Abstract
Layered double hydroxides (LDHs), known as a class of anionic clays, have attracted considerable attention recently due to their potential applications in different areas as catalyst materials, energy materials, and adsorbent materials for environmental remediation, especially for anionic pollutant removal. In this study, magnesium aluminum layered double hydroxide (MgAl-LDH) was synthesized by two methods: standard coprecipitation and urea hydrolysis. Their textural properties and morphologies were examined by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), thermogravimetry (TG) and differential (DTG) analysis, and point of zero charge (pHpzc). The specific surface area was calculated from BET adsorption equation. The results indicated that the crystallinity and the regularity of the samples prepared by urea hydrolysis were much preferable to those prepared by the coprecipitation method. Their sorption properties toward phosphate were investigated and the experimental evidence showed that, at the initial concentration of 100 mg L−1 and at room temperature, the LDH synthesized by urea hydrolysis had a percentage removal of 94.3 ± 1.12% toward phosphate ions while 74.1 ± 1.34% were uptaked by LDH synthesized by coprecipitation method, suggesting that the crystallinity affects the sorption capability. The sorption mechanism indicates that phosphate ions could be sorbed onto LDHs via electrostatic attraction, ligand exchange, and ion exchange.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Layered double hydroxides (LDHs), often identified as hydrotalcite-like compounds (HTlcs), are anionic clays constituted by a stacking of positively charged sheets (brucite-like layers) separated by interlayer spaces containing solvated anions. The positive charge of the layers is generated by the substitution of part of the divalent metal cations by trivalent ones. The formula of these anionic clays can be generalized to [M(II)1−x M(III)x(OH)2]x+(An−x/n).mH2O, where M(II) = Mg, Ni, Zn, Co, Mn, M(III) = Al, Cr, Fe, V, Co, the value of x is generally in the range 0.2–0.33, it is calculated as the molar ratio of M2+/(M2+ + M3+), An− is the compensating anion (CO32−, SO42−, NO3−, Cl−, organic anions), and m is the content of co-intercalated water (Cavani et al. 1991). LDHs could be easily synthesized on laboratory and industrial scales with a low cost. There are different ways of synthesizing LDHs with tailored physical and chemical properties suitable for many applications. LDHs could be prepared by coprecipitation method at variable and constant pH (Boclair et al. 1999; Zhao et al. 2002), urea hydrolysis (Costantino et al. 1998), sol-gel route (Jitianu et al. 2003), anion exchange (Mandal et al. 2009), and delamination and re-assembly (Liu et al. 2006), and by the calcination/reconstruction process (Miyata 1980). Standard coprecipitation of metallic salts in alkaline medium using NaOH and/or NaHCO3 or Na2CO3 reagents is most commonly used to prepare layered double hydroxides. This method is relatively easy but results often in particles with a very large distribution of size and strong agglomeration in aggregates (Xu et al. 2006a, b). Miyata (1980) and Adachi-Pagano et al. (2003) found that these aggregates are formed by strong edge–surface platelet interactions leading to the rigid spheroidal “sand rose” morphology; this rigid structure prevents accessibility to both the interlayers and external surface to produce less reactive LDHs, and such a morphology leads to poorly crystalline materials, very low specific surface areas, and nearly non-porosity. The use of urea as a precipitation agent in the presence of metallic salts at reaction temperature above 60 °C produces the progressive decomposition of urea in ammonium hydroxide leading to homogeneous precipitation, resulting in the formation of LDHs with good crystallinity degree and narrow distribution of particle size (Costantino et al. 1998; Ogawa and Kaiho 2002; Zeng et al. 2009).
Layered double hydroxides are promising materials for a large number of practical applications in catalysis, adsorption, pharmaceutics, corrosion, and other areas (Carja et al. 2002; Kang et al. 2018; Wei et al. 2013; Wang and Guo 2019). There has also been considerable interest in using LDHs to remove environmental contaminants since environmental pollution has emerged as an important issue in recent decades. Increasing interest has recently been diverted to evaluating the ability of LDHs to remove inorganic pollutants such as phosphate ions (Xing et al. 2008; Yang et al. 2014). The removal of phosphate ions through sorption processes onto various sorbents has been investigated for a long time. Among the sorbents investigated, the following are included: active carbon, silicates, iron oxides, dried plant particles, and etc. (Chiban et al. 2011, 2012).
The removal of phosphate by MgAl-LDH prepared by standard coprecipitation method has been evaluated in several studies. Nevertheless, to our knowledge, there is no paper investigating the effect of the synthesis method on sorption properties. Then, the purpose of this study was to prepare MgAl-LDHs, with the molar ratio of 3, by two methods: urea hydrolysis and standard coprecipitation, and to compare their structural features and textural properties. The effect of the synthesis method on phosphate removal from aqueous solution was also investigated. MgAl-LDHs synthesized by coprecipitation method are denoted LDH-Cop while those prepared by urea hydrolysis are denoted LDH-Urea, and LDH-P is a denotation of LDH-Urea after phosphate sorption.
Materials and methods
Synthesis of MgAl-LDHs
For all preparations, the reagents used were magnesium chloride (LobaChemie > 98%), aluminum chloride (AppliChem > 95%), sodium carbonate (LobaChemie > 99.5%), sodium hydroxide (LobaChemie > 98%), urea (AppliChem > 99%), and sodium dihydrogen phosphate (Sigma-Aldrich > 98%) which were of analytical grade.
Urea hydrolysis method
The same conditions described by Costantino et al. (1998) were used to synthesize LDHs by urea method: 9 g of AlCl3·6H2O, 22.9 g of MgCl2·6H2O, and 29.8 g of NH2CONH2 were added into a three-neck flask containing 150 mL of double-distilled water and stirred at 100 °C for 36 h. The solution pH was approximately 9 due to the release of ammonia. The solid was separated from the solution by centrifugation and washed several times with double-distilled water. Finally, they are dried at 80 °C in an oven overnight.
Standard coprecipitation method
The standard conditions were used to synthesize LDHs by coprecipitation method (Adachi-Pagano et al. 2003): 40 mL of an aqueous solution containing MgCl2·6H2O and AlCl3·6H2O (Mg2++ Al3+ = 1 M, Mg/Al = 3) was continuously added to 250 mL of double-distilled water, and the second solution of NaOH/Na2CO3 (2 M NaOH and 0.5 M Na2CO3) was dropwise added to adjust the pH to 10. The mixture was vigorously stirred at room temperature. After the complete addition of the metallic salts, the suspension was aged at room temperature for 24 h under stirring. The precipitate was repeatedly washed with double-distilled water until a negative Cl test of the supernatant was obtained by AgNO3 titration, then centrifuged and dried at 80 °C in an oven overnight.
Characterization of MgAl-LDHs
The crystallographic phase of MgAl-LDHs was investigated using an X’PERT PRO MPD diffractometer with Cu/Kα radiation (45 kV, 40 mA) at 0.0670° step size. Functional groups were analyzed using a VERTEX 70 DTGS spectrometer (FTIR) at 4 cm−1 resolution in the range 4000–400 cm−1. LDH samples were mixed with KBr at a mass ratio of 1:100 and finally powdered to prepare pellets. Quattro ESEM at accelerating voltage of 10 kV equipped with an energy-dispersive X-ray analyzer (CNRST, Morocco) was used to view the morphology of both samples by scanning electron microscopy and to determine the approximate composition of samples. The thermal analysis (TG and DTG) was carried out using a SETARAM LABSYS EVO device, in the following conditions: linear heating rate 10 °C min−1 from room temperature to 900 °C, dynamic air atmosphere, Al2O3 crucible, and sample weight approximately 20 mg. The specific surface area and porosity analysis were measured using a nitrogen adsorption-desorption technique at 77 K based on the Brunauer–Emmett–Teller (BET) isotherm model (Micromeritics/3 Flex). The point of zero charge (pHpzc) of the prepared LDHs was determined by batch equilibrium method, and 1 M NaOH/HCl solutions was used to adjust the initial pH values of 50 mL of 0.1 M NaCl to a pH range of 2–12. Then, 0.5 g of the adsorbent was added to each solution and the obtained suspension was shaken for 48 h. The adsorbent was filtered and the final solution pH was measured. The pHpzc was determined from the intersection of the curve of the final vs. initial pH (Sepehr et al. 2017).
Phosphate removal tests
A stock solution (100 mg/L) of phosphate ions was prepared from NaH2PO4.12H2O salt. Residual phosphate concentration experiments were performed in the batch experiment at room temperature (around 25 °C) using a solid/liquid ratio of 1.25 g-sorbent/L at initial pH value 5.4, and contact time varied from 2 to 30 min. In order to investigate how adsorption was affected by solution pH, the pH of the solution was adjusted from 4 to 12 using 0.1 M of NaOH or 0.1 M of HCl. The mixtures were shaken for 24 h and the sorbent was filtered out. The residual concentration of phosphate ions in the filtrate was determined using the ascorbic acid method (Rodier et al. 2009) by monitoring the absorbance at 700 nm using SELECTA spectrophotometer UV-2005. The percentage removal (%R) of phosphate ions from aqueous solutions was calculated as follows (1):
where C0 (mg/L) and Ct (mg/L) are the phosphate concentration in the liquid phase before and after sorption on LDH, respectively. Experiments are performed in duplicate and results are expressed as mean ± standard deviation of the mean
Results and discussion
Characterization of MgAl-LDHs
The XRD patterns of LDH-Cop and LDH-Urea were illustrated in Fig. 1. It could be seen that the diffraction peaks of sample synthesized by standard coprecipitation method are broad and asymmetric with relatively low diffraction intensities, indicating that disorder may be present in the stacking of the layers and materials are poorly crystallized. However, the diffraction peaks of LDH prepared by urea method are narrow and sharp, and show an excellent symmetry. These phenomena suggest that higher crystallinity and regularity exist in the structure of the layered materials synthesized by the urea method.
The crystallite size of the LDHs was calculated from the XRD result using Scherrer formula (Cullity 1978):
where D (nm) denotes the size of the crystalline phase, λ is the wavelength of Cu Kα radiation (λ = 0.15404 nm), and β is the full width at half maximum (FWHM) intensity in radians. The (003) reflection was used to define the FWHM intensity of the crystalline phase. All the diffraction peaks can be indexed on a hexagonal unit cell with a space group R-3 m, thus revealing that the LDHs are crystallized in a rhombohedral structure (Bruun Hansen and Koch 1995). The lattice parameters (a and c) of the hexagonal structure of MgAl-LDH are expressed as follows (Cullity 1978):
where d is the lattice spacing between two different crystallographic planes; h, k, and l are the Miller indexes of the reflection planes. Thus, the c parameter (which represents the thickness of three layers plus the interlayer space between them) was calculated as c = 3d(003). The dimension (which represents the shortest distance between two cations of the layer) was calculated as a = 2d(110). The cell parameters of both samples are included in Table 1. The results matched with those reported by other research (Oh et al. 2002).
IR analysis may be suitable to determine the presence of other anions in the interlayer spaces, and to identify the type of bonds formed by the anions and their orientation (Cavani et al. 1991). Figure 2 shows the IR spectra of the samples prepared by both coprecipitation and urea methods. The FT-IR spectrum for MgAl-LDHs was typical of hydrotalcite-like materials (Novillo et al. 2014). Broad absorption bands in the 3400–3600 cm−1 region can be assigned to OH stretching vibrations of hydroxyl groups. The band at 1621 cm−1 was attributed to the HOH bending vibration of physically adsorbed water (Titulaer et al. 1994). The carbonate anion in a symmetric environment is characterized by a D3h planar symmetry, with three IR active absorption bands, as well as in the case of the free carbonate anion. In most LDHs, the three bands are observed at 1358 cm−1 (υ3), 873 cm−1 (υ2), and 686 cm−1 (υ4) (Cavani et al. 1991). The bands at 540–650 cm−1 were ascribed to the vibration of Mg–O and Al–O groups in the layers (Khitous et al. 2016).
Figure 3 displays the SEM images of LDHs and the corresponding EDS spectrum. LDH-Urea image revealed large and regular hexagonal platelets of LDH. The morphology of the platelets reflected a good crystallinity; however, LDH-Cop image showed some deformed LDH particles. The presence of deformed particles confirmed the X-ray result of this sample. The EDS spectrum confirms that LDHs consisted of magnesium, aluminum, oxygen, carbon, and chlorine with a chemical composition that agreed well with MgAl-LDHs.
The TG-DTG curves of LDH-Cop and LDH-Urea are presented in Fig. 4. For both LDHs, the first weight loss in the TG-DTG curves is ascribed to the elimination of surface and interlayer water molecules. The second weight loss is due to the decomposition of carbonate anions and to the dehydroxylation of layers. At temperature 800 °C, the samples exhibited a net weight loss of 42% for LDH-Cop and 37% for LDH-Urea, with the second weight loss being larger than the first one. These results are in agreement with literature data for hydrotalcite-like materials (Tanasoi et al. 2009).
The typical nitrogen adsorption–desorption isotherms of MgAl-LDHs prepared by coprecipitation and urea methods were shown in Fig. 5. The specific surface area, pore volume, and average pore size were listed in Table 2. The average pore diameter and pore volume of LDH synthetized by urea hydrolysis were larger than that prepared by coprecipitation. The BET surface area of LDH-Urea was 91 m2/g, conversely, and the LDH-Cop was 50 m2/g. This result could be explained by the difference in crystallinity and regularity in the structure of the layered materials as confirmed by XRD and SEM analysis. For adsorbent, a large surface area can offer more active adsorption sites. Therefore, LDH-Urea might have a higher sorption capacity than LDH-Cop.
The point of zero charge (pHpzc) is the point at which the surface charge density of the material is zero. Therefore, the surface of LDH is positively charged when pH is below pHpzc and negatively charged for pH above pHpzc. As seen in Fig. 6, the results showed that the pHzpc values of LDH-Urea and LDH-Cop are around 7.3 and 7.7 respectively.
Efficiency of phosphate ion removal by MgAl-LDHs
The performance of the MgAl-LDH samples in the adsorption process of phosphate ion removal from aqueous solutions was investigated. Figure 7 shows that, at equilibrium time, LDH prepared by urea hydrolysis might remove 94.35 ± 1.12% of phosphate ions from aqueous solutions, while only 74.15 ± 1.34% of these ions could be removed by LDH prepared using coprecipitation method. Figure 8 shows that, in the range of pH values (4–12), LDH-Urea has much higher phosphate sorption capacity than LDH-Cop.
Sorption mechanisms
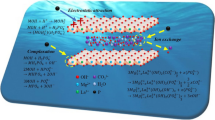
FTIR and XRD analyses of LDH-Urea after phosphate sorption could be helpful to understand the interaction mechanisms between phosphate ions and LDH adsorbents. The FTIR spectra of LDH-Urea after phosphate sorption (LDH-P) were shown in Fig. 2. The peak located at 1059 cm−1 was attributed to the bending vibration of adsorbed phosphate P–O (Liu et al. 2008). The new peak appeared after phosphate sorption onto LDHs, possibly indicating the information of inner-sphere surface complex (M–O–P) between phosphate ions and LDHs. Therefore, it was deduced that the surface hydroxyl groups (M–OH) of LDHs could be exchanged by the adsorbed phosphate ions. The XRD pattern of LDH-P was shown in Fig. 1; it can be seen that the characteristic peaks of the LDH structure appear in the XRD pattern of the sample, and the features at d003 (7.623 Å) and d006 (3.783 Å) are essentially retained after phosphate adsorbed, which indicates the sorption of phosphate on MgAl-LDH is by ion exchange. Consequently, these results are similar to those obtained by other teams of researchers (Xing et al. 2008; Yang et al. 2014). Since the pH of the medium is below the pHzpc of the adsorbent, an electrostatic attraction between negatively charged phosphate ions (H2PO4− and HPO42−) and electropositive LDH surface occurs. Then, as illustrated in Fig. 9, the phosphate ions could be adsorbed onto LDH particles via electrostatic attraction, ligand exchange, and ion exchange
Conclusion
The present investigation evaluated the structures and morphologies of MgAl layered double hydroxide particles prepared by two methods: standard coprecipitation and urea hydrolysis. The obtained materials were characterized and used for the removal of phosphate ions from aqueous solutions. The result from FT-IR and XRD analysis suggests that the intercalation of phosphate ions into LDHs is performed successfully. The XRD, FTIR, TG-DTG, SEM-EDS, and BET surface area analyses also show that LDH prepared by urea hydrolysis exhibits higher specific surface area and higher crystallinity and regularity in the structure counter to that prepared by standard coprecipitation. The phosphate removal performance of these samples was evaluated and showed that the synthesis method affected the amount of phosphate uptake onto MgAl-LDHs. Indeed, LDH prepared by urea hydrolysis has much higher phosphate sorption capacity than that prepared by standard coprecipitation method. The sorption mechanism of phosphate ions onto LDHs supposed to be electrostatic attraction, ligand exchange, and ion exchange. Therefore, MgAl-LDHs have potential for future applications in materials and physico-chemical processes at liquid/solid interfaces, especially for inorganic pollutant removal.
References
Adachi-Pagano M, Forano C, Besse J-P (2003) Synthesis of Al-rich hydrotalcite-like compounds by using the urea hydrolysis reaction-control of size and morphology. J Mater Chem 13:1988–1993
Boclair JW, Braterman PS, Jiang J, Lou S, Yarberry F (1999) Layered double hydroxide stability. 2. Formation of Cr(III)-containing layered double hydroxides directly from solution. Chem Mater 11:303–307
Bruun Hansen HC, Koch CB (1995) Synthesis and characterization of pyroaurite. Appl Clay Sci 10:5–19
Carja G, Nakamura R, Niiyama H (2002) Copper and iron substituted hydrotalcites: properties and catalyst precursors for methylamines synthesis. Appl Catal A Gen 236:91–102
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Chiban M, Soudani A, Sinan F, Tahrouch S, Persin M (2011) Characterization and application of dried plants to remove heavy metals, nitrate, and phosphate ions from industrial wastewaters. Clean Soil Air Water 39:376–383
Chiban M, Zerbet M, Sinan F (2012) Low-cost materials for phosphate removal from aqueous solutions, Handbook of phosphates: sources, properties and applications. Nova Science Publishers, Inc, New York, pp 1–42
Costantino U, Marmottini F, Nocchetti M, Vivani R (1998) New synthetic routes to hydrotalcite-like compounds−characterisation and properties of the obtained materials. Eur J Inorg Chem 1998:1439–1446
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley, Reading, pp 284–501
Jitianu M, Zaharescu M, Bãlãsoiu M, Jitianu A (2003) The sol-gel route in synthesis of Cr(III)-containing clays. Comparison between Mg-Cr and Ni-Cr anionic clays. J Sol-Gel Sci Technol 26:217–221
Kang HR, Da Costa Fernandes CJ, Da Silva RA, Constantino VRL, Koh IHJ, Zambuzzi WF (2018) Mg-Al and Zn-Al layered double hydroxides promote dynamic expression of marker genes in osteogenic differentiation by modulating mitogen-activated protein kinases. Adv Healthc Mater:7
Khitous M, Salem Z, Halliche D (2016) Removal of phosphate from industrial wastewater using uncalcined MgAl-NO3 layered double hydroxide: batch study and modeling. Desalin Water Treat 57:15920–15931
Liu Z, Ma R, Osada M, Iyi N, Ebina Y, Takada K, Sasaki T (2006) Synthesis, anion exchange, and delamination of Co−al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128:4872–4880
Liu H, Sun X, Yin C, Hu C (2008) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151:616–622
Mandal S, Tichit D, Lerner DA, Marcotte N (2009) azoic dye hosted in layered double hydroxide: physicochemical characterization of the intercalated materials. Langmuir 25:10980–10986
Miyata S (1980) Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clay Clay Miner 28:50–56
Novillo C, Guaya D, Allen-Perkins Avendaño A, Armijos C, Cortina JL, Cota I (2014) Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 138:72–79
Ogawa M, Kaiho H (2002) Homogeneous precipitation of uniform hydrotalcite particles. Langmuir 18:4240–4242. https://doi.org/10.1021/la0117045
Oh J-M, Hwang S-H, Choy J-H (2002) The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ionics 151:285–291
Rodier J, Legube B, Merlet N (2009) L’analyse de l’eau 9e édition Dunod, Paris
Sepehr MN, Al-Musawi TJ, Ghahramani E, Kazemian H, Zarrabi M (2017) Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab J Chem 10:611–623
Tanasoi S, Tanchoux N, Urdă A, Tichit D, Săndulescu I, Fajula F, Marcu I-C (2009) New Cu-based mixed oxides obtained from LDH precursors, catalysts for methane total oxidation. Appl Catal A Gen 363:135–142
Titulaer MK, Jansen JBH, Geus JW (1994) The quantity of reduced nickel in synthetic takovite: effects of preparation conditions and calcination temperature. Clay Clay Miner 42:249–258
Wang F, Guo Z (2019) Facile synthesis of superhydrophobic three-metal-component layered double hydroxide films on aluminum foils for highly improved corrosion inhibition. New J Chem 43:2289–2298
Wei J, Gao Z, Song Y, Yang W, Wang J, Li Z, Mann T, Zhang M, Liu L (2013) Solvothermal synthesis of Li-Al layered double hydroxides and their electrochemical performance. Mater Chem Phys 139:395–402
Xing K, Wang H, Guo L, Song W, Zhao Z (2008) Adsorption of tripolyphosphate from aqueous solution by Mg–Al–CO3-layered double hydroxides. Colloids Surf A Physicochem Eng Asp 328:15–20
Xu ZP, Stevenson G, Lu C-Q, Lu GQM (2006a) Dispersion and size control of layered double hydroxide nanoparticles in aqueous solutions. J Phys Chem B 110:16923–16929. https://doi.org/10.1021/jp062281o
Xu ZP, Stevenson GS, Lu C-Q, Lu GQM, Bartlett PF, Gray PP (2006b) Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J Am Chem Soc 128:36–37. https://doi.org/10.1021/ja056652a
Yang K, Yan L, Yang Y, Yu S, Shan R, Yu H, Zhu B, Du B (2014) Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: kinetics, isotherms and mechanisms. Sep Purif Technol 124:36–42
Zeng H-Y, Deng X, Wang Y-J, Liao K-B (2009) Preparation of Mg-Al hydrotalcite by urea method and its catalytic activity for transesterification. AICHE J 55:1229–1235. https://doi.org/10.1002/aic.11722
Zhao Y, Li F, Zhang R, Evans DG, Duan X (2002) Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem Mater 14:4286–4291
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benhiti, R., Ait Ichou, A., Zaghloul, A. et al. Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ Sci Pollut Res 27, 45767–45774 (2020). https://doi.org/10.1007/s11356-020-10444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10444-5