Abstract
Persistent organochlorine chemicals (OCs), including chlordane compounds (CHLs), DDTs, PCBs, and chlorinated dioxins and related compounds (DRCs), were examined in the adipose tissue and liver from 33 specimens of habu (Protobothrops flavoviridis), a species of venomous pit viper endemic to the Japanese Southwest Islands. The median concentrations of CHLs, DDTs, and PCBs in adipose tissue of 22 habus collected from an urban area were 4400 ng g−1 lipid weight (lw), 610 ng g−1 lw, and 1600 ng g−1 lw, respectively. Their DDT and PCB concentrations were higher in comparison with the specimens from a rural area. Liver of 10 specimens from the urban area were subjected to DRCs analysis, and PCDDs, PCDFs, and DL-PCBs were detected with median values of 1300, 350, and 150,000 pg g−1 lw, respectively. Among PCDD/F congeners, octa-CDD was detected at the highest concentrations in seven liver samples, but considerable concentrations of penta- and hexa-CDD/Fs were found in two samples. Relatively higher concentrations of PCB, DDTs, and PCDD/Fs were found in habus collected within 1 km of the boundary of military facilities, suggesting that OCs from some unknown sources of these OCs inside and/or around some of the facilities accumulated in habus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organochlorine chemicals (OCs), such as polychlorinated biphenyls (PCBs), chlordane compounds (CHLs), dichlorodiphenyltrichloroethane and its metabolites (DDTs), hexachlorocyclohexane isomers (HCHs), and hexachlorobenzene (HCB), were used worldwide during the 1960s and 1970s. The production and use of OCs were banned in many developed countries because of their bioaccumulative nature and chronic adverse effects on wildlife and humans by the 1970s and 1980s, and subsequently, these compounds were included in persistent organic pollutants (POPs), the reduction or elimination of whose release into the environment is aimed by the Stockholm Convention (Tanabe and Minh 2010). Despite the regulation, however, relatively high levels of OCs have been detected in wildlife, even in recent years (Kunisue et al. 2007, 2008; Aksoy et al. 2011; Tashiro et al. 2016; Iatrou et al. 2019). In addition, chlorinated dioxins and related compounds (DRCs) such as polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs), and dioxin-like PCBs (DL-PCBs), which are either included in PCB products or unintentionally generated in the production of chlorinated herbicides, papermaking processes, and the combustion of organic substances, have also been observed in many organisms (Kuehl et al. 1987; Fiedler 1996; Masunaga et al. 2001).
The Japanese Southwest Islands consist of about 200 islands under subtropical climate zones between Kyushu region in Japan and Taiwan. Over the past several decades, contaminations by OCs have been reported in Okinawa, the largest island (1204 km2) of the Southwest Islands. For instance, a monitoring study conducted in the 1970s revealed that up to 9 μg g−1 wet weight (ww) of PCBs was detected in fish samples collected off the Okinawan coast, and the authors suggested leaks from a U.S. military storage facility (Kinjo et al. 1977). Around the same time, 11,500 gal of pentachlorophenol (PCP) was dumped onto a limestone area of the island, which contaminated the groundwater used as drinking water for the residents at levels up to 6 mg L−1 (Oyama et al. 1972). Technical CHL products were extensively used in the island as a termiticide until 1986 and have been detected at high concentrations in coastal fish (Oshiro 1981; Oshiro et al. 2006; Tanabe et al. 2008). Recently, more than 100 drums containing harmful micropollutants including DRCs, PCBs, and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in their residual oil were discovered beneath a public sports ground in Okinawa (Okinawa City Hall 2013). Tashiro et al. (2016) analyzed liver and muscle samples of the introduced species inhabiting in Okinawa, the small Indian mongoose (Herpestes auropunctatus), and suggested the existence of specific PCB pollution sources around an area where military facilities are located. The aforementioned environmental pollution issues raise concerns about their adverse effects on wildlife and humans inhabiting the island. In addition, many of the Japanese Southwestern Islands are surrounded by fringing coral reefs, which support rich and highly productive coral ecosystems and serve as the foundation of coastal fishery and tourism industries (Roberts et al. 2002). There are also concerns about land-based coastal pollution, i.e., ecological effects of hazardous substances discharged through rivers or groundwater to the coastal environment. However, the information on the presence and the distribution of OCs in tropical and subtropical islands is still limited (Colombo et al. 2014), and further research activities are needed to fill the gaps and to support rich tropical ecosystem as well as health of people.
The habu (Protobothrops flavoviridis) is a species of venomous pit viper endemic to the Japanese Southwest Islands. This species of snake is terrestrial, mostly nocturnal, and often enters homes and other structures in search of rats and mice. Because snakes are relatively high in trophic level and have narrow home range, several studies have previously attempted to use them as inland bioindicators to elucidate site-specific pollution on OCs, heavy metals, and polycyclic aromatic hydrocarbons by analyzing tissue accumulation levels of these contaminants (Campbell and Campbell 2001; Jones et al. 2009; Ciliberti et al. 2011; Heydari Sereshk and Riyahi Bakhtiari 2014; Zhou et al. 2016; Lemaire et al. 2018). In the present study, we analyzed OCs in habus collected from urban and rural areas in Okinawa to reveal the regional pollution aspects.
Materials and methods
Study area
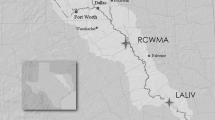
Two areas on Okinawa Island were selected for the present study, a rural area in the north of the island (Area I) and an urban area (Area II) in the south of the island (Fig. 1). Area I was the region that consisted of Higashi village, where population density is low (1.7 people km−2) and forests occupy a large portion (72%) of land use, and the town of Haebaru, where population density is also low (38 people km−2) and farmlands occupy a relatively large portion (17%) of land use. The towns and cities of Chatan, Ginowan, Urasoe, and Naha situated on the west coast of the central and southern parts of Okinawa were designated as the urban area (Area II)); this area has a greater population density (560 people km−2). In Area II, houses, commercial facilities, and several U.S. military facilities occupy the large part of land use. There is no waste disposal site in Area II, and there are no large-scale manufacturing facilities on the island.
Samples
Thirty-three habu snakes, which were captured in cage traps for the control programs of harmful animals by the local government or killed by vehicles on the road in Areas I and II, were collected during August 2013–November 2014. After measurement of snout–vent length (SVL) and weight, and dissection, tissue samples were frozen at − 20 °C until analysis. Abdominal adipose tissues (n = 32) of the specimens from both areas were subjected to analysis of organochlorine pesticides and PCBs, and liver samples (n = 10) from Area II were subjected to DRC analysis.
To use snakes as bioindicators for the assessment of regional pollution levels, the effect of sex, growth stage, and/or the size of each specimen on the concentration of the pollutants in its tissue should be considered. For example, previous studies on snakes have reported that female snake specimens accumulated lower levels of lipophilic contaminants than did male specimens, while higher concentrations of DDTs and PCBs were detected in immature snakes than in adult specimens (Fleet and Plapp Jr 1978; Santos et al. 1999). These results suggested that lipids and OC compounds can be transferred from the female to the eggs or embryos. Santos et al. (1999) also observed that concentrations of these compounds in adult snakes significantly increased with carcass weight, suggesting that the consumption of different preys depending on the size of the snake might also affect the concentration of the contaminants. In the case of habus, the increase rate of the body length drastically reduces when they are matured (Nishimura 1993), making it challenging to assume the age of an adult habu according to its size. Furthermore, unlike the development of skeletochronology for some snake species, including garter snakes, methods to determine the age of habus have not been implemented yet (Nishimura 1993), despite some early attempts (Minakami 1977; Hayashi and Tanaka 1981). Referring to Nishimura et al. (1991), who reported differences in prey items between the juvenile snakes with SVLs of less than 700 mm and the larger ones in Okinawa Island, the present study excluded small specimens from the samples for analyses to reduce possible disturbances in data analyses caused by the extreme size variation and/or differences in the growth stages of specimens. However, we could not explore the relationship between the concentrations of OCs and age of habu in the present study.
Chemical analysis
Organochlorine pesticides (CHLs, DDTs, HCHs, and HCB) and PCBs were analyzed following the method described by Tanabe et al. (1998) and the Japan Environment Agency (1998). Tissue samples (1–5 g) were ground with anhydrous sodium sulfate and extracted in a Soxhlet apparatus with a mixture of hexane and diethyl ether. After concentrating the extract, an aliquot was dried at 80 °C to determine the lipid content. The remaining extract was loaded onto 20 g dry Florisil (Florisil PR; Wako Chemicals USA, Inc., USA) packed in a glass column, dried with nitrogen stream, and then eluted with a water–acetonitrile mixture to remove fat. The eluate was then concentrated after being partitioned with hexane, treated with sulfuric acid, and passed through an activated Florisil column. The first fraction eluted with hexane contained PCBs, HCB, p,p′-DDE, and trans-nonachlor, and the second fraction eluted with 15% diethyl ether in hexane contained p,p′-DDT, p,p′-DDD, HCH isomers (α-, β-, and γ-), cis-nonachlor, trans-nonachlor, cis-chlordane, trans-chlordane, and oxychlordane. Total CHLs (trans-nonachlor, cis-nonachlor, trans-chlordane, cis-chlordane, oxychlordane), DDTs (p,p′-DDE, p,p′-DDT, and p,p′-DDD), HCHs (α-, β-, and γ-isomers), HCB, and PCBs were quantified using a gas chromatograph (GC; Shimadzu GC-2010) equipped with an electron capture detector (ECD) and an automatic injector (Shimadzu AOC-20i). The GC-ECD capillary column was a HP-5 (Hewlett Packard) fused silica capillary column (0.32 mm × 30 m, film thickness 0.25 μm). The oven temperature was programmed to increase from 70 to 160 °C at a rate of 10 °C/min and held for 10 min. The temperature was then increased to 260 °C at a rate of 2 °C/min with a final hold of 20 min. The temperatures of the injector and detector were set at 260 and 280 °C, respectively. High purity nitrogen was used as both carrier and makeup gases, and the flow rate of carrier gas was 1.2 mL min−1.
The concentration of each compound was quantified by comparing the peak area of each sample to that of the corresponding external standard. For the quantitative determination of PCBs, an equivalent mixture of Kanechlor 300, 400, 500, and 600 was used as a standard. Identification and quantification of major peaks in GC-ECD chromatograms were confirmed using a GC-MSD (Shimadzu QP-2010 plus), which achieved similar results as GC-ECD.
PCDD/Fs and DL-PCBs (mono-ortho and non-ortho PCBs) in habu liver samples were analyzed based on the methods reported by our previous studies (Kunisue et al. 2006; Goto et al. 2017), with slight modification. Briefly, a freeze-dried liver tissue was extracted using a high-speed solvent extractor with acetone/hexane mixture (1:1 v/v). The crude extract was concentrated and solvent-exchanged into hexane using a rotary evaporator. This sample extract was spiked with 13C12-labeled internal standards (PCDD/F and DL-PCB congeners) and then purified by gel permeation chromatography (GPC) and multi-layer silica gel column chromatography. The purified extract was separated into two fractions using an activated carbon-impregnated silica gel column. The mono-ortho PCB fraction and another dioxin fraction (containing PCDD/Fs and non-ortho PCBs) were spiked with 13C12-labeled CB-157 and 1,2,3,4-/1,2,3,7,8,9-CDDs, respectively, and these two solutions were concentrated under nitrogen stream. The final solution was analyzed using a gas chromatograph (Agilent 6890N GC; Agilent Technologies) coupled with magnetic sector high-resolution mass spectrometer (JMS-800D; JEOL).
Concentrations of PCDD/Fs and DL-PCBs in the liver samples were determined using the isotope dilution method to the corresponding 13C12-labeled internal standards. Toxic equivalents (TEQs) of 2,3,7,8-substituted PCDD/F congeners and DL-PCBs were calculated based on toxic equivalency factors (TEFs) for mammals proposed by the World Health Organization (van den Berg et al. 2006).
Quality assurance and quality control
The measurements of procedural blank were performed with every batch of samples and the results were below the limit of detection (LOD). The recoveries of spiked organochlorine pesticides and PCBs into the samples were 97.5 ± 1.4% for CHLs, 99.8 ± 4.5% for DDTs, 87.9 ± 1.9% for HCHs, 93.4 ± 6.2% for HCB, and 99.2 ± 2.3% for PCBs. The LODs for these compounds were all around 0.5 ng g−1 lipid weight based on a signal-to-noise ratio (S/N) of 5.0. Recoveries of 13C12-labeled PCDD/Fs and DL-PCBs were 91.3 ± 11.2% and 95.8 ± 12.9%, respectively. Reported concentrations were not corrected for recovery rates. The Center for Marine Environmental Studies (CMES) of Ehime University participated in the Intercomparison Exercise for Persistent Organochlorine Contaminants in Marine Mammal Blubber organized by the National Institute of Standards and Technology (Gaithersburg, MD, USA) and Marine Mammal Health and Stranding Response Program of the National Oceanic and Atmospheric Administration’s National Marine Fisheries Service (Silver Spring, MD, USA) for quality assurance and quality control.
Statistical analyses
Mann–Whitney U test was used for the comparison of OC concentrations and biological parameters of specimen between Areas I and II, and between the sexes. The Pearson product–moment correlation test was utilized to check for significant correlations between OC concentrations and biological parameters of the specimen. A p value of less than 0.05 was considered to indicate statistical significance. These analyses were executed using SPSS Statistics (version 22; IBM Corp.).
Results and discussion
Occurrence of OCs in habus
CHLs, DDTs, PCBs, HCBs, HCB, and DRCs were detected in all the analyzed samples. There were no statistical differences in the body weight, SVL, or the fraction of adipose tissue in the body weight of habu between Area I (Table 1) and Area II (Table 2), and no correlation was found between these biological parameters and OC concentrations. In the present study, most of the habus collected were males (Tables 1 and 2), but there was no significant difference in the detected concentration of each OC between male and female snakes. Consequently, male and female data have been pooled to improve the reliability of statistical comparisons.
Concentration of PCBs and organochlorine pesticides in habu adipose tissues
All the habus accumulated CHLs (Tables 1 and 2). The median concentration of CHLs in samples from Area II (4400 ng g−1 lw) was higher than that from Area I (110 ng g−1 lw); however, the difference between the two areas was not statistically significant due to the high variation of the CHL concentrations in Area I (Fig. 2). The compositions of CHLs in most of habus showed higher proportions of trans-nonachlor, which is more persistent in the environment, and of oxychlordane, which is an accumulative metabolite of chlordanes in animal bodies. Among the recent studies analyzing pollutants in snakes, Zhou et al. (2016) detected CHLs with a range of 1.3–8.2 ng g−1 lw in short-tailed mamushi snakes (Gloydius brevicaudus) from a paddy field in the Yangtze River Delta, an area with rapid economic development in China, which were far less than the level in habus in the present study. On the other hand, elevated concentrations of CHLs in snake species were reported in 1970s–1980s, including the study on garter snakes (Thamnophis sirtalis) collected from Spider Island in Lake Michigan in the USA in 1978, whose maximum trans-nonachlor concentration was 5.6 μg g−1 lw (Heinz et al. 1980). Some of the habus from Areas I and II contained even higher concentrations of trans-nonachlor than these snakes in the 1970s. Technical CHL products were widely used in wooden buildings such as dwelling houses on the island for termite control. The amount of CHLs contained in the products sold in the Okinawa Prefecture in 1979 was as much as 59,000 kg (Oshiro 1981), and the mean concentration of CHLs in breast milk of 22 Okinawan women in 1983 was 6.0 μg g−1 ww, which was three times higher than that in Osaka Prefecture (Oshiro et al. 1986). The elevated concentrations of CHLs in habus could be attributed to the residues of such products.
DDTs and PCBs were also detected from all the specimens (Tables 1 and 2). Concentrations of DDTs and PCBs in samples from Area II (median—610 ng g−1 lw for DDTs, 1600 ng g−1 lw for PCBs) were statistically higher than those from Area I (median—150 ng g−1 lw for DDTs, 110 ng g−1 lw for PCBs; Fig. 2). In the case of the viperine water snakes (Natrix maura) collected in the Ebro Delta of Spain during 1991–1992, where large amounts of organochlorine pesticides were used for rice crops until the 1970s and a PCB manufacturing plant was located upstream of a river, mean concentrations of DDTs and PCBs were 1800 ng g−1 lw and 770 ng g−1 lw, respectively (Santos et al. 1999). More recently, Zhou et al. (2016) reported relatively lower concentrations of DDTs and PCBs for mamushi snakes in China, which ranged from 260 to 490 ng g−1 lw for DDTs and 59–180 ng g−1 lw for PCBs. Concentrations of DDTs and PCBs observed in habu snakes from Area II were comparable with the values found in the snakes from the Ebro Delta in the 1990s, indicating that relatively large amounts of these compounds were emitted into the environment of this area. On the other hand, more serious OC contamination was recorded in snake species from Europe and the USA during the 1970s and 1980s. For example, the maximum PCB concentration in garter snakes collected from Spider Island in Lake Michigan in 1978 was 100 μg g−1 lw (Heinz et al. 1980). Niethammer et al. (1984) analyzed a series of animals collected from an island in oxbow lakes of the Mississippi River in 1980. The geometric mean concentrations of DDTs and PCBs were 270 and 12 μg g−1 lw for cottonmouth (Agkistrodon piscivorus) and 190 and 6.5 μg g−1 lw for water snake species (Nerodia cyclopion and N. rhombifera), respectively. In the present study, the contamination levels in habus were much lower than those in such highly contaminated snakes immediately after the regulation of production and use of these compounds. Among DDTs, p,p′-DDE was detected and predominant in all the samples from Areas I and II. Most of habus contained low concentrations of p,p′-DDT, and only two of them accumulated p,p′-DDD at detectable concentrations. The ratio of p,p′-DDE/DDTs was between 86 and 100% in all the samples, except the one from Area I with low concentrations of both p,p′-DDE (20 ng g−1 lw) and p,p′-DDT (8.6 ng g−1 lw). These results suggest that habus are exposed to DDTs from prey and soil in most cases rather than direct exposure to leaked technical DDT products on the island.
All the specimens accumulated HCHs and HCB; however, their concentrations were lower than those of CHLs, DDTs, and PCBs in most samples (Tables 1 and 2). The differences of HCHs and HCB concentrations between the samples from Areas I and II were not significant. As their values showed no apparent differences compared with those in the snakes reported in other recent studies (Zhou et al. 2016), these compounds were excluded in further discussions on specific sources on the island.
Concentration of DRCs in habu livers
PCDDs, PCDFs, and DL-PCBs were detected in all habu livers with median values of 1300, 350, and 150,000 pg g−1 lw, respectively (Table 3). To our knowledge, there are no data on DRCs in snakes from Japan. When comparing total TEQs detected in habu livers with data reported in livers of Japanese wild terrestrial mammals, the TEQ levels in habus (30–4400 pg TEQs g−1) were consistent with those in raccoon dogs (Nyctereutes procyonoides, 250–3300 pg TEQs g−1) (Kunisue et al. 2006) and higher than those in raccoons (Procyon lotor, 130–360 pg TEQs g−1), wild boars (Sus scrofa, 50–100 pg TEQs g−1), and cats (Felis silvestris catus, 9.4–110 pg TEQs g−1) (Someya et al. 2007), indicating that habus in Area II have been accumulating relatively high levels of DRCs. In addition to the potential toxic effects of DRCs on habu, possible impacts on endemic and migratory raptorial avian species such as gray-faced buzzards that migrate to Okinawa in the winter and feed on small animals including snakes are also of concern. For instance, based on the dose of TCDD that significantly affected the fertility and hatching success on pheasants, a no-observed-adverse-effect concentration (NOAEC) of 14,000 pg TCDD kg−1 day−1 was adopted for the risk assessment of the dietary exposure of the great blue heron (Ardea herodias) to PCDD/DFs (Seston et al. (2011). Although the frequency of habus as a prey item for avian species in Okinawa is unknown, the accumulation of DRCs in Okinawan birds should be studied in the future.
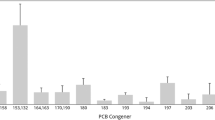
Octa-CDD was detected in nine habus. Its concentration was the highest among PCDD/Fs in seven specimens, while considerable concentrations of penta- and hexa-CDD/Fs were found in specimens 12 and 13. This result suggests that multiple PCDD/F contamination sources are present in Area II, i.e., the seven specimens with high octa-CDD levels might be affected by PCP, and specimens 12 and 13 may have accumulated penta- and hexa-CDD/Fs from combustion and/or impurities of technical PCB mixtures.
Specific sources of OCs in Area II
Unlike Area I, Area II is densely populated with many houses and commercial facilities, and adjacent military facilities occupy a large proportion of the study area. Previous studies on environmental and biological samples suggest environmental pollutions by OCs in this area. For instance, Yoshinaga et al. (1978) analyzed seashore sediments to assess the environmental impacts of spill incidents of chemical products from military facilities located within Area II. Seven out of nine sediments sampled in 1977 from the sites off the coast of Area II contained PCBs and pesticide residues. Maximum concentrations of PCBs and p,p′-DDD were 0.65 and 0.14 μg g−1 dw, respectively, whereas none of the three sediments from the sites in the north of the island close to Area I contained PCBs and pesticide residues. Currently, the annual environmental monitoring by the local authority continuously reports detections of OCs (e.g., 0.01 μg g−1 dw of PCBs and 0.024 μg g−1 dw of DDTs in 2017) in sediments at the mouth of a river in Area II. This river is directly discharged from the territory of a military airport to the ocean, in contrast to the seashore sediments from the sites around the north of the island that contain no detectable OCs (Okinawa Prefectural Government 2018). Our previous study on OCs in muscle tissues of mongooses from the island demonstrated higher PCB concentrations in the urban area including Area II of the present study than in a control area (Tashiro et al. 2016). On the other hand, the higher concentrations of DDTs and PCBs detected in habus from Area II in comparison with those from Area I are unlikely to be attributed to the differences in their prey between the areas, referring to the analyses of frequencies of prey items for habus which showed no significant differences between the two regions that includes Areas I and II, respectively (Nishimura et al. 1991). It is suggested that higher concentrations of DDTs and PCBs detected in habus from Area II compared with those from Area I are reflecting the environmental pollution by these compounds in Area II.
Specimens 11, 12, and 19 accumulated the highest concentrations of PCDDs, PCDFs, and DL-PCBs in their livers, respectively, among the 10 habus analyzed for DRCs. Also, specimen 11 showed the second highest concentrations of PCDFs in the liver and PCBs in the adipose tissue, and specimen 12 accumulated the second highest concentrations of PCDDs and DL-PCBs in the liver. The sampling points for these three highly contaminated specimens were in residential areas surrounded by military facilities. Although DRCs are released into the environment from several types of sources, and commercial PCB products with various physical properties derived from their homologue compositions were used for various purposes (e.g., as heating mediums, insulating oils, and paint components) (Erickson and Kaley II 2011), these areas did not include facilities for civilians that could be considered as potential DRCs and/or PCBs sources, such as large-scale factories, chemical storage facilities, power stations, waste disposal sites, or large structures such as bridges to which a large amount of paint could be applied (Fig. 3). In addition, because the geology of this region consists of limestone, there are no surface rivers that can transport OCs from any source in the upstream area to the downstream area. Given these local geographical conditions, the sources of the elevated concentrations of DRCs and PCBs detected in these three specimens are likely to be related to the military facilities. Further, we analyzed the relationships between detected concentrations of OCs and the distance from the sampling point to the nearest military facility boundary for each specimen in Area II, and the results are illustrated in Fig. 4. Relatively higher concentrations of PCB, DDTs, and PCDD/Fs were found in habus collected within 1 km of the boundary. To date, there have been no data published on the home range of habu in Okinawa, but it is generally understood that these snakes do not travel further than 100 m per day (unpublished data by local authority). In that context, it is possible that habu collected near military facilities could cross boundaries and enter the facility, or eat prey that have done so, suggesting that OCs from some unknown sources inside and/or around some of the facilities accumulated in these habus in Area II.
Conclusions
The present study demonstrated a novel approach to biomonitoring of POPs using a single species of venomous pit viper, habu. The characteristics of snake species, which are relatively high in trophic level and have narrow ranges of inhabitation, allowed us to suggest the location of the specific pollution sources. As venomous snake species are often captured by residents and/or local authorities, the specimens for biomonitoring can be obtained easily and constantly. Moreover, this approach, where such advantageous snakes are used, is expected to be applicable to pollution studies in other regions, especially in cases where the pollution sources are difficult to identify, such as inadequately buried OC-containing waste, or when pollutants are transported through groundwater systems, following the surveillances using other organisms with larger home ranges (e.g., mammals and birds).
Results of the analyses in the present study indicated the existence of the environmental contamination by OCs in a part of Okinawa Island. For the purpose of preventing farther spread of contamination and pollution remediation, as well as to analyze the soil and groundwater around the sites in the present study, it will be necessary to conduct surveys on OCs including DRCs inside nearby military facilities to elucidate whether pollution sources are actually present and implement countermeasures.
References
Aksoy A, Das YK, Yavuz O, Guvenc D, Atmaca E, Agaoglu S (2011) Organochlorine pesticide and polychlorinated biphenyls levels in fish and mussel in Van region, Turkey. Bull Environ Contam Toxicol 87:65–69. https://doi.org/10.1007/s00128-011-0286-z
Campbell KR, Campbell TS (2001) The accumulation and effects of environmental contaminants on snakes: a review. Environ Monit Assess 70:253–301. https://doi.org/10.1023/a:1010731409732
Ciliberti A, Berny P, Delignette-Muller ML, de Buffrénil V (2011) The Nile monitor (Varanus niloticus; Squamata: Varanidae) as a sentinel species for lead and cadmium contamination in sub-Saharan wetlands. Sci Total Environ 409:4735–4745. https://doi.org/10.1016/j.scitotenv.2011.07.028
Colombo A, Bettinetti R, Strona G, Cambria F, Fanelli R, Zubair Z, Galli P (2014) Maldives: an archipelago that burns. A first survey of PCDD/Fs and DL-PCBs from human activities. Sci Total Environ 497–498:499–507. https://doi.org/10.1016/j.scitotenv.2014.08.013
Erickson MD, Kaley RG II (2011) Applications of polychlorinated biphenyls. Environ Sci Pollut Res 18:135–151. https://doi.org/10.1007/s11356-010-0392-1
Fiedler H (1996) Sources of PCDD/PCDF and impact on the environment. Chemosphere 32:55–64. https://doi.org/10.1016/0045-6535(95)00228-6
Fleet RR, Plapp FW Jr (1978) DDT residues in snakes decline since DDT ban. Bull Environ Contam Toxicol 19:383–388. https://doi.org/10.1007/BF01685814
Goto A, Tue NM, Someya M, Isobe T, Takahashi S, Takahashi S, Tanabe S, Kunisue T (2017) Occurrence of natural mixed halogenated dibenzo-p-dioxins: specific distribution and profiles in mussels from Seto Inland Sea, Japan. Environ Sci Technol 51:11771–11779. https://doi.org/10.1021/acs.est.7b03738
Hayashi Y, Tanaka H (1981) Age determination in the venomous snake, habu, Trimeresurus flavoviridis. Jpn J Exp Med 51:209–213 (in Japanese)
Heinz GH, Haseltine SD, Hall RJ, Krynitsky AJ (1980) Organochlorine and mercury residues in snakes from Pilot and Spider Islands, Lake Michigan—1978. Bull Environ Contam Toxicol 25:738–743. https://doi.org/10.1007/BF01985601
Heydari Sereshk Z, Riyahi Bakhtiari A (2014) Distribution patterns of PAHs in different tissues of annulated sea snake (Hydrophis cyanocinctus) and short sea snake (Lapemis curtus) from the Hara Protected Area on the North Coast of the Persian Gulf, Iran. Ecotox Environ Safe 109:116–123. https://doi.org/10.1016/j.ecoenv.2014.06.004
Iatrou EI, Tsygankov V, Seryodkin I, Tzatzarakis MN, Vakonaki E, Barbounis E, Zakharenko AM, Chaika VV, Sergievich AA, Tsatsakis AM, Golokhvast K (2019) Monitoring of environmental persistent organic pollutants in hair samples collected from wild terrestrial mammals of Primorsky Krai, Russia. Environ Sci Pollut Res 26:7640–7650. https://doi.org/10.1007/s11356-019-04171-9
Japan Environment Agency (1998) Endocrine disrupting chemicals interim investigation manual (water, sediment, and aquatic organisms). Water Quality Management Division, Water Quality Conservation Bureau, Japan Environment Agency, Tokyo (in Japanese)
Jones DE, Magnin-Bissel G, Holladay SD (2009) Detection of polycyclic aromatic hydrocarbons in the shed skins of corn snakes (Elaphe guttata). Ecotox Environ Safe 72:2033–2035. https://doi.org/10.1016/j.ecoenv.2008.11.001
Kinjo K, Yamashiro Y, Uehara T (1977) Monitoring of PCBs and heavy metals in fish (3). Report of Okinawa Prefectural Institute of Health and Environment 11:74–75 (in Japanese)
Kuehl DW, Butterworth BC, De Vita VM, Suer CP (1987) Environmental contamination by polychlorinated dibenzo-p dioxins and dibenzofurans associated with pulp and paper mill discharge. Biomed Environ Mass Spectrom 14:443–447. https://doi.org/10.1002/bms.1200140811
Kunisue T, Watanabe MX, Iwata H, Tsubota T, Yamada F, Yasuda M, Tanabe S (2006) PCDDs, PCDFs, and coplanar PCBs in wild terrestrial mammals from Japan: congener specific accumulation and hepatic sequestration. Environ Pollut 140:525–535. https://doi.org/10.1016/j.envpol.2005.07.020
Kunisue T, Takayanagi N, Tsubota T, Tanabe S (2007) Persistent organochlorines in raccoon dogs (Nyctereutes procyonoides) from Japan: hepatic sequestration of oxychlordane. Chemosphere 66:203–211. https://doi.org/10.1016/j.chemosphere.2006.06.001
Kunisue T, Takayanagi N, Isobe T, Takahashi S, Nakatsu S, Tsubota T, Okumoto K, Bushisue S, Shindo K, Tanabe S (2008) Regional trend and tissue distribution of brominated flame retardants and persistent organochlorines in raccoon dogs (Nyctereutes procyonoides) from Japan. Environ Sci Technol 42:685–691. https://doi.org/10.1021/es071565z
Lemaire J, Bustamante P, Olivier A, Lourdais O, Michaud B, Boissinot A, Galán P, Brischoux F (2018) Determinants of mercury contamination in viperine snakes, Natrix maura, in Western Europe. Sci Total Environ 635:20–25. https://doi.org/10.1016/j.scitotenv.2018.04.029
Masunaga S, Takasuga T, Nakanishi J (2001) Dioxin and dioxin-like PCB impurities in some Japanese agrochemical formulations. Chemosphere 44:873–885. https://doi.org/10.1016/S0045-6535(00)00310-6
Minakami K (1977) Age determination of the yellow-green pit viper, habu by the vertebral centrum. Zool Mag 86:82–86 (in Japanese)
Niethammer KR, White DH, Baskett TS, Sayre MW (1984) Presence and biomagnification of organochlorine chemical residues in oxbow lakes of Northeastern Louisiana. Arch Environ Contam Toxicol 13:63–74. https://doi.org/10.1007/BF01055647
Nishimura M (1993) Estimation of age and growth of habu, Trimeresurus flavoviridis (serpents: Viperidae), in the Okinawa Islands. Jpn J Ecol 43:83–90 (in Japanese)
Nishimura M, Araki Y, Ueda H, Kawashima Y (1991) Frequencies of prey items of habu, Trimeresurus flavoviridis (viperidae), in the Okinawa Islands 1. Snake 23:81–83
Okinawa City Hall (2013) Investigation report on the soil pollution at a football field in Okinawa City. Okinawa City Hall, Okinawa (in Japanese)
Okinawa Prefectural Government (2018) Annual Report on the Environment in Okinawa 2018. Okinawa Prefectural Government, Okinawa (in Japanese)
Oshiro Z (1981) Chlordane and environmental pollution (1)—analytical method and contamination status. Report of Okinawa Prefectural Institute of Health and Environment 14:1–16 (in Japanese)
Oshiro Z, Yamashiro O, Shiroma H, IKema J, Oyama M, Kinjo K (1986) Contamination of chlordanes in man resided in Okinawa. Report of Okinawa Prefectural Institute of Health and Environment 20:77–86 (in Japanese)
Oshiro Y, Uechi S, Asato N, Kinjo K, Yoshida N, Toguchi A, Tamaki F, Futenma T, Miyagi T, Uehara T (2006) Environmental survey and monitoring of chemicals in Okinawa (1995–2004). Report of Okinawa Prefectural Institute of Health and Environment 40:179–186 (in Japanese)
Oyama C, Moriyama C, Ikema S, Chibana Y, Oshiro S, Kinjo Y, Tagashira M, Oyama M, Kinjo K, Yamashiro Y, Oshiro N (1972) PCP contamination of water supply in southern part of Okinawa Island. Report of Okinawa Prefectural Institute of Health and Environment 6:78–88 (in Japanese)
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne FC, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284. https://doi.org/10.1126/science.1067728
Santos X, Pastor D, Llorente GA, Albaigrs J (1999) Organochlorine levels in viperine snake Natrix maura carcasses from the Ebro Delta (NE Spain): sexual and size-related differences. Chemosphere 39:2641–2650. https://doi.org/10.1016/S0045-6535(99)00199-X
Seston RM, Fredricks TB, Tazelaar DL, Coefield SJ, Bradley PW, Roark SA, Newsted JL, Kay DP, Zwiernik MJ, Giesy JP (2011) Dietary exposure of great blue heron (Ardea herodias) to PCDD/DFs in the Tittabawassee River flood plain, MI. Ecotox Environ Safe 74:494–503. https://doi.org/10.1016/j.ecoenv.2010.10.024
Someya M, Kunisue T, Tashiro Y, Asakawa M, Iwata H, Tanabe S (2007) Contamination status and accumulation features of dioxins and related compounds in terrestrial mammals from Japan. Organohalogen Compounds 69:1721–1724
Tanabe S, Minh TB (2010) Dioxins and organohalogen contaminants in the Asia-Pacific region. Ecotoxicology 19:463–478. https://doi.org/10.1007/s10646-009-0445-8
Tanabe S, Senthilkumar K, Kannan K, Subramanian AN (1998) Accumulation features of polychlorinated biphenyls and organochlorine pesticides in resident and migratory birds from South India. Arch Environ Contam Toxicol 34:387–397. https://doi.org/10.1007/s002449900335
Tanabe S, Takahashi S, Malarvannan G, Ikemoto T, Anan Y, Kunisue T, Isobe T, Agusa T (2008) Survey on hazardous chemicals in aquatic organisms inhabiting Nansei Shoto Islands: report on the contamination status of fish and shellfish. In: Japan WWF (ed) Wildlife contamination assessment of Nansei Shoto Islands (2005–2007). WWFJ, Tokyo, pp 25–46
Tashiro Y, Goto A, Kunisue T, Kurahashi T, Tanabe S (2016) Contamination status of PCBs and organochlorine pesticides in the Okinawa Island, Japan: utilization of small Indian mongoose (Herpestes auropunctatus) as a bioindicator. J Environ Chem 26:115–122. https://doi.org/10.5985/jec.26.115
Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93:223–241. https://doi.org/10.1093/toxsci/kfl055
Yoshinaga A, Oyama M, Oshiro Z, Ikema S, Chibana Y, Sakugawa H, Shimoji K, Miyagi K, Takeuchi T, Takara T (1978) Survey results of drainages from U.S. military bases in 1977. Report of Okinawa Prefectural Institute of Health and Environment 12:44–51 (in Japanese)
Zhou Y, Asplund L, Yin G, Athanassiadis I, Wideqvist U, Bignert A, Qiu Y, Zhu Z, Zhao J, Bergman Å (2016) Extensive organohalogen contamination in wildlife from a site in the Yangtze River Delta. Sci Total Environ 554–555:320–328. https://doi.org/10.1016/j.scitotenv.2016.02.176
Acknowledgment
Part of this research was carried out by the Grant-in-Aid for Scientific Research (C) Project No. 25340062 and the Ministry of Education, Culture, Sports, Science and Technology , Japan (MEXT) to a project on Joint Usage/Research Center—Leading Academia in Marine and Environment Pollution Research (LaMer). Authors are grateful to Mr. Yoshiyasu Iha and Dr. Gloria Esther Luyo Acero de Tashiro for valuable comments on the research design and manuscript. We also received the cooperation of the Okinawa Prefectural Institute for Hygiene and Environment and local residents in collecting samples for the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tashiro, Y., Goto, A., Kunisue, T. et al. Contamination of habu (Protobothrops flavoviridis) in Okinawa, Japan by persistent organochlorine chemicals. Environ Sci Pollut Res 28, 1018–1028 (2021). https://doi.org/10.1007/s11356-020-10510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10510-y