fig. 3, reaching close to 40% color removal. This can be explained by MB coordination with the metal centers of NiFe2O4 nanoparticles. However, almost complete decolorization by NiFe2O4@GO is attained under UV irradiation. These experimental results demonstrate that the nanoenabled GO composites are photoactive and induce MB degradation through photocatalytic mechanisms (AlSalka et al. 2019).
Understanding the effects of initial dye concentration and catalyst loading
Industrial effluents contain different concentrations of organic dyes depending if those are directly treated after the dye bath or are the results of blending with other water effluents produced during the manufacturing process. The effect of initial MB concentration on photocatalytic decolorization performance of 0.5 g L−1 of nanocomposite NiFe2O4@GO was evaluated. As can be observed in Fig. 4, the percentage of decolorization at a given treatment time is lowered for increasing concentrations of MB. This trend can be explained by two main factors. First, the light transport is hindered by increasing concentration of the dye that absorbs delivered photons (Spasiano et al. 2015; Zuorro et al. 2019). This effect results in a lower photogeneration of oxidants according to reaction (1) due to the virtually lower amounts of photons that reach the surface of the NiFe2O4@GO catalyst. Second, not only the amounts of oxidants potentially generated decrease due to the competitive absorption of delivered photons but also the ratio between target pollutant molecules and photogenerated oxidants (Lin et al. 2016b; Wang et al. 2018). In other words, the larger quantities of organic molecules compete for the available amount of oxidant species (i.e., hvb+ and •OH). These combined effects result in slower photocatalytic decolorization for increasing MB concentrations. Note that according to the experimental results shown in Fig. 4, these effects become more noticeable when the initial dye concentration exceeds 0.04 mM. Higher dye concentrations would require higher photon doses and longer hydraulic retention times in photocatalytic converters (Heck et al. 2019). In this frame, it can be concluded that dye effluents can be treated when not exceeding concentration of 0.05 mM of MB.
The dose of catalyst is a variable that has high impact in terms of capital expenditures associated with the cost of the catalyst requirements. Defining required dose of catalyst for optimum operation is required to ensure minimum capital and operational expenditures when considering scaled-up photoreactors (Fagan et al. 2016; Spasiano et al. 2015). Figure 5 shows the impact of NiFe2O4@GO catalyst dose in the photocatalytic decolorization of 0.04-mM solutions of MB. Decolorization performance is enhanced by increasing the catalyst dose from 0.1 to 0.5 g L−1. This trend can be explained by the increased availability of catalytic sites resulting in higher production of photogenerated hvb+ and •OH (Booshehri et al. 2017; Deng et al. 2018). It is important to highlight that these heterogeneous oxidation processes occur on the surface or near the surface of the nanocatalyst. The adsorption capabilities of the nanocatalysts enable pollutant degradation through a hook and destroy mechanism (Lee et al. 2018; Zhao et al. 2016), which targets dye molecules concentrated near the catalytic centers. However, it can be observed in Fig. 5 that higher doses of photocatalyst drastically decrease its degradation capabilities. These deleterious effects observed at higher catalyst dosage can be explained by different factors that occur simultaneously at high catalyst concentrations. The solution opacity at higher catalyst doses diminishes effective light delivery and radiation penetration in the solution, which renders a small volume of slurry solution capable of being photoactivated (Tugaoen et al. 2017). In a similar fashion, enhanced light-scattering effects also reduce UV light penetration and therefore the amount of photogenerated oxidants (Marcelino and Amorim 2019; Medidi et al. 2018). Despite the increased availability of catalytic centers with high catalyst dose, the lower quantum yield due to hindered light transport diminishes treatment performance. Some authors suggest that nanoparticles agglomerate at higher catalyst dosage; however, such effect was not observed here due to the ferromagnetic properties of the nanocomposites that prevent their agglomeration (Dominguez et al. 2017; Saire-Saire et al. 2019). According to the experimental results, an optimum catalyst dosage of 0.5 g L−1 was identified.
Describing the influence of initial solution pH on NiFe2O4@GO nanocomposite activity
Solution pH is a relevant variable that affects the surface charge of the photocatalytic materials as well as the speciation of target pollutants in solution. Material characterization indicates that the pHPZC of NiFe2O4@GO was 7.2. The photocatalytic NiFe2O4@GO nanocomposite surface is positively charged below circumneutral pH conditions, whereas it remains negatively charged under more alkaline conditions when pH > pHPZC (Perreault et al. 2015). On the other hand, methylene blue is a cationic organic dye that remains positively charged in solution (de Luna et al. 2019a). As shown in Fig. 6, the photocatalytic activity of 0.5 g L−1 NiFe2O4@GO for MB decolorization increases from 48.03 at pH 3.0 to 84.35% at pH 7.0 and 90.84% at pH 10.0. The slow degradation kinetics in acidic media suggests that the reaction solution is more favorable in alkaline conditions than in acidic and neutral surroundings. This experimental trend can be explained by the electrostatic repulsion at acidic pH between the positively charged NiFe2O4@GO and the positively charged MB, whereas operational pH above pHPZC favors electrostatic attraction and adsorption of dye on the nanocomposite surface, which enhances degradation kinetics following a hook and destroy approach mediated by photogenerated oxidants hvb+ and •OH.
Evaluating the impact of co-existing ions in the water matrix
Dye baths are composed of organic dyes and different electrolytes as adjuvants. The most commonly found anionic species found in solution are sulfates, chloride, and carbonates. In order to evaluate the possible deleterious effects of ions in complex water matrices, experiments in the presence of 500 mg L−1 of these different sodium salts were performed. Figure 7 shows that sodium sulfate has a null effect on MB decolorization, which is explained by its inert character. Chloride anion is an ubiquitous electrolyte in different water sources including textile effluents. With chloride ions, a slight decrease in decolorization kinetics is noted that attains 83% decolorization after 120 min of photocatalytic treatment vs the 91% observed in ultrapure water. The decrease in dye removal efficiency can be explained by the oxidation of chloride by photogenerated oxidants to produce active chlorine species such as Cl2 (E° = 1.36 V vs standard hydrogen electrode or SHE), HClO (E° = 1.49 V vs SHE), and ClO− (E° = 0.89 V vs SHE) (Kanakaraju et al. 2015; Mostafa et al. 2018). These species can oxidize the organics in the bulk of the solution but are considered weaker oxidants compared with •OH (E° = 2.80 V vs SHE) (Villaluz et al. 2019). In this context, the slight slower decolorization can be attributed to chloride oxidation that consumes hydroxyl radical yielding weaker oxidants that also contribute to the overall decolorization of the solution. On the other hand, a more adverse effect was noted in the presence of carbonates, which resulted in only 70% decolorization at the end of the run. This effect can be explained by the scavenging action of carbonates that consume photogenerated •OH (Fagan et al. 2016; Marcelino and Amorim 2019). Both bicarbonate (k = 8.5 × 106 M−1 s−1) and carbonate (k = 3.9 × 108 M−1 s−1) have a high kinetic rate constant with •OH, yielding as product the weaker oxidant known as carbonate radical (CO3•− , E° = 1.78 V vs SHE) following reactions (4) and (5).
It is important to remark that despite these effects, a relevant decolorization degree was achieved under all water matrix conditions considered. These results suggest the highly promising character of the NiFe2O4@GO nanocatalysts.
Identifying the oxidants involved through the selective use of scavenging species
Experimental results suggest that the photoactivity of the nanocomposite is enhanced due to a dual role of graphene oxide sheets. First, the graphene oxide facilitates the target pollutant molecules to be concentrated close to the photocatalyst surface by using a hook and destroy approach through a sequential adsorption–photocatalytic destruction mechanism (Lee et al. 2018; Li et al. 2020). Second, the highly conductive properties of the graphene oxide support contribute to diminish the rate of recombination reaction (3) by transporting electrons away from the NiFe2O4 surface, thereby promoting the charge carrier separation (Byrne et al. 2018; Moreira et al. 2018). Stability of charge carriers can be demonstrated by photoluminescence studies or proven by the higher photocatalytic degradation performance observed (Chang et al. 2011; Pan et al. 2011). Electrons stabilized on the graphene oxide sheet can yield superoxide radical (O2•−) via charge transfer reaction with dissolved oxygen following reaction (6), whereas the photogenerated hole by reaction (1) can directly oxidize MB or react with water yielding •OH from reaction (2).
In order to elucidate the role of reactive oxidant species and other photogenerated oxidants in the mechanistic decolorization of MB, decolorization experiments were conducted in the presence of selective scavengers as summarized in Fig. 8 (de Luna et al. 2019b). Benzoquinone, used as superoxide scavenger, diminished decolorization by only 10% and reveal the negligible effects of (i) direct charge transfer reduction processes and (ii) weaker oxidants such as superoxide radicals generated from the reaction between electron and dissolved oxygen according to reaction (6). A notable 27% decline in dye removal performance was noted when using tert-butyl alcohol as •OH scavenger. Similarly, a 35% decrease in MB decolorization was observed in the presence of ammonium oxalate as hole scavenger. These results suggest that the degradation mechanism of MB by NiFe2O4@GO catalysts is dually driven by oxidation mediated by •OH and hvb+.
Conclusions
NiFe2O4@GO was successfully prepared by using the solution combustion method. NiFe2O4@GO presents excellent photocatalytic activity under UV light irradiation due to charge carrier stabilization induced by the conductivity of graphene oxide, which allows charge carrier separation with diminished recombination. Furthermore, the combination of both nanomaterials enabled a hook and destroy mechanism where adsorption on the nanocomposite surface enhances pollutant degradation performance by overcoming mass transport limitations. Almost complete decolorization was attained within 120 min of UV irradiation with low catalyst dose of 0.5 g L−1. Maximum MB concentration for efficient photodegradation by NiFe2O4@GO composite was 0.04 mM. Higher concentrations would require longer operational times associated with lower kinetics due to limited photon transport through the highly colored solutions. Understanding of the role of competing ions in a solution was explored. The scavenging effect of carbonate was defined as the main cause of the deceleration of photocatalytic degradation kinetics in the presence of this oxyanion. Radical scavenging experiments reveal the dual contribution of photogenerated holes and hydroxyl radicals as main reactive oxidant species.
References
AlSalka Y, Granone LI, Ramadan W, Hakki A, Dillert R, Bahnemann DW (2019) Iron-based photocatalytic and photoelectrocatalytic nano-structures: facts, perspectives, and expectations. Appl Catal B Environ 244:1065–1095. https://doi.org/10.1016/j.apcatb.2018.12.014
Apul OG, Wang Q, Zhou Y, Karanfil T (2012) Adsorption of aromatic organic contaminants by graphene nanosheets: comparison with carbon nanotubes and activated carbon. Water Res 47:1648–1654. https://doi.org/10.1016/j.watres.2012.12.031
Batista LMB, dos Santos AJ, da Silva DR, Alves AP d M, Garcia-Segura S, Martínez-Huitle CA (2017) Solar photocatalytic application of NbO2OH as alternative photocatalyst for water treatment. Sci Total Environ 596–597:79–86. https://doi.org/10.1016/j.scitotenv.2017.04.019
Booshehri AY, Polo-Lopez MI, Castro-Alférez M, He P, Xu R, Rong W, Malato S, Fernández-Ibáñez P (2017) Assessment of solar photocatalysis using Ag/BiVO4 at pilot solar compound parabolic collector for inactivation of pathogens in well water and secondary effluents. Catal Today 281:124–134. https://doi.org/10.1016/j.cattod.2016.08.016
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166–167:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Byrne C, Subramanian G, Pillai SC (2018) Recent advances in photocatalysis for environmental applications. J Environ Chem Eng 6:3531–3555. https://doi.org/10.1016/j.jece.2017.07.080
Chang WS, Li YCM, Chung TW, Lin YS, Huang CM (2011) Toluene decomposition using silver vanadate/SBA-15 photocatalysts: DRIFTS study of surface chemistry and recyclability. Appl Catal A Gen 407:224–230. https://doi.org/10.1016/j.apcata.2011.08.043
Chirik P, Morris R (2015) Getting down to earth: the renaissance of catalysis with abundant metals. Acc Chem Res 48:2495. https://doi.org/10.1021/acs.accounts.5b00385
de Luna MDG, Lin JC-T, Gotostos MJN, Lu M (2016) Photocatalytic oxidation of acetaminophen using carbon self-doped titanium dioxide. Sustain Environ Res 26:161–167. https://doi.org/10.1016/j.serj.2016.02.001
de Luna MDG, Laciste MT, Tolosa NC, Lu M (2018) Effect of catalyst calcination temperature in the visible light photocatalytic oxidation of gaseous formaldehyde by multi-element doped titanium dioxide. Environ Sci Pollut Res 25:15216–15225
de Luna MDG, Garcia-Segura S, Mercado CH, Lin YT, Lu MC (2019a) Doping TiO2 with CuSO4 enhances visible light photocatalytic activity for organic pollutant degradation. Environ Sci Pollut Res 27:24604–24613. https://doi.org/10.1007/s11356-019-05789-5
de Luna MDG, Paragas LKB, Doong RA (2019b) Insights into the rapid elimination of antibiotics from aqueous media by tunable C3N4 photocatalysts: effects of dopant amount, co-existing ions and reactive oxygen species. Sci Total Environ 669:1053–1061. https://doi.org/10.1016/j.scitotenv.2019.03.003
Deng F, Zhang Q, Yang L, Luo X, Wang A, Luo S, Dionysiou DD (2018) Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl Catal B Environ 238:61–69. https://doi.org/10.1016/j.apcatb.2018.05.004
Dominguez S, Huebra M, Han C, Campo P, Nadagouda MN, Rivero MJ, Ortiz I, Dionysiou D (2017) Magnetically recoverable TiO2-WO3 photocatalyst to oxidize bisphenol A from model wastewater under simulated solar light. Environ Sci Pollut Res 24:12589–12598. https://doi.org/10.1007/s11356-016-7564-6
Dos Santos AJ, Batista LMB, Martínez-Huitle CA, Alves AP d M, Garcia-Segura S (2019) Niobium oxide catalysts as emerging material for textile wastewater reuse: photocatalytic decolorization of azo dyes. Catalysts 9:1070. https://doi.org/10.3390/catal9121070
Ersan G, Kaya Y, Ersan MS, Apul OG, Karanfil T (2019) Adsorption kinetics and aggregation for three classes of carbonaceous adsorbents in the presence of natural organic matter. Chemosphere 229:515–524. https://doi.org/10.1016/j.chemosphere.2019.05.014
Fagan R, Mccormack DE, Dionysiou DD, Pillai SC (2016) A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater Sci Semicond Process 42:2–14. https://doi.org/10.1016/j.mssp.2015.07.052
Gautam S, Shandilya P, Singh VP, Raizada P, Singh P (2016) Solar photocatalytic mineralization of antibiotics using magnetically separable NiFe2O4 supported onto graphene sand composite and bentonite. J Water Process Eng 14:86–100. https://doi.org/10.1016/j.jwpe.2016.10.008
He D, Peng Z, Gong W, Luo Y, Zhao P, Kong L (2015) Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv 5:11966–11972. https://doi.org/10.1039/c4ra14511a
Heck KN, Garcia-Segura S, Westerhoff P, Wong MS (2019) Catalytic converters for water treatment. Acc Chem Res 52:906–915. https://doi.org/10.1021/acs.accounts.8b00642
Hou Y, Lv S, Liu L, Liu X (2020) High-quality preparation of graphene oxide via the Hummers’ method: understanding the roles of the intercalator, oxidant, and graphite particle size. Ceram Int 46:2392–2402. https://doi.org/10.1016/j.ceramint.2019.09.231
Kanakaraju D, Motti CA, Glass BD, Oelgemöller M (2015) TiO2 photocatalysis of naproxen: effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere 139:579–588. https://doi.org/10.1016/j.chemosphere.2015.07.070
Khedr TM, El-sheikh SM, Ismail AA, Badawy WA, Bahnemann DW (2017) Highly active non-metals doped mixed-phase TiO2 for photocatalytic oxidation of ibuprofen under visible light. J. Photochem. Photobiol. A Chem 346:530–540. https://doi.org/10.1016/j.jphotochem.2017.07.004
Kumar A, Rout L, Achary LSK, Dhaka RS, Dash P (2017) Greener route for synthesis of aryl and alkyl-14H-dibenzo [a.j] xanthenes using graphene oxide-copper ferrite nanocomposite as a recyclable heterogeneous catalyst. Sci Rep 7:42975. https://doi.org/10.1038/srep42975
Lee C, Javed H, Zhang D, Kim H, Westerhoff P, Li Q, Alvarez PJJ (2018) Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ Sci Technol 52:4285–4293. https://doi.org/10.1021/acs.est.7b06508
Li L, Zheng X, Chi Y, Wang Y, Sun X, Yue Q, Gao B, Xu S (2020) Molecularly imprinted carbon nanosheets supported TiO2: strong selectivity and synergic adsorption-photocatalysis for antibiotics removal. J Hazard Mater 383:121211. https://doi.org/10.1016/j.jhazmat.2019.121211
Lin JCT, de Luna MDG, Aranzamendez GL, Lu MC (2016a) Degradations of acetaminophen via a K2S2O8-doped TiO2 photocatalyst under visible light irradiation. Chemosphere 155:388–394. https://doi.org/10.1016/j.chemosphere.2016.04.059
Lin JC, de Luna MDG, Gotostos MJN, Lu M-C (2016b) Effects of doping amounts of potassium ferricyanide with titanium dioxide and calcination durations on visible-light degradation of pharmaceuticals. Environ Sci Pollut Res 22:22721–22733. https://doi.org/10.1007/s11356-016-7470-y
Lin JC, Sopajaree K, Jitjanesuwan T, Lu M (2018) Application of visible light on copper-doped titanium dioxide catalyzing degradation of chlorophenols. Sep Purif Technol 191:233–243. https://doi.org/10.1016/j.seppur.2017.09.027
Liu SQ, Xiao B, Feng LR, Zhou SS, Chen ZG, Liu CB, Chen F, Wu ZY, Xu N, Oh WC, Da Meng Z (2013) Graphene oxide enhances the Fenton-like photocatalytic activity of nickel ferrite for degradation of dyes under visible light irradiation. Carbon N Y 64:197–206. https://doi.org/10.1016/j.carbon.2013.07.052
Loeb SK, Alvarez PJJ, Brame JA, Cates EL, Choi W, Crittenden J, Dionysiou DD, Li Q, Li-puma G, Quan X, Sedlak DL, Waite TD, Westerhoff P, Kim J-H (2019) The technology horizon for photocatalytic water treatment: sunrise or sunset? Environ Sci Technol 53:2937–2947. https://doi.org/10.1021/acs.est.8b05041
Mansour F, Alnouri SY, Al-Hindi M, Azizi F, Linke P (2018) Screening and cost assessment strategies for end-of-pipe zero liquid discharge systems. J Clean Prod 179:460–477. https://doi.org/10.1016/j.jclepro.2018.01.064
Marcelino RBP, Amorim CC (2019) Towards visible-light photocatalysis for environmental applications: band-gap engineering versus photons absorption—a review. Environ Sci Pollut Res 26:4155–4170
Medidi S, Markapurapu S, Kotupalli MR, Chinnam RKR, Susarla VM, Gandham HB, Sanasi PD (2018) Visible light photocatalytic degradation of methylene blue and malachite green dyes with CuWO4-GO nano composite. Mod Res Catal 07:17–34. https://doi.org/10.4236/mrc.2018.72002
Moreira NFF, Narciso-da-Rocha C, Polo-López MI, Pastrana-Martínez LM, Faria JL, Manaia CM, Fernández-Ibáñez P, Nunes OC, Silva AMT (2018) Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res 135:195–206. https://doi.org/10.1016/j.watres.2018.01.064
Mostafa E, Reinsberg P, Garcia-Segura S, Baltruschat H (2018) Chlorine species evolution during electrochlorination on boron-doped diamond anodes: in-situ electrogeneration of Cl2, Cl2O and ClO2. Electrochim Acta 281. https://doi.org/10.1016/j.electacta.2018.05.099
Muzyka R, Kwoka M, Smȩdowski Ł, Díez N, Gryglewicz G (2017) Oxidation of graphite by different modified Hummers methods. Xinxing Tan Cailiao/New Carbon Mater 32:15–20. https://doi.org/10.1016/S1872-5805(17)60102-1
Pan GT, Huang CM, Peng PY, Yang TCK (2011) Nano-scaled silver vanadates loaded on mesoporous silica: characterization and photocatalytic activity. Catal Today 164:377–383. https://doi.org/10.1016/j.cattod.2010.12.037
Perreault F, Fonseca De Faria A, Elimelech M (2015) Environmental applications of graphene-based nanomaterials. Chem Soc Rev 44:5861–5896. https://doi.org/10.1039/c5cs00021a
Saire-Saire S, Barbosa ECM, Garcia D, Andrade LH, Garcia-Segura S, Camargo PHC, Alarcon H (2019) Green synthesis of Au decorated CoFe2O4 nanoparticles for catalytic reduction of 4-nitrophenol and dimethylphenylsilane oxidation. RSC Adv 9:22116–22123. https://doi.org/10.1039/c9ra04222a
Sivakumar P, Ramesh R, Ramanand A, Ponnusamy S, Muthamizhchelvan C (2012) Synthesis, studies and growth mechanism of ferromagnetic NiFe2O4 nanosheet. Appl Surf Sci 258:6648–6652. https://doi.org/10.1016/j.apsusc.2012.03.099
Soares PA, Silva TFCV, Ramos Arcy A, Souza SMAGU, Boaventura RAR, Vilar VJP (2016) Assessment of AOPs as a polishing step in the decolourisation of bio-treated textile wastewater: technical and economic considerations. J Photochem Photobiol A Chem 317:26–38. https://doi.org/10.1016/j.jphotochem.2015.10.017
Spasiano D, Marotta R, Malato S, Fernandez-Ibañez P, Di Somma I (2015) Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl Catal B Environ 170–171:90–123. https://doi.org/10.1016/j.apcatb.2014.12.050
Sun M, Wang Y, Shao Y, He Y, Zeng Q, Liang H, Yan T, Du B (2017) Fabrication of a novel Z-scheme g-C3N4/Bi4O7 heterojunction photocatalyst with enhanced visible light-driven activity toward organic pollutants. J Colloid Interface Sci 501:123–132. https://doi.org/10.1016/j.jcis.2017.04.047
Thema FT, Moloto MJ, Dikio ED, Nyangiwe NN, Kotsedi L, Maaza M, Khenfouch M (2013) Synthesis and characterization of graphene thin films by chemical reduction of exfoliated and intercalated graphite oxide. J Chem 3:150536–150536. https://doi.org/10.1155/2013/150536
Tugaoen HON, Garcia-Segura S, Hristovski K, Westerhoff P (2017) Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci Total Environ 599–600:1524–1551. https://doi.org/10.1016/j.scitotenv.2017.04.238
Tugaoen HON, Garcia-Segura S, Hristovski K, Westerhoff P (2018) Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci Total Environ 613–614:1331–1338. https://doi.org/10.1016/j.scitotenv.2017.09.242
Villaluz FJA, de Luna MDG, Colades JI, Garcia-Segura S, Lu MC (2019) Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron (II) sulfate-doped nano-titania. Process Saf Environ Prot 125:121–128. https://doi.org/10.1016/j.psep.2019.03.001
Wang D, Pillai SC, Ho SH, Zeng J, Li Y, Dionysiou DD (2018) Plasmonic-based nanomaterials for environmental remediation. Appl Catal B Environ 237:721–741. https://doi.org/10.1016/j.apcatb.2018.05.094
Zhang Y, Liu J, Zhang Y, Liu J, Duan Y (2017) Facile synthesis of hierarchical nanocomposites of aligned polyaniline nanorods on reduced graphene oxide nanosheets for microwave absorbing materials. RSC Adv 7:54031–54038. https://doi.org/10.1039/c7ra08794b
Zhao GY, Liu LJ, Li JR, Liu Q (2016) Efficient removal of dye MB: through the combined action of adsorption and photodegradation from NiFe2O4/Ag3PO4. J Alloys Compd 664:169–174. https://doi.org/10.1016/j.jallcom.2016.01.004
Zuorro A, Lavecchia R, Monaco MM, Iervolino G, Vaiano V (2019) Photocatalytic degradation of azo dye Reactive Violet 5 on Fe-doped titania catalysts under visible light irradiation. Catalysts 9:645
Credit author statement
YJS, CDD, and MDGDL conceptualized the study. YJS, CDD, and MDGDL acquired the funding. MDGDL, CDD, and YJS supervised the work. MDGDL and YJS designed the experiments. ARBB performed the experiments. MDGDL, ARBB, and SGS organized the results. ARBB, SGS, and MDGDL analyzed the data. SGS, ARBB, and MDGDL wrote the draft manuscript. MDGDL and SGS reviewed and revised the manuscript.
Funding
The authors would like to thank the Ministry of Science and Technology, Taiwan (Contract No. MOST 106-2221-E-022-002-MY3), and the Department of Science and Technology, Philippines, for providing financial support for this research undertaking.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nickel ferrite nanoenabled graphene oxide catalysts were synthesized.
• The hook and destroy approach improved degradation performance.
• Photogenerated holes and hydroxyl radicals were identified as main oxidants.
Rights and permissions
About this article
Cite this article
Bayantong, A.R.B., Shih, YJ., Dong, CD. et al. Nickel ferrite nanoenabled graphene oxide (NiFe2O4@GO) as photoactive nanocomposites for water treatment. Environ Sci Pollut Res 28, 5472–5481 (2021). https://doi.org/10.1007/s11356-020-10545-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10545-1





 UV irradiation.
UV irradiation.  0.5 mg L−1 of GO in the dark.
0.5 mg L−1 of GO in the dark.  0.5 mg L−1 of GO under UV irradiation.
0.5 mg L−1 of GO under UV irradiation.  0.5 mg L−1 of NiFe2O4@GO in the dark.
0.5 mg L−1 of NiFe2O4@GO in the dark.  0.5 mg L−1 of NiFe2O4@GO under UV irradiation
0.5 mg L−1 of NiFe2O4@GO under UV irradiation
 0.01 mM.
0.01 mM.  0.02 mM.
0.02 mM.  0.03 mM.
0.03 mM.  0.04 mM.
0.04 mM.  0.05 mM
0.05 mM
 0.1 g L−1.
0.1 g L−1.  0.2 g L−1.
0.2 g L−1.  0.5 g L−1. ♦ 0.8 g L−1.
0.5 g L−1. ♦ 0.8 g L−1.  1.0 g L−1
1.0 g L−1
 3.0.
3.0.  7.0.
7.0.  10
10
 None. ○ Sodium sulfate.
None. ○ Sodium sulfate.  Sodium chloride.
Sodium chloride.  Sodium carbonate
Sodium carbonate
 None.○ Superoxide radical.
None.○ Superoxide radical.  Hydroxyl radical.
Hydroxyl radical.  Photogenerated holes
Photogenerated holes