Abstract
This study investigates the contents of lead, zinc, and cadmium in 109 near-surface soil samples collected around the abandoned mine of Fedj Lahdoum, northern Tunisia, to assess the risk of pollution they generate. The study involved some analytical procedures such as pH measurements, X-ray diffraction techniques, sequential fractionation, and geostatistical mapping using the ordinary Kriging techniques. The sequential fractionation revealed that the bioavailability of Pb, Zn, and Cd follows the orders F5 > F3 » F4 > F2 » F1, F5 > F3 » F4 » F2 > F1 and F5 > F2 » F4 > F1, respectively; their associations with organic matter and residual sulfides (F4) are relatively low. However, their high cumulated contents are dominantly associated with the residual (F5) and reducible (F3) fractions. The geostatistical mapping was endeavored to predict the spatial distribution of the studied heavy metals at unsampled sites and to produce a cumulated risk map of soil pollution. The latter is discussed with emphasis of the main factors responsible for the scattering of the pollution as much as the landscape conditions, the chemical composition of the mine tailings, the surface drainage of meteoric water and the wind. This study provides insight into the delineation of the spatial spreading of Pb, Zn, and Cd around the abandoned mine Fedj Lahdoum and their surrounding urban areas. It reveals that the mine infrastructure areas encompassing both extraction and processing and tailing deposition areas are the main sources of contamination. And the landscape conditions together with the surface drainage of meteoric water and the wind are the main factors responsible for the scattering of the pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities have a direct and positive economic outcome, but in return, abundant and uncontrollable heavy metal releases have contaminated wide areas of the earth surface. The ore extraction using froth flotation process cumulated worldwide massive sulfide tailings that exert a significant environmental impact on soils and hazardous to ecosystems and biodiversity.
Heavy metals such as lead (Pb), cadmium (Cd), zinc (Zn), antimony (Sb), arsenic (As), mercury (Hg), strontium (Sr)… are toxic pollutants usually accumulated within tailings of abandoned mines (Bhattacharya et al. 2006). Alongside mining sites, the amount of metal pollutants is 100- to 1000-fold higher than the average concentration in a given soil. When tailings are well exposed to air and both meteoric and surface water, heavy metal release is significantly enhanced (Ritcey 2005; Smuda et al. 2008). Once these pollutants are released into the surrounding environment, they engender negative impacts on soil, basement, water, natural habitat, and local biodiversity.
The processes of emission and distribution of heavy metal pollutants in different environmental compartments have been discussed worldwide (Guillet et al. 1980; Alloway 1995; Colandini 1997; Mason et al. 1999; Blanchard 2000; Hou et al. 2019; Delmas-Gadras 2000; Semlali 2000; Bril 2001; Jung et al. 2001; Marcos 2001; Abollino et al. 2002; Colinet 2003; Kotoky et al. 2003; Algan et al. 2004; Boussen et al. 2010; Sebei et al. 2017). These pollutants can disperse in the atmosphere and eventually fall, dry or wet on the ground especially by rainwater infiltration. Their destinations are conditioned by the hydrodynamic and the lithological characteristics of the ground that impact the distance and the speed of their migration. In fact, the mobility of an element means its capacity to move in the soil through the compartments where it is less intensely retained (Juste 1988). In the ground, heavy metals are associated with different compartments; during pedogenesis, they are redistributed among the different horizons based on the physicochemical conditions and natural geochemical functioning (Semlali 2000). Hence, a clear understanding of the ground characteristics is essential for a better prediction of the eventual migration of elements and the risk of contamination they may cause to soil and groundwater.
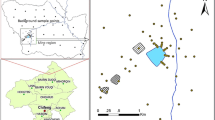
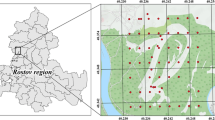
Worldwide, the tailings of abandoned mines are often very hazardous sources of pollution since they contain high amounts of metal pollutants. Particularly in northern Tunisia, the abandoned mine of Fedj Lahdoum (Fig. 1) contains considerable tailings with high contents of Pb, Zn, Cd, and Sr. The mine wastes are spilled around the exploited sites of Fedj Lahdoum adjacent to an exclusively rural area characterized by extensive agriculture based on cereal breeding. The cultivated area is subject to a high risk of pollution by heavy metal pollutants that can be exacerbated by development related to agricultural activities and water drainage coming from rainfall and irrigation.
The main purpose of this work is to present an updated investigation of the concentrations of Pb, Zn, and Cd in the urban soils around the abandoned mine of Fedj Lahdoum in northern Tunisia and to define the phases of heavy metals within the sampled soils using sequential fractionation and geostatistical mapping. The spatial distribution of these heavy metals within soils in the watershed of Fedj Lahdoum are interpreted with emphasis of the main factors responsible for the scattering of the pollution such as the landscape conditions, the morphology and the chemical composition of the mine tailings, the surface drainage of meteoric water and the wind.
Study area
The mine of Fedj Lahdoum is one of the oldest Pb-Zn mine in Tunisia (first exploitation in 1892). It is located in the so-called diapirs zone at ~ 120 km to the southwest of Tunis and ~ 6 km to the northwest of Al Krib city (Fig. 1). The Pb-Zn ores of Fedj Lahdoum evaluated to ~ 500,000 tons are considered economically interesting in Tunisia. They occurred within the transition between the Cretaceous (Cenomanian-Turonian) rocks and the Triassic evaporites of diapiric structures that pierce the sedimentary cover (Charef et al. 1987). The mine site, located at ~ 20 km southwest of Teboursouk, occupies the highest part of the Jouaouda Mountains which makes part of the Medjerda watershed. To the east and to the northwest of the mine site, the cultivated lowlands of Garat Al Krib and Teboursouk plain are drained, respectively, by the wadi Rmal and wadi Zitouna that constitute two tributaries of the Medjerda wadi to the north (Fig. 1).
The first exploration of Fedj Lahdoum area began at the mid-nineteenth century by Joseph Faure then followed by several detailed geologic studies (e.g., Laatar 1980; Perthuisot 1981; Charef et al. 1987; Kassa 1990; Bouhlel 1993; Boussen et al. 2010; Sebei et al. 2017 and others). Metal extraction was ceased in 2004 and about 157,270 m3 (~ 0.39 Mt) of mining wastes were scattered over an area of ~ 10 ha.
Materials and methods
Soil sampling
A total of 109 near-surface soil samples (0–20 cm depths) were collected from the terrains surrounding the site of Fedj Lahdoum Pb-Zn mine (Fig. 2). The soil samples were packed in polyethylene bags before being shipped to the laboratory. All samples were systematically air-dried and disaggregated before being sieved using a 2-mm diameter polyethylene sieve.
Analytical procedures
The pH measurements were done for soil/water suspensions prepared at a ratio of 1:2.5 (v/v) by melting 10 mg of soil in ultrapure water, using WTW digital pH meter (Soil Survey Staff 2004). The contents of calcium carbonate have been estimated using the classic Bernard calcimeter method according to the AFNOR NF P 94-048 (1996) norm. The X-ray diffraction techniques (X-Pert-MPD pro PANalytical automatic X-ray diffractometer with Cu Kα radiation) were used for identifying the mineral species. The scan speed was 0.02°/s and the range of 2θ was 2–70°.
The analysis of Zn, Pb, and Cd is done by atomic absorption spectroscopy (AAS) using a spectrophotometer of thermo-elemental type. The soil samples were previously dissolved by triacid attack (using HClO4, HNO3, and HF) and the analytical control was based on the BCR-032 (Natural Moroccan phosphorite). The recovery of the standard sample ranges from 95 to 105%.
The exchangeable fraction of metals in soil can be estimated either by single extraction methods such as chemical dissolution (e.g., Chao 1972) or by sequential extraction procedures (Tessier et al. 1979; Sposito et al. 1982; Ure et al. 1993). The sequential fractionation procedures are widely used to estimate chemical forms of heavy metals in soils (Walter and Cuevas 1999). Nevertheless, the efficiency of the method of extraction depends on the mineral to be extracted, and the resulted extract is not specific for a given mineral (Martin et al. 1987). Heavy metals in soils and plants are generally associated with different fractions. The Tessier protocol applies consecutive selective chemical reagents in five extraction steps to extract the pollutants associated with the consecutive soil fractions: F1 > F2 > F3 > F4 > F5 which represent, respectively, exchangeable, carbonate-bound metals, reducible, organic matter, and residual sulfides and residual fractions.
In this study, five successive extractions were performed following Tessier et al. (1979) and Lespagnol (2003) methods; F1: extraction with MgCl2, 1 M, pH 7 for 1 h with shaking, F2: extraction with CH3COONa, 1 M, pH 5 with CH3COOH, for 5 h with shaking. F3: extraction with NH2OH·HCl, 0.04 M at 25% (v/v) of CH3COOH under 96 °C for 6 h, F4: first extraction with 3 ml of HNO3, 0.02 M, 5 ml H2O2 30%, pH 2 with HNO3 under 85 °C for 3 h, second extraction with 5 ml CH3OONH4 in 20% (v/v) of HNO3, diluted to 20 ml, with 30 min of shaking, F5: during the last extraction (F5), the final residual was dissolved for complete determination using the digestion procedure of Tessier et al. (1979).
In this study, 24 soil samples were selected to establish the geochemical distribution of Pb, Zn, and Cd within the above-defined five fractions of soil. The order of bioavailability of Pb, Zn, and Cd is F5 > F3 » F4 > F2 » F1, F5 > F3 » F4 » F2 > F1 and F5 > F2 » F4 > F1, respectively (Fig. 5). However, the order of their cumulated labile fractions is F5 > F3 > F2 > F4 > F1 (Fig. 6). It appears that Cd is not associated with the reducible fraction and heavy metals associated with organic matter and residual sulfides are relatively low. However, the high cumulated contents of the three studied elements are dominantly associated with the residual (F5) and reducible (F3) fractions.
The heavy metal content in the samples is computed as a recovery percentage based on the sum of F1–F5 fractions using the formula:
The recovery values are found between 0.85 and 1.15.
Geostatistical analysis and data processing
The Kriging technique is one of the most employed geostatistical methods used in spatial interpolation and mapping of various sets of geospatial data. Since it is resulted in logical estimations and it allows pertinent analyses and interpretation, this technique is widely used in the prediction of the spatial distribution of geolocated data. It is one of the successful methods for mapping heavy metal concentration in soils and estimating the risk of pollution they generate (e.g., Amini et al. 2005; Franco et al. 2006; Wu et al. 2007; Zhang et al. 2009; Burak et al. 2010 ; Luo et al. 2010; Wang et al. 2012; Hani et al. 2014).
In this work, the data Kriging is established using ArcGIS software. The data interpolation aims to show the spatial variations of the contents of three soil contaminants (Pb, Zn, and Cd) in the watershed of Fedj Lahdoum (Table 1) and to estimate their concentrations at unsampled sites. Indeed, the estimated concentrations are prediction results; the root mean square error (RMSE) criterion is used to estimate their accuracy and to choose the best fitted variogram model for each element, and thereby a final risk map of pollution in the studied watershed can be established. The flow chart of Fig. 3 illustrates the successive steps of the geostatistical analysis methods (described in literature and followed in this study) used to map the risk associated with heavy metal contamination in soils (Mehrjardi et al. 2008; Omran 2012; Marko et al. 2014). Geostatistics in thematic mapping are applied to geolocated variables to model their variation in space across the study area and examine their prospective correlation. They use variogram function to fit an appropriate model that may describe the spatial correlation of the studied variables. The data Kriging is, however, computerized under user-defined parameters for the spatial interpolation (Webster and Oliver 2001). The variogram computed for paired data values relates the two following parameters: The semi-variance defined as half the average of the squared difference between the paired data values{Z(xi),Z(xi + h)} and the lag h, which is the distance between them (Atkinson and Lloyd 2014). The classical variogram estimator proposed by Matheron (1963) still a robust prediction Kriging tool commonly applied for discrete geolocated data such these of soil samples. This semi variogram is computed as follows:
Z(xi) and Z(xi + h) are the values attributed to the variable at locations (xi) and (xi + h), respectively, and N(h) represents the number of pairs of the sample points separated by h.
Generally, the appropriate fitted variogram is selected after examining and comparing the sets of computed variograms. The user can estimate the correlation and the relationship between the sample points by observing the variogram and evaluating the following parameters (Webster and Oliver 2001): the range (distance at which the model first flattens out), the nugget (value at which the semi-variogram intercepts the y-value), and the sill (value at which the model first flattens out).
In fact, there are many different methods of Kriging as much as the ordinary Kriging (OK) which serves to estimate a value at a point of a region for which a variogram is known. To model the spatial variability of heavy metal contents in the watershed of Fedj Lahdoum, we used the OK based on the following equation:
where \( \hat{Z}\left({x}_0\right) \) and Z(xi) are the estimated values of the variable Z at the location x0 and xi, respectively, γi is the weight of Z(xi), and n is the number of values used for the estimation.
The OK is used in this study due to its relatively simple application and its good prediction accuracy compared to other alternative Kriging methods (Omran 2012; Yao et al. 2012). The variogram used to fit the model obtained from Kriging is evaluated based on the three parameters highlighted above: the nugget, the sill, and the range. The nugget is commonly used to express non-spatial variation and the variation at distances smaller than the sampling interval. Range is defined as the lag distance where the variance approaches the sill asymptotically (Trangmar et al. 1985; Omran 2012).
To compare and evaluate different interpolation techniques, the cross-validation methodology can be used (Isaaks and Srivastava 1989). After that, it is possible to validate the optimal methods to be used for the studied elements to get the best predictions of the spatial variability for each data set. Cross-validation can be performed using GIS softwares (e.g., ArcGis of ESRI); the autocorrelation model is computed using all data input and allow deleting omitted values and replace them by new predicted ones. Typically, the normality of the data must be ensured before proceeding to the interpolation to remove or reduce the skewness and extreme values which can affect the variogram structure. In several similar studies, data are normalized using the logarithmic and Box–Cox transformations (e.g., Zhang and Zhang 1996; McGrath et al. 2004; Liu et al. 2015). Nevertheless, the exploratory spatial data analysis (ESDA) tool of ArcGIS software was used in this study for data processing. Box–Cox and logarithmic transformation were applied to reduce the effect of the outliers and to solve the non-normality problem. The Box–Cox transformation was employed for Pb and Zn and logarithmic transformation for Cd, using the specific power parameter of 0.1. The values of the skewness coefficient of raw data after transformation are shown in Table 2. All parameters were observed to have a smaller skewness after transformation.
Finally, the RMSE derived from the comparison of predicted value to the observed value will be used to evaluate the accuracy of the plotted models. RMSE can be calculated as follows:
Z(pi) and Z(oi) are the predicted and the observed value for location i, respectively. n is the number of data points. Conventionally, the lower the RMSE, the better the prediction results that are accurate.
Results
Soil characterization
Clay minerals, quartz, feldspar, dolomite, and galena are significantly present within all soil samples which exhibit X-ray diffraction patterns similar to that of Fej45 sample presented in Fig. 4. However, the occurrence of sphalerite seems to be restricted in some locations. Among the clay minerals, chlorite and kaolinite seem to be the most represented, while illite and smectite appear rarely.
Pb and Zn occur within all soil samples with concentrations varying from 1 to 3457 mg kg−1 and 2 to 14,180 mg kg−1, respectively (Table 1). Cd concentration reaches 186 mg kg−1 in Fej44 but it is still relatively low. The highlighted total contents of Pb, Zn, and Cd within the studied samples indicate that the soils around the Fedj Lahdoum mine exhibit variable amounts of contamination, and they can be classified from uncontaminated to highly contaminated soils (Table 1). The pollution levels were defined based on the Tunisian soil pollution standards (INNORPI 2003). Nevertheless, the quantity of detected heavy metals (in mg kg−1) mostly exceeded the Tunisian pollution norms in soils set at 300 for Zn, 100 for Pb, and 3 for Cd. The highest values are detected within the soils sampled near the exploited mineralized bodies and close to the areas of ore processing.
The pH values (Table 1) of sample suspensions range between 8.12 and 8.74 indicating alkaline soils. The mean pH value (8.38) relates to the raised percentage of the carbonates within these soils (20–38% CaCO3) (Table 1).
Heavy metals in the substrate
The heavy metal average percentage in the soil sample fractions was evaluated using Tessier et al. (1979) procedure; metal distribution is studied through five major geochemical forms attributed to the following fractions (Fig. 5):
-
The exchangeable fraction contains the lower heavy metal average percentage in the following order Cd > Zn > Pb. The cadmium content is ~ 8%; however, those of zinc and lead are near 2%.
-
The carbonate bound fraction in which the order of heavy metal average percentage is Cd > Pb > Zn. The contents of the three elements within this fraction are 32%, 10%, and 6%, respectively.
-
The reducible fraction which contains high contents of bioavailable Pb and Zn (an average of 23%) while no Cd was detected.
-
The organic matter and sulfide fraction despite its affinity with heavy metals shows relatively lower contents (12%) compared to the residual (46%) and reducible (23%) fractions. In this fraction, the order of heavy metal average percentage is Zn > Pb > Cd.
-
The residual fraction encloses high percentage of heavy metals with an average of 46% and the order is Cd > Pb > Zn.
The percentages of lead (Pb), zinc (Zn), and cadmium (Cd) exchangeable fractions obtained from the soil samples of Fedj Lahdoum mine after sequential extraction of Tessier et al. (1979)
The heavy metal contents detected in the residual fractions (~ 46%) seems to be stable and did not participate in the diagenetic and biological processes in natural conditions (Martin et al. 1987). By contrast, the heavy metals associated with the other fractions (labile fractions) were more reactive and could be remobilized. The percentages of the cumulated labile fractions of both (Pb), zinc (Zn), and cadmium (Cd) within the five soil fractions obtained from the soil samples of Fedj Lahdoum mine show the same distribution as for each element taken apart (Figs. 5 and 6). In fact, these fractions are sensitive to the chemical, physical, and biochemical substances found in soils.
The percentages of the cumulated labile fractions of both (Pb), zinc (Zn), and cadmium (Cd) within the five soil fractions obtained from the soil samples of Fedj Lahdoum mine after sequential extraction of Tessier et al. (1979)
Prediction maps of heavy metal spatial distribution
The OK was applied to normalized data sets to infer interpolated values of some heavy metal content in the soils of Fedj Lahdoum mine. The RMSE is the criterion used for selecting the best fitted variogram model for each heavy metal. For Pb case, the smallest RMSE criterion is obtained by circular model with the Box–Cox transformation. For Cd, the best fitted variogram is obtained by exponential model with logarithmic transformation. And for Zn, the exponential model with the Box–Cox transformation gives good results (Table 3).
The Nug/Sill ratio is common parameters used to classify spatial dependence of soil properties. According to many authors (e.g., Chien et al. 1997; Chang et al. 1998; Liu et al. 2005 and others), there are three classes of spatial dependence of a variable: strong, moderate, and weak corresponding to the Nug/Sill ratio of 25%, 75%, and > 75%, respectively. In fact, this spatial dependence may be affected by intrinsic and extrinsic factors of the soil. These soil factors relate, respectively, to strong and weak spatial dependence of soil properties (Cambardella et al. 1994; Liu et al. 2005). In this study, results relative to Zn and Cd show strong spatial dependence; however, those of Pb exhibit moderate spatial dependence (Table 4).
The cross-validation is used to inspect if the fitted semi variogram model predicts accurately the heavy metal at unsampled locations. The accuracy of the model and their parameters is subjected to validation by statistic results. The ratio between predicted and measured values should be a straight line with 1:1 slope. The deviation of the predicted values from the measured values is evaluated using the “prediction error”. The smaller the mean error (tend to zero), the greater the prediction result accuracy. Conventionally an accurate prediction is characterized by very small RMSE, an average standard error close to 0 and a root mean square standardized prediction error close to 1 (Johnston et al. 2001; Marko et al. 2014). The example of cross-validation performed for the OK of Pb element (Fig. 7) provides good correlation between the predicted and measured values. The maps of the predicted distribution of Pb, Zn, and Cd contents in the Fedj Lahdoum watershed (Figs. 8, 9, and 10) are drawn after OK of their relative data sets. For each map, the concentration of the studied heavy metal is reclassified into four classes (low, moderate, considerable, and high) based on the Interim Canadian Environmental Quality Criteria for Contaminated Sites (CCME [Canadian Council of Ministers of the Environment] 1991) (Table 5).
The superimposition of the predicted distributions of these three elements in one layer permits to produce a map of potential soil pollution risk (Fig. 11) which can be used to predict the cumulated contamination and point out the pollution risk near the Fedj Lahdoum mine. Consequently, the “Raster Calculator” tool was used to overlap the three layers (Pb, Zn, and Cd) and to generate an output layer illustrating the potential risk of soil pollution. Since the obtained map illustrates the distribution of the cumulated concentrations of heavy metals in soils for a large land surface, it is used to identify the high metal concentration zones and it can help in the detection of the possible sources of soil contamination. It seems that the central part of the mapped area (Fig. 11) constitutes the most contaminated zone of the studied watershed. This zone matches with the Fedj Lahdoum mine infrastructure areas encompassing both extraction, processing, and tailing deposition areas.
Discussion
Distribution of heavy metals and contamination assessment
Heavy metals are natural components of the Earth’s crust and they occur in the soil environment as trace elements. Heavy metals in soils are present in different fractions with different degrees of availability to plants (Morillo et al. 2009), surface, and underground water. Their bioavailability is, however, conditioned by their chemical mobility and soil physicochemical characteristics. Plants exhibit presumable tolerance for heavy metals (Viehweger 2014) since they use some elements (Zn, copper (Cu), manganese (Mn), nickel (Ni), and cobalt (Co)) as micronutrients necessary for their biological function while other elements are toxic even with low concentrations (Pb, Cd, and Hg). Heavy metal pollution in soils and ecosystems constitutes an important environmental problem resulting from natural processes and increasing anthropogenic activities. Heavy metals accumulated in soils and water may be transferred to the food chain by plant uptake and threaten the human and animal health. The risk levels associated with heavy metal concentrations in soil are defined by some analytical approaches (Ferguson et al. 2000; Carlon et al. 2007) aiming to define a threshold and standard values for each hazardous element to identify soil contamination and remediation needs (Tóth et al. 2016). If the concentration of an element is above the guideline value, the assessed area is considered contaminated and the concentration level will present an ecological or health risks. For example, according to the permissible limits of heavy metal concentrations in soil (in mg kg−1) established for the European Union countries (Amlinger et al. 2004), the concentrations of Zn, Pb, and Cd in soil should be 40–450, 40–450, and 0.4–4 respectively; however, in Tunisia, the limits are set at 300, 100, and 3, respectively.
According to the Tunisian standards, we notice that among the 109 soil samples, 39 exhibit Zn concentrations above the limit of 450 mg kg−1 and are consequently considered Zn-polluted soils. According to the same reasoning, 24 samples are Pb polluted and 71 samples are Cd polluted (Table 1). Generally, results do not show a clear spatial correlation between the three analyzed elements and only four samples (Fej1, Fej46, Fej54 and Fej57) were highlighted to be polluted with both the three elements. According to our knowledge of the study area and the mine activity history, the soil contamination near these sampled sites is of different origins. The contamination near Fej1 located beside the mine access road is assumed to be the result of accidental spills of ores during their transport by truck outside the site (ore processing was done in the mine of Lakhouet for a period). Fej46 is located to the vicinity of the exploited area; the high concentration of heavy metals seems to be linked to the presence of a mineralized body. Fej54 is located near the treatment plant and it exhibits the highest concentration of Zn (14,180 mg kg−1). The high amount of pollution highlighted within the Fej57 is explained by the fact that this sample is taken from the outlet of the E-NE-oriented talweg (tributary of wadi el Mour to the west) that drain the mine tailings located upstream and spread the pollution toward the west (Figs. 8, 9, 10, and 11).
The map of Fig. 11 reveals that most contaminated area matches with the extraction and processing area located in the central part of the watershed of Fedj Lahdoum. The highest contents of heavy metals are restricted close to the mining area, smelters, and the mine tailing dam. The contamination exhibits a clear spreading to the southeastern part of the studied area; however, soils located upstream or away from contamination sources contain relatively low levels of heavy metals. Heavy metal contents in the mine tailings are commonly very highs, well exposed to meteoric water and wind. The infiltration and drainage of meteoric water through the mine tailings easily mobilize dissolved and particulate forms of heavy metals and transfer them to drained terrains and groundwater. In addition, wind can mobilize and disperse particle-bound heavy metals to random environmental locations. So, the leaching of the mine tailings by meteoric water and the spreading of particle-bound heavy metals by wind seem to be two main pollution process near mining areas since they can transfer pollutants in particulate and/or dissolved form (Conesa et al. 2006; Tembo et al. 2006). Heavy metals spreading from mining wastes can be related to two main reasons: (1) the physicochemical properties of the mine tailings involving high heavy metal contents, thin size of wastes, and sensitive erosion zone considered as source of soluble metals that can be mobilized to contaminate surrounding areas. According to Babbou-Abdelmalak et al. (2011), the average contents of Zn, Pb, and Cd (in mg kg−1) within the wastes of Fedj Lahdoum mine are 14,600, 2100, and 62.1, respectively. (2) Dusts transported by wind can scatter the pollution by strewing pollutants far from their sources as described in other similar mine sites (e.g., Conesa et al. 2006; Boussen et al. 2010; Babbou-Abdelmalak et al. 2011; Sebei et al. 2017).
Jebel Fedj Lahdoum exhibits a mountainous landscape with NNE-trending culminations of about 850–950 m. The latter are made of Cenomanian-Turonian limestones and encompass several Pb-Zn mineralization bodies of banded, vein, and massive types (Charef and Sheppard 1987). The eastern side of Jebel Fedj Lahdoum is abruptly sloped toward 650 m and it encompasses several ravines draining the surface runoff toward the ESE direction. This landscape condition is responsible for the erosion and the leaching of Cenomanian-Turonian series located in relatively highlands and the spreading of the heavy metals by means of the surface drainage system toward the eastern lowlands (Figs. 8, 9, 10, and 11).
In addition, particle-bound heavy metals can be transferred and resuspended naturally as dust storms affecting the ambient concentration and deposition of heavy metal pollutants (Jeričević et al. 2012). The wind resuspension of heavy metal may explain the occurrence of pollution in some restricted location apart from the exploitation and processing areas and not connected to the surface drainage. The amount of wind pollution around mining area depends on many factors as much as the speed and frequency of winds, the heavy metal content in sterile slag, and the quantity and the composition of wind-mobilized material (Brotons et al. 2010). The dominant wind direction in the study area is NW with 2.7 m s−1 average speed. Generally, the contamination pattern follows the dominant wind direction which can be locally influenced by the orography. The mine of Fedj Lahdoum is located in an ENW-oriented talweg system. Their bordering hills system seems to prevent the dispersion of pollution northward and southward; however, the pollutants spreading toward the SE seem to be privileged.
Geochemical forms of metals in soil
The F1 fraction includes the metals retained near the soil surface due to electrostatic interaction. This fraction is the most bioavailable and labile metal forms. Moreover, F2, F3, and F4 fractions are also potentially available to plants and are mobile in the environment. The species in the four first fractions are the bioavailable fractions; however, the last one will be referred as the residual fraction.
Lead
The lead concentration in the earth’s crust is 10 to 30 mg kg−1 (USDHHS 1999). Lead found in the soil is not mobile, limited to the upper horizons and is not absorbed by plants because it is not a plant nutrient (Verheye 2009; Sharma and Dubey 2005). However, increased Pb content in soils has serious risk to groundwater. The main Pb content in the soil was preferentially associated with the residual fraction and it ranges from 29 to 49% of the total Pb (43%). The reducible Pb fraction ranges from 30 to 39% of the total Pb. Eleven percent of the compounds were found to be organic matter and sulfide residual fraction with ~ 10% of carbonate-bound metal fractions and ~ 2% exchangeable fractions. The chemical association of this element in the different phases follows the order F5 > F3 » F4 > F2 » F1 (Fig. 5). Numerous works highlighted that the Pb residual fraction seems to be the most increased in contaminated soils (Ramos et al. 1994; Karczewska 1996; Ahumada et al. 1999). Bioavailable Pb in the soils of Fedj Lahdoum mine is relatively high (57%), and due to its mobility in the environment, it constitutes a high risk for underground water.
Zinc
Zinc is a trace element with a content of 75 mg kg−1 in the earth’s crust. It is vital for plant growth and many plant functions (Hafeez et al. 2013). Zinc toxicity is naturally rare under normal field conditions; however, it can occur near industrial zones. The most common form of mobile zinc in soil is Zn2+ which is easily adsorbed on the mineral components. Among the other heavy metals contained in soil, Zn is relatively easily soluble. This metal is preferentially associated with the residual fraction like the lead case (Fig. 5). Its amount ranges between 41 and 50%; however, the amount of reducible Zn ranges from 31 to 38%. About 13% of the total Zn is associated with sulfide fraction and organic matter, ~ 6% is associated to the fraction bound to carbonates, and ~ 2% with the exchangeable fraction. This metal is distributed in five fractions as follows: F5 > F3 » F4 » F2 > F1.
Kabala and Singh (2001) reported that Zn is found in association with the Fe oxides. The fact that the percentage of Zn within the residual fraction is high argues for an evolution to Zn unavailability in these soils (Shaheen and Rinklebe 2013). According to other authors (e.g., Karczewska 1996; Wilson and Pyatt 2007), Zn mobility in soils is greater than the Pb one.
Cadmium
Galena (PbS) occurs commonly in association with sphalerite (ZnS) (Klein and Philpotts 2013; Ndimofor 2018). Sphalerite is the chief host of cadmium in zinc deposits (Schwartz 2000) since it allows the Cd substitution for zinc. The Cd average concentration is 0.15–0.2 mg kg−1 in earth’s crust (Adriano 2001) and 0.3–0.62 mg kg−1 in the world’s soils (Ure and Berrow 1982). Regarding these concentrations, the Cd content highlighted within the analyzed soil samples (Table 1) is high and may engender a serious environmental risk given its high toxicity.
Cd is preferentially associated with the residual fraction as much as Pb and Zn (Fig. 5). The residual Cd content ranges from 38 to 54%. Cd associated with the carbonate-bound fraction makes about 32% of the total Cd. Moreover, a significant association was observed between the organic matter and sulfide (12%). About 8% of the total Cd is found in association with the exchangeable fraction. However, Cd was not detected in the reducible fraction. The order of Cd contents in the different soil fractions is F5 > F2 » F4 > F1. The increased content of Cd in the residual phases argues for possible transfer of Cd to the food chain via the uptake of the water retained in soil by plants or by any other mechanism (Petit and Rucandio 1999).
Conclusion
The study of soils sampled around the mine site of Fedj Lahdoum revealed high concentrations of Pb, Zn, and Cd within the residual fractions. Considering the total metal concentrations and mobile fractions, these metals engendered increased contaminations of soil in the following order of contamination Zn > Pb > Cd. The high amounts of contamination are located close to the mine and smelters which argue that the zones of mineral extraction and processing are the principal sources of contamination. The landscape of Fedj Lahdoum area, the morphology, and the chemical composition of the mine tailings and the surface drainage of meteoric water are main factors responsible for the scattering of the pollution to the southeastern part of the watershed of Fedj Lahdoum. The wind is also a contributor factor responsible for the spreading of heavy metals especially toward the SE of the mine.
Since no treatment method was applied for these mine tailings and considering the pollution impact it engenders for the surrounding ecosystem, the application of an appropriate treatment method is an emergency action to endeavor for the elimination and/or the reduction of the pollution risk.
References
Abollino O, Aceto M, Malandrino M, Mentasti E, Sarzanini C, Barberis R (2002) Distribution and mobility of metals in contaminated sites, investigation of pollutant profiles. Environ Pollut 119(2):177–193
Adriano DC (2001) Bioavailability of trace metals. In: Trace elements in terrestrial environments. Springer, New York, pp 61–89
AFNOR N 94–048 (1996) Soils: recognition and testing-determination of carbonate: calcimeter method
Ahumada I, Mendoza J, Ascar L (1999) Sequential extraction of heavy metals in soils irrigated with wastewater. Commun Soil Sci Plant Anal 30(9–10):1507–1519
Algan O, Balkis N, Namikcagatay M, Sari E (2004) The sources of metal contents in the shelf sediments from the Marmara Sea, Turkey. Environ Geol 46(6–7):932–950
Alloway BJ (1995) In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, p 308
Amini M, Afyuni M, Khademi H, Abbaspour KC, Schulin R (2005) Mapping risk of cadmium and lead contamination to human health in soils of Central Iran. Sci Total Environ 347:64–77
Amlinger F, Favoino E, Pollak M, Peyr S, Centemero M, Caima V (2004) Heavy metals and organic compounds from wastes used as organic fertilisers. Study on behalf of the European Commission, Directorate-General Environment, ENV.A,2
Atkinson PM, Lloyd CD (2014) Geostatistical models and spatial interpolation. In: Handbook of Regional Science, pp 1461–1476
Babbou-Abdelmalak C, Sebei A, Chaabani F (2011) Incurred environmental risks and potential contamination sources in an abandoned mine site. Afr J Environ Sci Technol 5:11,894–11,915
Bhattacharya A, Routh J, Jacks G, Bhattacharya P, Mörth M (2006) Environmental assessment of abandoned mine tailings in Adak, Västerbotten district (northern Sweden). Appl Geochem 21(10):1760–1780
Blanchard C (2000) Caractérisation de la mobilisation potentille des polluants inorganiques dans les sols pollués [Characterization of the potential mobilization of inorganic pollutants in polluted soils]. Dissertation, INSA Lyon
Bouhlel S (1993) Gîtologie, minéralogie et essai de modélisation des minéralisations à F-Ba-Sr-Pb-Zn associés aux carbonates (Jurassique et Crétacé) et aux diapirs triasiques : gisement de Stah, Kohl, Zriba, Guebli, Boujaber et Fedj Lahdoum (Tunisie septentrionale) [Gitology, mineralogy and attempt of modeling of F-Ba-Sr-Pb-Zn mineralization associated with (Jurassic and Cretaceous) carbonates and Triassic diapirs: Stah, Kohl, Zriba, Guebli, Boujaber and Fedj Lahdoum deposits (Northern Tunisia)]. Dissertation, University of Tunis El Manar
Boussen S, Sebei A, Soubrand-Colin M, Bril H, Chaabani F, Adeljaouad S (2010) Mobilization of lead-zinc rich particles from mine tailings in northern Tunisia by aeolian and run-off processes. Bulletin de la Société Géologique de France 181:5459–5471
Bril H, Floc’h J.P (2001) Le devenir des métaux provenant des anciennes mines : l’exemple du massif central français [the fate of metals from old mines; the example of the French Massif Central]. Géologues 130.131:233–241
Brotons JM, Diaz AR, Sarria FA, Serrato FB (2010) Wind erosion on mining waste in southeast Spain. Land Degrad Dev 21(2010):196–209. https://doi.org/10.1002/ldr.948
Burak DL, Fontes MPF, Santos NT, Monteiro LVS, Martins E, Becquer T (2010) Geochemistry and spatial distribution of heavy metals in Oxisols in a mineralized region of the Brazilian Central Plateau. Geoderma 160:131–142
Cambardella CA, Moorman TB, Novak JM, Parkin TB, Turco RF, Konopka AE (1994) Field-scale variability of soil properties in Central Iowa soils. Soil Sci Soc Am J 58(5):1501–1511
Carlon C, D'Alessandro M, Swartjes F (2007) Derivation methods of soil screening values in Europe: a review of national procedures towards harmonization. European Commission Joint Research Centre, Ispra Italy, EUR 22805 EN–2007 320
CCME [Canadian Council of Ministers of the Environment] (1991) Interim Canadian environmental quality criteria for contaminated sites. Report CCME EPC-CS34. CCME, Ottawa. 20 pp
Chang YH, Scrimshaw MD, Emmerson RHC, Lester JN (1998) Geostatistical analysis of sampling uncertainty at the Tollesbury managed retreat site in Blackwater Estuary, Essex, UK: kriging and cokriging approach to minimize sampling density. Sci Total Environ 221(1):43–57
Chao TT (1972) Selective Dissolution of Manganese Oxides from Soils and Sediments with Acidified Hydroxylamine Hydrochloride 1. Soil Sci Soc Am J 36(5):764–768
Charef A, Sheppard SM (1987) Pb-Zn mineralization associated with diapirism: fluid inclusion and stable isotope (H, C, O) evidence for the origin and evolution of the fluids at Fedj-El-Adoum, Tunisia. Chem Geol 61(1–4):113–134
Charef A, Bouhlel S, Sheppard SMF (1987) The strata bound Pb-Zn deposit and salt bearing diaper of Fedj el Hadoum (Northern Tunisia Geological frame, fluid inclusions, isotopic data and genetic model). 8th Regional Meeting of sedimentology:145–146.
Chien YL, Lee DY, Guo HY, Houng KH (1997) Geostatistical analysis of soil properties of mid-west Taiwan soils. Soil Sci 162(4):291–298 162, 291–297
Colandini V (1997) Effets des structures réservoirs à revêtement poreux sur les eaux de ruissellement pluviales: qualité des eaux et devenir des métaux lourds [Effects of a porous pavement with reservoir structure on runoff water: water quality and fate of heavy metals]. Dissertation, University of Pau and Pays de l’Adour, 161 p
Colinet G (2003) Eléments traces métalliques dans les sols: Contribution des déterminants de leur distribution spatiale en région limoneuse Belge [Metallic trace elements in soils: Contribution of the determinants of their spatial distribution in the Belgian silty region]. Dissertation, University of Gembloux, Belgium, 139 p
Conesa HM, Faz A, Arnaldos R (2006) Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena-La Union mining district SE Spain. Sci Total Environ 366:1–11
Delmas-Gadras C (2000) Influence des conditions physico-chimiques sur la mobilité du plomb et du zinc dans un sol et un sédiment routier. [Influence of physico-chemical conditions on the mobility of lead and zinc in a soil and a sediment from road area]. Dissertation, University of Pau and Pays de l’Adour
Ferguson C, Vegter J, Kasamas H (2000) Assessing risks from contaminated sites: Policy and practice in European countries. In Contaminated Soil 2000: Seventh International FZK/TNO conference on contaminated soil:1–3, Thomas Telford Ltd
Franco C, Soares A, Delgado J (2006) Geostatistical modeling of heavy metal contamination in the topsoil of Guadiamar river margins (S Spain) using a stochastic simulation technique. Geoderma 136(3–4):852–864
Guillet B, Jeanroy E, Rouiller J, Souchier B (1980) Le cycle biogéochimique et la dynamique du comportement des éléments traces (Cu, Pb, Zn, Ni, Co et Cr) dans les pédogenèses organiques acides: L’exemple des sols brunifiés et podzoliques sur le granite des Ballons, Vosges méridionales [The biogeochemical cycle and the behavior dynamics of trace elements (Cu, Pb, Zn, Ni, Co and Cr) in the organic acid pedogenesis: the example of browned and podzolic soils on the granite of Balloons, Southern Vosges]. Centre of Biological Pedology CNRS Nancy (ed), Technical note 27,55p
Hafeez B, Khanif YM, Saleem M (2013) Role of zinc in plant nutrition-a review. J Exp Agric Int:374–391
Hani A, Sinaei N, Gholami A (2014) Spatial variability of heavy metals in the soils of Ahwaz using geostatistical methods. Int J Environ Sci Dev 5(3):295–298
Hou S, Zheng N, Tang L, Ji X, Li Y, Hua X (2019) Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ Int 128:430–437
INNORPI Institut National de Normalization et de la Propriété Industrielle [National Institute for Standardization and Industrial Property] (2003) Soil quality, vocabulary. Part 4: Terms and definitions related to rehabilitation of soils and sites NT 91,12p
Isaaks EH, Srivastava RM (1989) An introduction to applied geostatistics. Oxford University Press, New York, p 561
Jeričević A, Ilyin I, Vidič S (2012) Modeling of heavy metals: study of impacts due to climate change. In: National security and human health implications of climate change. Springer, Dordrecht, pp 175–189
Johnston K, Hoef JMV, Krivoruchko K, Lucas N (2001) ArcGIS®9: using ArcGIS® geostatistical analyst. ESRI. http://dusk.geo.orst.edu/gis/geostat_analyst.pdf., 2001. Accessed 23 Mar 2019.
Jung MC, Ahn SJ, Chon TH (2001) Environmental contamination and sequential extraction of trace elements from mine wastes around various metalliferous mines in Korea. Geosyst Eng 4:50–60
Juste C (1988) Appréciation de la mobilité et de la biodisponibilité des éléments en traces du sol [Assessment of trace element mobility and bioavailability in soil]. Science du sol 26(2):103–112
Kabala C, Singh BR (2001) Fractionation and mobility of Cu, Pb and Zn in soil profiles in the vicinity of a Cu smelter. J Environ Qual 30:485–492
Karczewska A (1996) Metal species distribution in top- and sub-soil in an area affected by copper smelter emissions. Appl Geochem 11:35–42
Kassa S (1990) Caractérisation thermo-optique et physico-chimique des fluides en inclusions dans les minéraux; application aux célestites de la zone de transition de la mine de Pb-Zn de Fedj Lahdoum (Tunisie septentrionale) [Thermo-optical and physico-chemical characterization of fluid inclusions in minerals; application to celestites from the transition zone of the Pb-Zn mine of Fedj Lahdoum]. Dissertation, University of Tunis El Manar
Klein C, Philpotts AR (2013) Earth materials: introduction to mineralogy and petrology. Cambridge University Press
Kotoky P, Bora BJ, Baruah NK, Baruah J, Baruah P, Borah GC (2003) Chemical fractionation of heavy metal in soils around oil installations, Assam. Chem Speciat Bioavailab 15:115–126
Laatar E (1980) Gisement de plomb-zinc et diapirisme du Trias salifère en Tunisie septentrionale: les concentrations péridiapiriques du district minier de Nefate-Fedj El Adoum (région de Téboursouk) [Lead-zinc deposit and diapirism of the saliferous Triassic in northern Tunisia: peridiapiric concentrations in the mining district of Nefate-Fedj El Adoum (Teboursouk region)]. Dissertation, University of Pierre and Marie Curie, Paris VI
Lespagnol G (2003) Lixiviation du Chrome, du Cuivre et de l’Arsenic (CCA) à partir de sols contaminés sur des sites de traitement du bois [Leaching of Chromium, Copper and Arsenic (CCA) from contaminated soils at wood treatment sites]. Dissertation, University of Jean Monnet
Liu X, Wu J, Xu J (2005) Characterizing the risk assessment of heavy metals and sampling uncertainty analysis in paddy field by geostatistics and GIS. Environ Pollut 141:257–264
Liu G, Niu J, Zhang C, Guo G (2015) Accuracy and uncertainty analysis of soil Bbf spatial distribution estimation at a coking plant-contaminated site based on normalization geostatistical technologies. Environ Sci Pollut Res 22:20121–20,130
Luo W, Lu Y, Zhang Y, Fu W, Wang B, Jiao W, Wang G, Tong X, Giesy JP (2010) Watershed-scale assessment of arsenic and metal contamination in the surface soils surrounding Miyun Reservoir, Beijing, China. J Environ Manag 91:2599–2607
Marcos L (2001) Etude expérimentale et modélisation du transfert du zinc et du plomb dans milieux sableux modèles [Experimental study and modeling of the transfer of zinc and lead in model sandy environments]. Dissertation, Univeristy of Nantes
Marko K, Al-Amri NS, Elfeki AMM (2014) Geostatistical analysis using GIS for mapping groundwater quality: case study in the recharge area of Wadi Usfan, western Saudi Arabia. Arab J Geosci 7(12):5239–5252
Martin JM, Nirel P, Thomas AJ (1987) Sequential extraction techniques: promises and problems. Mar Chem 22:313–341
Mason Y, Ammann AA, Ulrich A, Sigg LM (1999) Behavior of heavy metals, nutriments and major components during roof runoff infiltration. Environ Sci Technol 33:1588–1597
Matheron G (1963) Principles of geostatistics. Econ Geogr 58:1246–1266
McGrath D, Zhang C, Carton OT (2004) Geostatistical analyses and hazard assessment on soil lead in Silvermines area, Ireland. Environ Pollut 127(2):239–248
Mehrjardi RT, Jahromi MZ, Mahmodi S, Heidari A (2008) Spatial distribution of groundwater quality with geostatistics case study: Yazd-Ardakan Plain. World Appl Sci J 41:9–17
Morillo J, Usero J, Garcia I (2009) Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. J Coast Res 23:353–361
Ndimofor A (2018) The fundamentals of crystallography and mineralogy. Spears Media Press. ISBN: 978–1–942,876-24-3
Omran EE (2012) improving the prediction accuracy of soil mapping through geostatistics. Scientific Research. Int J Geosci 3:574–590
Perthuisot V (1981) Diapirism in northern Tunisia. J Struct Geol 3:231–235
Petit MD, Rucandio MI (1999) Sequential extractions for determination of cadmium distribution in coal fly ash., soil and sediment samples. Anal Chim Acta 401:283–291
Ramos L, Hernandez LM, Gonzales MJ (1994) Sequential fractionation of copper, lead, cadmium and zinc in soils from Donana National Park. J Environ Qual 23:50–57
Ritcey GM (2005) Tailings management in gold plants. Hydrometallurgy 78(1–2):3–20
Schwartz MO (2000) Cadmium in zinc deposits: economic geology of a polluting element. Int Geol Rev 42(5):445–469
Sebei A, Helali MA, Oueslati W, Abdelmalek C, Chaabani F (2017) Bioavailability of Pb, Zn, Cu, Cd, Ni and Cr in the sediments of the Tessa River: a mining area in the North-West Tunisia. J Afr Earth Sci 137:1–8
Semlali R (2000) Localisation, dynamique et estimation de flux d’éléments traces métalliques dans les sols [Location, dynamics and estimation of flows of metallic trace elements in soils]. Dissertation, University of Paris-Saclay
Shaheen SM, Rinklebe J (2013) Geochemical fractions of chromium, copper, and zinc and their vertical distribution in floodplain soil profiles along the Central Elbe River, Germany. Geoderma 228:142–159
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Smuda J, Dold B, Spangenberg JE, Pfeifer HR (2008) Geochemistry and stable isotope composition of fresh alkaline porphyry copper tailings: implications on sources and mobility of elements during transport and early stages of deposition. Chem Geol 256(1–2):62–76
Soil Survey Staff (2004) Soil survey laboratory methods manual. Soil Survey Investigations Report 42, USDA-NRCS, Washington, DC, USA
Sposito G, Lund J, Change AC (1982) Trace metal chemistry in arid-zone filed soils amended with sewage sludge: fractionation of Ni, Cu, Zn, Cd and Pb in solid phases. Soil Sci Soc Am J 46:260–264
Tembo BD, Sichilongo K, Cernak J (2006) Distribution of copper, lead, cadmium and zinc concentrations in soils around Kabwe town in Zambia. Chemosphere 63:497–501
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for speciation of particulate trace metals. Anal Chem 51:884–851
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Trangmar BB, Yost RS, Uehara G (1985) Application of geostatistics to spatial studies of soil properties. In: Brady NC (ed) Advances in Agronomy, vol 38. Academic Press Inc., Orlando, pp 45–94
Ure AM, Berrow ML (1982) The elemental constituents of soil. Environ Chem 2:94–204
Ure A, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the CEC. Int J Envir Analyt Chem:135–151
USDHHS (1999) Toxicological profile for lead, United States Department of Health and Human Services, Atlanta
Verheye WH (2009) Land use, land cover and soil sciences-volume IV: land use management and case studies. EOLSS Publications
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55(1):35
Walter I, Cuevas G (1999) Chemical fractionation of heavy metals in a soil amended with repeated sewage sludge application. Sci Total Environ 226:113–119
Wang M, Bai Y, Chen W, Markert B, Peng C, Ouyang Z (2012) A GIS technology based potential eco-risk assessment of metals in urban soils in Beijing, China. Environ Pollut 161:235–242
Webster R, Oliver MA (2001) Geostatistics for Environmental Scientists, 2nd edn. Wiley
Wilson B, Pyatt FB (2007) Heavy metal dispersion, persistence and bio-accumulation around an ancient copper mine situated in Anglesey, UK. Ecotoxicol Environ Saf 66:224–231
Wu C, Wu J, Luo Y, Zhang H, Teng Y (2007) Statistical and geostatistical characterization of heavy metal concentrations in a contaminated area taking into account soil map units. Geoderma1 44:171–179
Yao XL, Fu BJ, Lu YH et al (2012) The multi-scale spatial variance of soil moisture in the semi-arid Loess Plateau of China. J Soils Sediments 12:694–703
Zhang C, Zhang S (1996) A robust-symmetric mean: a new way of mean calculation for environmental data. GeoJournal 40.1(2):209–212
Zhang C, Tang Y, Luo L, Xu W (2009) Outlier identification and visualization for Pb concentrations in urban soils and its implications for identification of potential contaminated land. Environ Pollut 157:3083–3090
Acknowledgments
The authors thank Dr. Philippe Garrigues the Managing Editor of ESPR for handling the manuscript and Dr. Ivan Perković and the other anonymous reviewers for their criticisms and comments that helped us to improve this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sebei, A., Chaabani, A., Abdelmalek-Babbou, C. et al. Evaluation of pollution by heavy metals of an abandoned Pb-Zn mine in northern Tunisia using sequential fractionation and geostatistical mapping. Environ Sci Pollut Res 27, 43942–43957 (2020). https://doi.org/10.1007/s11356-020-10101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10101-x