Abstract
Fe-Mn/TiO2 catalysts were prepared through the wet impregnation process to selective catalytic reduction of NO by NH3 at low temperature, and series of experiments were conducted to investigate the effects of key precursors on their SCR performance. Ferric nitrate, ferrous sulfate, and ferrous chloride were chosen as Fe precursors while manganese nitrate, manganese acetate, and manganese chloride as Mn precursors. These precursors had been commonly used to prepare Fe-Mn/TiO2 catalysts by numerous researchers. The results showed that there were distinct differences in NO conversion efficiencies at low temperature of catalysts prepared with different precursors. Catalysts prepared with ferric nitrate and manganese nitrate precursors exhibited the best catalytic performance at low temperature, while three kinds of catalysts prepared with manganese chloride precursors exhibited significantly low catalytic activity. All catalysts were characterized by XRD, SEM, H2-TPR, NH3-TPD, and XPS. The results indicated that when the catalysts were prepared with manganese nitrate or manganese acetate as precursors, Mn4+ contents and Oβ/(Oβ + Oα) ratios decreased in an order of ferric nitrate > ferrous sulfate > ferrous chloride, which was consistent with the change of catalytic activities of the corresponding catalysts at low temperature. It can be found that the excellent catalytic performance of Fe(A)-Mn(a)/TiO2 was ascribed to high redox property and enrichment of Mn4+species and surface chemical labile oxygen groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that nitrogen oxides (NOx) emitted from stationary and mobile sources are serious gaseous pollutants, which have resulted in the severe impact on the atmospheric environment and human health. At present, there are a lot of NOx removal technologies for practical applications. Among them, selective catalytic reduction (SCR) with NH3 (or urea) is the most attractive one due to high reduction efficiency and low running cost. It has been widely used to remove NOx from industrial boilers, diesel cars, and ocean-going vessels (Cimino et al. 2016), in which catalysts in SCR systems play a critical role in creating an efficient reaction and controlling the construction cost (Fan et al. 2017). Up to date, two kinds of SCR catalysts, V2O5-WO3/TiO2 and V2O5-MoO3/TiO2, have been used extensively in commercial projects. However, there are still some shortcomings, such as narrow catalytic temperature window (300–450 °C) (Zhu et al. 2017) and toxic effect of V2O5 (Xu et al. 2018a, b), limiting their application in more and more cases. Especially when the flue gas temperature is much below 300 °C, it is very difficult to achieve a high NOx removal efficiency for the commercial catalyst products. Thus, it is of great importance to develop an environmentally friendly SCR catalyst with excellent catalytic activity at low and middle temperatures.
During the past decades, researchers around the world had put a great deal of effort to develop high-performance low-temperature SCR catalysts. Several kinds of transition metals, such as Mn, Co, Fe, Cu, and Ce, had been introduced to prepare SCR catalysts (Qiu et al. 2016; Gao et al. 2019; Wang et al. 2019a, b; Liu et al. 2019). Since manganese oxides (Mn3O4, Mn2O3, MnO2, MnO) have multiple valences, labile oxygen, and diversiform oxidation states, MnOx-based catalysts have attracted numerous researchers’ interests (Li et al. 2017; Yang et al. 2019; Wu et al. 2007). In NH3-SCR reactions, MnOx would undergo oxidation–reduction cycles, which reflected the ease of changing oxidation states of Mn ions (Kapteijn et al. 1994). Meanwhile, it was reported that TiO2 was a favorable candidate as SCR support due to its good catalytic activity and sulfur resistance (Kantcheva 2001). Therefore, Mn/TiO2 catalysts had been investigated extensively, and the results demonstrated that it was one of the most promising SCR catalysts for low-temperature application (Pena et al. 2004; Li et al. 2017; Yang et al. 2019; Wu et al. 2007). But there are still some challenges for MnOx-based catalysts to deal with, such as poor N2 selectivity and easy deactivation by H2O. A feasible way to promote the NH3-SCR performance of Mn/TiO2 catalysts was to introduce other modified metals. Several kinds of bimetallic Mn-based catalysts, such as Fe-Mn/TiO2 (Qi and Yang 2003; Putluru et al. 2015; Zhang et al. 2018; Lin et al. 2018), Sm-Mn/TiO2 (Sun et al. 2018), Cu-Mn/TiO2 (Li et al. 2019), Ce-Mn/TiO2 (Pan et al. 2018), Nd-Mn/TiO2 (Huang et al. 2019), Sb-Mn/TiO2 (Yang et al. 2016), and W-Mn/TiO2 (Geng et al. 2018), had already been studied, and the results implied that there were obvious synergetic effects between MnOx and other metal oxides in enhancing the SCR performance. Typically, the addition of Fe into MnOx-based catalysts supported on TiO2 could improve the catalytic activity significantly at low temperature. The reason might lie in that co-existing ferric oxides could be in favor to obtain a high dispersion of MnOx on supports, thus enhancing the oxidation of NO into NO2 (Qi and Yang 2003; Wu et al. 2007; Putluru et al. 2015; Deng et al. 2016). To some extent, Fe-Mn/TiO2 catalysts were considered as one promising candidate for low-temperature SCR application.
It was reported that the types of metal precursors would induce different metal oxidation states during the preparation of SCR catalysts, which would exert a great impact to catalyst activities (Xu et al. 2018b; Kapteijn et al. 1994; Li et al. 2007; Huang et al. 2018). Previous studies showed that Mn/TiO2 catalysts prepared from manganese nitrate exhibited better performance within 100–200 °C than that prepared from manganese acetate precursors (Pena et al. 2004). It was ascribed to a higher surface area of the catalysts prepared from manganese nitrate precursors and the formation of MnO2 as active species. But an opposite result was reported that Mn/TiO2 catalysts prepared from manganese acetate precursors could present higher low-temperature activity compared to that made from manganese nitrate (Li et al. 2007). An explanation was given that surface Mn concentration and surface Mn2O3 species were a little higher in the catalysts prepared from manganese acetate precursors. Similar conclusion could also be found in other researchers’ work. A comparative study on the effects of three different precursors, manganese (II) nitrate, manganese (II) acetate, and manganese (III) acetate, on Mn/TiO2 catalytic performance had been done (Hwang et al. 2016). Experimental results showed that Mn/TiO2 catalysts prepared from manganese (III) acetate precursors had the highest denitrification efficiency, which was ascribed to the most enriched Mn concentration and MnO2 species, together with strong acid sites on catalyst surface.
Undoubtedly, as bimetallic MnOx-based catalysts, the physicochemical properties of Fe-Mn/TiO2 catalysts were directly related to both ferric and manganese precursors. It was reported that compared with other nitrates of transition metals such as Cu, Ni, and Cr, ferric nitrate as Fe precursor could be in favor of improving NH3-SCR activity of Fe-Mn/TiO2 catalyst, but the related mechanism had not been explored in detail (Wu et al. 2008). Hitherto, the major efforts had focused on Fe-Mn/TiO2 in terms of preparation methods, other metal modification, calcination temperature, and catalytic behavior. To the best of our knowledge, few studies have been done to investigate the influence of ferric and manganese precursors on physicochemical properties of Fe-Mn/TiO2 catalysts and their corresponding SCR performance. Therefore, it is very meaningful to conduct systematic experiments to study the effects of key precursors on the catalytic activity of Fe-Mn/TiO2 catalysts at low temperature. In the present work, Fe-Mn/TiO2 catalysts were prepared with ferric precursors (ferric nitrate, ferrous sulfate, ferrous chloride) and manganese precursors (manganese nitrate, manganese acetate, manganese chloride) via the wet impregnation process. The reason for the choice of these six precursors lied in that they were commonly used to prepare Fe-Mn/TiO2 catalysts in previous studies, and their catalytic performance was evaluated in a temperature range of 60–300 °C (Xu et al. 2018a, b; Chen et al. 2018; Li et al. 2007; Hwang et al. 2016; Zhang et al. 2012). N2 adsorption/desorption, X-ray diffraction (XRD), scanning electron microscopy (SEM), H2 temperature-programmed reduction (H2-TPR), NH3 temperature-programmed desorption (NH3-TPD), and X-ray photoelectron spectroscopy (XPS) were employed to characterize the physicochemical properties of the prepared catalysts. And the influences of ferric and manganese precursors on SCR performance were also analyzed in detail.
Experimental
Catalyst preparation
Fe-Mn/TiO2 catalysts were prepared by wet impregnation of TiO2 (P25) with various ferric precursors (Fe(NO3)3·9H2O or FeSO4·7H2O or FeCl2·4H2O) and manganese precursors (Mn(NO3)2·4H2O or Mn(CH3COO)2·4H2O or MnCl2·4H2O) successively, followed by stirring thoroughly at 80 °C for 2 h. Then ammonia was added dropwise until solution pH reached 10. The mixture solution was dried at 120 °C overnight and calcined at 500 °C for 3 h in air. Finally, catalyst powders were pressed, crushed, and sieved to 40–60 mesh before evaluation.
In this study, the molar ratio of Fe/Mn/Ti was fixed at 2:6:15. The prepared catalysts were denoted as Fe(x)-Mn(y)/TiO2, where x represents A (ferric nitrate), B (ferrous sulfate), and C (ferrous chloride) and y represents a (manganese nitrate), b (manganese acetate), and c (manganese chloride). For example, Fe(A)-Mn(a)/TiO2 corresponds to the catalyst prepared with Fe(NO3)3·9H2O and Mn(NO3)2·4H2O as precursor solutions.
Catalyst activity test
The SCR performance of all prepared catalysts was evaluated using a fixed-bed quartz reactor (inner diameter 9 mm) at a reaction temperature range of 60–300 °C under atmospheric pressure. The reactor was equipped with a temperature-programming controller. A catalyst of 1.5 g was used for catalytic assessment with a gas hourly space velocity (GHSV) of 25,000 h−1. The total flow rate of simulated flue gas was 1.1 L/min, and the gas components were 0.08% NO, 0.08% NH3, 5% O2, 10% H2O (when used), 0.01% SO2 (when used), and N2 as balance gas. An online flue gas analyzer (MRU, Germany) was used to monitor NO concentration in flue gas. The concentrations of N2O, SO2, NH3, and H2O in the outlet gas were measured by a Fourier-transform infrared spectrometer (Thermo fisher, USA). NO conversion efficiency and N2 selectivity were calculated as Eqs. (1) and (2), respectively.
Catalyst characterization
The textural properties of the prepared catalysts, including the specific surface area, total pore volume, and average pore diameter, were measured at − 196 °C) using a N2 adsorption analyzer (Quantachrome, USA) following a static-volumetric method. The specific surface areas were calculated via the Brunauer–Emmett–Teller (BET) model from the adsorption data. XRD patterns were obtained using an X-ray diffractometer (PANalytical B.V., Netherland) using Cu Kα as radiation source at 40 kV and 30 mA. The angle 2θ was scanned over a range of 10–80° at a step size of 0.02°. The surface morphology and structure of the catalysts were observed using SEM (Zeiss, Germany). H2-TPR profiles were measured using a chemisorption analyzer (Micromeritics, USA). NH3-TPD experiments were conducted using a chemisorption analyzer (Micromeritics, USA), and the TPD profiles were obtained under a He atmosphere (50 mL/min) from 100 to 500 °C (ramp rate of 10 °C/min). XPS profiles were collected using a surface analysis photoelectron spectrometer (ESCALAB, USA), with Al Kα as radiation source at 300 W.
Results and discussion
Effects on SCR performance
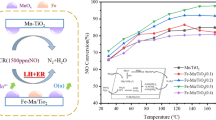
Figure 1 illustrated NO conversion efficiencies of the prepared Fe-Mn/TiO2 catalysts across a temperature window of 60–300 °C. It could be observed that all of the catalysts exhibited good catalytic performance at reaction temperature above 240 °C. But when reaction temperature was below 240 °C, there were distinct differences in NO conversion efficiencies of the catalysts prepared with different precursors. It indicated that both Fe and Mn precursors influenced SCR performance apparently. According to the results, NO removal performance of the prepared catalysts at low temperature was approximately in an order of Fe(A)-Mn(a)/TiO2 > Fe(A)-Mn(b)/TiO2 > Fe(B)-Mn(a)/TiO2 ≈ Fe(B)-Mn(b)/TiO2 > Fe(C)-Mn(b)/TiO2 ≈ Fe(C)-Mn(a)/TiO2 > Fe(A)-Mn(c)/TiO2 ≈ Fe(B)-Mn(c)/TiO2 ≈ Fe(C)-Mn(c)/TiO2. Clearly, the catalysts prepared with ferric nitrate and manganese nitrate precursors exhibited superior catalytic performance over other catalysts at low temperature. But some previous studies showed that the catalytic performance of the catalyst prepared with ferric nitrate and manganese acetate could be superior over that of the catalyst prepared with ferric nitrate and manganese nitrate. To some extent, it demonstrated that not only the precursors but also the preparation process would influence the catalytic performance, obviously. Mu et al. had prepared Fe-Mn/TiO2 catalysts with ferric nitrate and manganese acetate as precursors by an impregnation method (Mu et al. 2018). By simple comparison, it could be seen that the catalytic performance of Fe(A)-Mn(b)/TiO2 prepared in this work was similar to that without the assistance of ethylene glycol (EG) in Mu’s work. In other words, Mu et al. improved the catalytic performance of Fe(A)-Mn(b)/TiO2 by adding EG in the precursor solution while that could be achieved similarly by replacing manganese acetate with manganese nitrate in our work. The results showed that the light-off temperature (where NO conversion achieved 50%) for the Fe(A)-Mn(a)/TiO2 catalyst was ~70 °C, and it achieved 100% NO conversion efficiency at 100 °C with a wide temperature window from 75 to 300 °C.
As shown in Fig. 1, three kinds of catalysts prepared with manganese chloride precursors exhibited significantly lower catalytic activity at low temperature. It could be ascribed to a lower degree of manganese chloride decomposition.
For the catalysts prepared using manganese nitrate and manganese acetate precursors, it could be seen that the catalytic activities at low temperature for catalysts prepared using different ferric precursors were in an order of ferric nitrate > ferrous sulfate > ferrous chloride. Besides, the results indicated that when ferrous sulfate or ferrous chloride was used as precursor, there were few differences in catalytic performance between the catalysts prepared using manganese nitrate and manganese acetate precursors. But a distinct difference existed in Fe(x)-Mn(a)/TiO2 catalysts at low temperature. It implied that ferric precursors imposed a great effect on the catalytic performance of Fe-Mn/TiO2 catalysts at low temperature, which was possibly related with the degree of ferric precursor decomposition and the dispersion of MnOx species. Here, the performance of Fe-Mn/TiO2 catalysts prepared with various precursors was quite different from that in previous comparison work on the effects of precursors on Mn/TiO2 catalyst (Li et al. 2007). It was obvious that the presence of transition metal Fe had changed the influences of single Mn precursor on catalytic activity at low temperature.
Since the Fe(A)-Mn(a)/TiO2 catalyst displayed the excellent NO conversion, thus, it was selected to study the N2 selectivity during the NH3-SCR reaction, and the results are presented in Fig. 2. As shown in Fig. 2, N2 selectivity of Fe(A)-Mn(a)/TiO2 catalyst was 88% at 100 °C, while NO conversion efficiency reached 100% at the same time. But there was a decreasing trend of N2 selectivity with the increase of the reaction temperature. This phenomenon was ascribed to the generation of more unwanted N2O through some side reactions (Han et al. 2019). It is known that Mn is the main active phase for SCR reactions; so, the formation routines of N2O with Fe(A)-Mn(a)/TiO2 as catalyst in our experiments is similar to those with Mn/TiO2 as catalysts. Wang et al. had investigated the sources of N and O in formed N2O over MnOx/TiO2 catalysts in the NH3-SCR process (Wang et al. 2019b). Their experimental results showed that both NH3 oxidation reaction (2NH3 + 2O2 → N2O + 3H2O) and nonselective catalytic reduction (NSCR) of NO with NH3 (4NO + 4NH3 + 3O2 → 4N2O + 6H2O) accounted for N2O formation. Other researchers pointed out that the reaction rate of these side reactions would increase with the increase of reaction temperature, so it was normal that the production of N2O would increase with the temperature increasing (Pârvulescu et al. 1998). Therefore, it was still a common issue that, for Mn-based catalysts, N2 selectivity would decrease with the increase of reaction temperature.
N2 adsorption/desorption
N2 adsorption–desorption isotherms of Fe(x)-Mn(y)/TiO2 catalysts are shown in Fig. 3. According to the IUPAC classification, the adsorption isotherms of the catalysts were type IV, suggesting that the prepared catalysts belonged to mesoporous materials. The specific surface areas, pore volumes, and pore sizes are summarized in Table S1. The results showed that the physical properties of the catalysts were quite different from each other. The specific surface areas of the five kinds of catalysts prepared with chloride precursors were less than 45 m2/g, which exhibited the greatest loss of specific surface areas compared with the other four kinds of catalysts. The reason for the latter catalysts possessing higher specific surface areas might be attributed to MnOx species being highly dispersed over the catalysts. It was in favor of improving the catalytic activity. The result suggested that the changes in textural properties correlated to the catalytic performance to some degree, though they were not the main factors influencing the catalytic activity at low temperature.
XRD
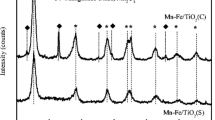
Figure 4 presents the XRD patterns of the prepared Fe(x)-Mn(y)/TiO2 catalysts. In the XRD patterns, the peaks at 2θ of 25.3°, 36.9°, 37.9°, 38.7°, 48.0°, 54.0°, 55.0°, and 62.7° could be attributed to anatase TiO2 (PDF#21–1272). Though the diffraction peaks of TiO2 were apparent in all catalysts, the diffraction peaks of Fe and Mn oxides could only be detected in the patterns of five kinds of catalysts prepared with chloride precursors. It indicated that Fe and Mn oxides existed in the form of crystal states, and their sizes were relatively large, which would impose some adverse effects on catalytic performance. Therefore, the catalytic activities of catalysts prepared with chloride precursors were obviously inferior to those with other kinds of precursors. This agreed well with the aforementioned results of SCR activity and N2 adsorption/desorption tests.
In other words, it could be known from Fig. 4 that for the catalysts prepared using non-chloride precursors, Fe and Mn oxides were well dispersed in amorphous or crystallite states with very small particle sizes on anatase TiO2 support. That was because nitrates, sulfate, and acetate could decompose into metal oxides easily during the calcination process, and the presence of transition metal Fe was beneficial to promote the transformation of MnOx from crystalline state to amorphous state (Mu et al. 2018). In addition, the addition of Fe could improve the dispersion of Mn oxides on the supports, which might also have a correlation with the increasing surface area. A well dispersion of active species would be beneficial to obtain a well distribution of active sites, thus enhancing the catalytic activity (Yang et al. 2019; Wu et al. 2019). Therefore, the high dispersion property of the active species was a preferable aspect for the selection of precursors for preparing Fe-Mn/TiO2 catalysts.
H2-TPR
The reducibility of the metal oxides could be investigated via H2-TPR. It was known that ease of reduction of metals was in favor of low-temperature SCR activity. The redox properties of the prepared catalysts were illustrated in Fig. 5, and the reduction peak temperatures and H2 consumption values are listed in Table S2. It could be seen that there were three reduction peaks in the profile of Fe(A)-Mn(a)/TiO2 catalyst. The low-temperature reduction peak center was at about 296 °C, which could be assigned to the reduction of MnO2 to Mn2O3 (Putluru et al. 2015; Wu et al. 2015). The peak centered at about 418 °C in the profile belonged to the reduction of Mn2O3 to MnO or Fe2O3 to Fe, while the peak centered at about 554 °C belonged to the reduction of Mn3O4 to MnO (Putluru et al. 2015). The redox ability of samples could be determined by the low-temperature peak centers. Fe(A)-Mn(a)/TiO2 catalyst was being reduced at relatively lower temperature compared with other catalysts. Apparently, with manganese nitrate or manganese acetate as precursor, the low-temperature peak centers for catalysts with different ferric precursors were in an order of ferric nitrate > ferrous sulfate > ferrous chloride. The variation trend was consistent with that of H2 consumption values of these samples as shown in Table S2. With the same manganese precursor, H2 consumption values for catalysts with different ferric precursors were also in the order of ferric nitrate > ferrous sulfate > ferrous chloride. This trend was also corresponding with the varieties of Mn/Ti ratios and contents of surface-adsorbed oxygen on the surface of catalysts. It suggested that more manganese species incorporated into TiO2 crystalline phase and higher abundance of active oxygen species were contributed to the enhancement of low-temperature redox ability for Fe(a)-Mn(y)/TiO2 catalyst (Xu et al. 2018b). The shift of low-temperature peak centers for catalysts prepared with ferrous sulfate and ferrous chloride was also possibly due to a formation of larger particle crystals and a decrease of surface-adsorbed oxygen (Wu et al. 2015). Since lower reduction temperature and greater H2 consumption signified the stronger redox behavior and oxygen storage capacity of the catalysts, which was of great importance for SCR reactions (Zhang et al. 2018), the choice of Fe and Mn precursors would lead to the shift of low-temperature peak centers and influence the H2 consumption for Fe-Mn/TiO2 catalysts, further influencing their catalytic performance at low temperature (Wang et al. 2013). As to the catalysts prepared with manganese chloride and/or ferrous chloride as precursors, the low-temperature peak centers were obviously much higher than those of other catalysts. It implied that to a great extent, the low-temperature reducibility and catalytic activity of catalysts prepared with chloride precursors were related to a low decomposition degree of chloride species and a low dispersion degree of MnOx over supports.
NH3-TPD
NH3-TPD was frequently adopted for determining the amounts and strength of surface acid sites of solid-phase catalysts. It was considered that the presence of acid sites would favor NO conversion due to the preferential adsorption of NH3 on these sites thus initiating SCR reaction. Thus, the SCR activity of catalysts might correlate with the amount of total acid sites (Lewis and Brönsted acid sites) and acid strength (Tmax of ammonia desorption). Figure 6 shows the NH3-TPD desorption patterns of the prepared Fe-Mn/TiO2 catalysts. Generally, the total amounts of adsorbed ammonia were determined from the areas under TPD curves. It could be seen from Fig. 6 that most of the catalysts had two broad desorption peaks in a wide temperature range, which was due to the variability of adsorbed NH3 species with different thermal stabilities (Xiong et al. 2015). These two peaks could be assigned to physically adsorbed ammonia (desorption temperature below 300 °C) and chemically adsorbed ammonia (desorption temperature above 300 °C). At low reaction temperature, the amount of physically adsorbed ammonia would play a key role in evaluating the catalytic activities of Fe-Mn/TiO2 catalysts. As shown in Fig. 6, Fe(A)-Mn(a)/TiO2 and Fe(B)-Mn(a)/TiO2 catalysts exhibited much better ammonia adsorption properties than the other catalysts in the temperature range of 100–300 °C, which was advantageous to the adsorption of ammonia. However, as to the Fe(B)-Mn(a)/TiO2 catalyst, it was considered that the inhibiting action of ammonia occurred on the fast SCR activity at low temperature (Grossale et al. 2009). It was mainly ascribed to either a competitive adsorption of NH3 onto the metal sites which are involved in NO activation, or an adverse electronic interaction of adsorbed NH3 with metal oxidization centers (Nova et al. 2006). For Fe(x)-Mn(b)/TiO2 catalysts, Fe(B)-Mn(b)/TiO2 catalysts had adsorbed much more ammonia than Fe(A)-Mn(b)/TiO2 and Fe(C)-Mn(b)/TiO2 catalysts, and it also suggested that ferrous sulfate as precursor was beneficial to enhance the surface acidity. Fe(x)-Mn(c)/TiO2 catalysts adsorbed more or less the same ammonia in the whole temperature range, indicating that the ammonia adsorption properties were possibly determined by manganese chloride precursors.
The total surface acidity for each sample could be calculated according to the integral peak area of the corresponding TPD profile, and the results are shown in Table S3. Among of the prepared samples, Fe(B)-Mn(y)/TiO2 catalysts exhibited a higher total surface acidity than the catalysts prepared with the same manganese precursors. Except for the catalysts prepared with manganese acetate precursors, the total surface acidities for the other catalysts prepared with different ferric precursors decreased in an order of ferrous sulfate > ferric nitrate > ferrous chloride. It indicated that it was easy to obtain a relatively higher total surface acidity by adopting ferrous sulfate to prepare Fe-Mn/TiO2 catalysts. Since NO removal performance of Fe(A)-Mn(y)/TiO2 catalysts at low temperature was much higher than that of Fe(b)-Mn(y) catalysts, it suggested that the total surface acidity was not the determinant factor for SCR performance of the prepared Fe-Mn/TiO2 catalysts.
Overall, the results showed that the acidity property of the catalysts was closely related with the redox property, and it was necessary to achieve a proper balance between acidity property and redox property to obtain an optimal catalytic activity (Wang et al. 2018; Tang et al. 2016).
XPS
XPS analysis was performed to further explore the chemical species and surface atomic composition of the catalysts prepared using different Fe and Mn precursors. Figures 7, 8 and 9 displayed the spectra of Fe 2p, Mn 2p, and O 1s on the catalysts. According to the measurement results, surface atomic concentrations ratios of Fe, Mn, and O are calculated and listed in Table S2. The surface concentration ratios of Mn/Ti on Fe(A)-Mn(a)/TiO2, Fe(A)-Mn(b)/TiO2, and Fe(B)-Mn(b)/TiO2 catalysts were more than 50%, suggesting a high dispersion of MnOx in these catalysts, which was beneficial to improve the catalytic performance.
As shown in Fig. 7, there were two main peaks in XPS spectra of Fe 2p. These two peaks observed for all samples were assigned to Fe 2p3/2 at 710.9 eV and Fe 2p1/2 at 723.9 eV. It indicated that Fe species in these samples were in Fe3+ oxidation states. The results showed that the changes in the chemical states of Fe species were not a main reason for the difference in the SCR activities of these catalysts, because the corresponding binding energies did not show a significant variation (Mu et al. 2018).
Two main peaks assigned to Mn 2p3/2 and Mn 2p1/2 could be observed from Fig. 8 for all samples in a binding energy range of 641 ~ 653 eV. The overlapping peaks of Mn 2p3/2 could be deconvoluted into several peaks with Shirley-type background to identify the surface MnOx phases, and the results are shown in Table S4. Then it could be obtained that 2p3/2 binding energy peaks of MnO2 (Mn4+) and Mn2O3 (Mn3+) appeared at about 642.6 eV and 641.2 eV, respectively, while the binding energy peaks at 644.7 eV represented manganese nitrate (Mu et al. 2018; Hwang et al. 2016; Xu et al. 2018b). The SCR activities of MnOx at low temperature had been investigated by Kapteijn et al. (1994), and they found that NOx removal efficiency for manganese oxides decreased in an order of MnO2 > Mn5O8 > Mn2O3 > Mn3O4. It demonstrated that MnO2 (Mn4+) would play a key role in catalytic removal of NO. As shown in Table S2, Fe(A)-Mn(a)/TiO2 catalyst prepared in this work had the most enriched Mn4+ species (MnO2) over TiO2 supports. So it was normal to obtain an excellent catalytic performance at low temperature for Fe(A)-Mn(a)/TiO2 catalysts. In addition, for the catalysts prepared using manganese nitrate or manganese acetate precursors, Mn4+ contents were in an order of ferric nitrate > ferrous sulfate > ferrous chloride, which was consistent with the change of catalytic activities at low temperature for these catalysts. It demonstrated that the content of Mn4+ species over TiO2 supports was essential for the catalytic activity of Fe-Mn/TiO2 catalysts.
XPS spectra of O 1s in the prepared catalysts were presented in Fig. 9. The sub-bands at a binding energy peak of 529.8 eV could be assigned to the lattice oxygen O2− (denoted as Oα), and the sub-bands at a binding energy peak of 531.2 eV could be assigned to the surface chemisorbed labile oxygen (denoted as Oβ). Here, Oγ referred to the defect oxide and/or hydroxyl-like groups. The quantitative data of Oβ/(Oβ + Oα) ratios of the catalysts were calculated by the relative peak areas, and they are also presented in Table S4. It could be seen that Fe(A)-Mn(a) catalyst had the highest ratio of Oβ/(Oβ + Oα). Some previous studies had pointed out that surface chemisorbed labile oxygen (Oβ) was of great importance to the SCR process at low temperature. It had been considered as the most active oxygen because it was of high mobility and played a critical role in oxidation reaction. Therefore, the high ratio of Oβ/(Oβ + Oα) was conducive to oxidizing NO into NO2, then further enhancing the low-temperature catalytic performance via a fast SCR route. The effect of Oβ/(Oβ + Oα) ratios on SCR performance was similar to that of Mn4+ contents over TiO2 supports. For the catalysts prepared using manganese nitrate or manganese acetate precursors, the Oβ/(Oβ + Oα) ratio decreased in an order of ferric nitrate > ferrous sulfate > ferrous chloride, which was also consistent with the change of catalytic activities at low temperature for the corresponding catalysts. It demonstrated that the Oβ/(Oβ + Oα) ratio was another important factor influencing the catalytic activity of Fe-Mn/TiO2 catalysts greatly.
Effects of H2O and SO2
In general, flue gas exhausted from stationary and mobile sources contained a certain amount of SO2 and H2O, so it was important to evaluate the effects of SO2 and H2O on the activity of NH3-SCR.
The resistance of H2O and SO2 over the Fe(A)-Mn(a)/TiO2 catalyst at 200 °C is presented in Fig. 10. At first, 10% H2O was introduced, there was a slight drop in NO conversion efficiency. However, after stopping injection of water vapor, NO conversion efficiency recovered to 100% within 30 min. It indicated that Fe(A)-Mn(a)/TiO2 catalysts possessed a good resistance to H2O at low temperature. However, when 0.01% of SO2 was added to the reaction gas mixtures, a rapid decrease of NO conversion was observed, indicating that the inhibition effect of SO2 was presented on the SCR reaction over the Fe(A)-Mn(a)/TiO2 catalysts. In addition, NO conversion efficiency did not recover after the shutoff of SO2 injection. This demonstrated that the poisoning effects of sulfur on Fe(A)-Mn(a)/TiO2 catalyst were difficult to reverse. The causes of catalyst deactivation might have resulted from the sulfation of Mn and the formation of ammonium salts, which resulted in some adverse effects on SCR properties. Much more efforts will be devoted to improve the sulfur resistance of the catalyst in our future research (Li et al. 2019).
Conclusion
In this work, Fe-Mn/TiO2 catalysts were prepared via the wet impregnation method, and the effects of various Fe and Mn precursors on NH3-SCR performance were investigated. The results indicated that both Fe and Mn precursors influenced low-temperature SCR performance obviously. Overall, the catalytic activity below 200 °C was in the orders of manganese nitrate > manganese acetate > manganese chloride and ferric nitrate > ferrous sulfate > ferrous chloride. The results indicated that chloride precursors were not suitable for preparing Fe-Mn/TiO2 catalysts due to a low decomposition degree of chloride species during calcination process. On the other hand, it was difficult for catalysts prepared with chloride precursors to obtain a high BET area and high dispersion of Fe and Mn active species over supports. H2-TPR tests showed that, Fe(A)-Mn(a)/TiO2 catalyst could be reduced at relatively lower temperatures compared with other catalysts, which was ascribed to high contents of MnOx and surface chemisorbed labile oxygen on TiO2 support when manganese nitrate and ferric nitrate were adopted as precursors. XPS analysis implied that both Mn4+ species (MnO2) over TiO2 surface and Oβ/(Oβ + Oα) ratios imposed great impact on the catalytic performance of Fe-Mn/TiO2 catalysts at low temperature. When the catalysts were prepared with manganese nitrate or manganese acetate precursors, Mn4+ contents and Oβ/(Oβ + Oα) ratios decreased in an order of ferric nitrate > ferrous sulfate > ferrous chloride, which was consistent with the change of catalytic activities at low temperature for the corresponding catalysts. The results demonstrated that the choice of Fe and Mn precursors was vital for preparing Fe-Mn/TiO2 catalysts, because it would probably affect the Mn4+ content, the surface chemisorbed labile oxygen content, the redox property and the dispersion of active species over support, thus influencing the catalytic performance at low temperature obviously. The Fe(A)-Mn(a)/TiO2 catalysts showed a good resistance to H2O at low temperature. However, the N2 selectivity and resistance to SO2 needs to be further improved.
References
Cimino S, Lisi L, Tortorelli M (2016) Low temperature SCR on supported MnOx catalysts for marine exhaust gas cleaning: effect of KCl poisoning. Chem Eng J 283:223–230
Chen L, Yuan F, Li Z, Niu X, Zhu Y (2018) Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NO removal over the Co0.2CexMn0.8-xTi10 (x = 0–0.40) oxides catalysts. Chem Eng J 354:393–406
Deng S, Zhuang K, Xu B, Ding Y, Yu L, Fan Y (2016) Promotional effect of iron oxide on the catalytic properties of Fe-MnOx/TiO2 (anatase) catalysts for the SCR reaction at low temperatures. Catal Sci Technol 6:1772–1778
Fan Y, Ling W, Huang B, Dong L, Yu C, Xi H (2017) The synergistic effects of cerium presence in the framework and the surface resistance to SO2 and H2O in NH3-SCR. J Ind Eng Chem 56:108–119
Gao Y, Luan T, Zhang S, Jiang W, Feng W, Jiang H (2019) Comprehensive comparison between nanocatalysts of Mn-Co/TiO2 and Mn-Fe/TiO2 for NO catalytic conversion: an insight from nanostructure, performance, kinetics, and thermodynamics. Catalysts 9:175
Geng Y, Shan W, Yang S, Liu F (2018) W-modified Mn-Ti mixed oxide catalyst for the selective catalytic reduction of NO with NH3. Ind Eng Chem Res 57:9112–9119
Grossale A, Nova I, Tronconi E (2009) Ammonia blocking of the “fast SCR” reactivity over a commercial Fe-zeolite catalyst for diesel exhaust after treatment. J Catal 265:141–147
Han Z, Yu Q, Teng Z, Wu B, Xue Z, Qin Q (2019) Effects of manganese content and calcination temperature on Mn/Zr-PILM catalyst for low-temperature selective catalytic reduction of NOx by NH3 in metallurgical sintering flue gas. Environ Sci Pollut Res Int 26:12920–12927
Huang J, Huang H, Liu L, Jiang H (2018) Revisit the effect of manganese oxidation state on activity in low-temperature NO-SCR. Catal Today 446:49–57
Huang J, Huang H, Jiang H, Liu L (2019) The promotional role of Nd on Mn/TiO2 catalyst for the low-temperature NH3-SCR of NOx. Catal Today 332:49–58
Hwang S, Jo SH, Kim J, Shin MC, Chun HH, Park H, Lee H (2016) Catalytic activity of MnOx/TiO2 catalysts synthesized with different manganese precursors for the selective catalytic reduction of nitrogen oxides. React Kinet Mech Catal 117:583–591
Kantcheva M (2001) Identification, stability, and reactivity of NOx species adsorbed on titania-supported manganese catalysts. J Catal 204:479–494
Kapteijn F, Vanlangeveld AD, Moulijn JA, Andreini A, Vuurman MA, Turek AM, Wachs IE (1994) Alumina-supported manganese oxide catalysts: I. characterization: effect of precursor and loading. J Catal 150:94–104
Li J, Chen J, Ke R, Luo C, Hao J (2007) Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts. Catal Commun 8:1896–1900
Li L, Sun B, Sun J, Yu S, Ge C, Tang C, Dong L (2017) Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR. Catal Commun 100:98–102
Li F, Xie J, Qi K, Gong P, He F (2019) Evaluating the intermetallic interaction of Fe or Cu doped Mn/TiO2 catalysts: SCR activity and sulfur tolerance. Catal Lett 149:788–797
Lin L, Lee C, Zhang Y, Bai H (2018) Aerosol-assisted deposition of Mn-Fe oxide catalyst on TiO2 for superior selective catalytic reduction of NO with NH3 at low temperatures. Catal Commun 111:36–41
Liu K, He H, Yu Y, Yan Z, Yang W, Shan W (2019) Quantitative study of the NH3-SCR pathway and the active site distribution over CeWOx at low temperatures. J Catal 369:372–381
Mu J, Li X, Sun W, Fan S, Wang X, Wang L, Zhang D (2018) Enhancement of low-temperature catalytic activity over a highly dispersed Fe-Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind Eng Chem Res 57:10159–10169
Nova I, Ciardelli C, Tronconi E, Chatterjee D, Bandl KB (2006) NH3-SCR of NO over a V-based catalyst: low-T redox kinetics with NH3 inhibition. AICHE J 52(9):3222–3233
Pan Y, Shen Y, Jin Q, Zhu S (2018) Promotional effect of Ba additives on MnCeOx/TiO2 catalysts for NH3-SCR of NO at low temperature. J Mater Res 33:2414–2422
Pârvulescu V, Grange P, Delmon B (1998) Catalytic removal of NO. Catal Today 46:233–316
Pena DA, Uphade BS, Smirniotis PG (2004) TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: I. evaluation and characterization of first row transition metals. J Catal 221:421–431
Putluru SSR, Schill L, Jensen AD, Siret B, Tabaries F, Fehrmann R (2015) Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation—promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B-Environ 165:628–635
Qi G, Yang RT (2003) Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl Catal B-Environ 44:217–225
Qiu L, Wang Y, Pang D, Ouyang F, Zhang C, Cao G (2016) Characterization and catalytic activity of Mn-Co/TiO2 catalysts for NO oxidation to NO2 at low temperature. Catalysts 6:9
Sun C, Liu H, Chen W, Chen D, Yu S, Liu A, Feng S (2018) Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem Eng J 347:27–40
Tang C, Zhang H, Dong L (2016) Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal Sci Technol 6:1248–1264
Wang H, Chen X, Gao S, Wu Z, Liu Y, Weng X (2013) Deactivation mechanism of Ce/TiO2 selective catalytic reduction catalysts by the loading of sodium and calcium salts. Catal Sci Technol 3:715–722
Wang T, Wan Z, Yang X, Zhang X, Niu X, Sun B (2018) Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the fast SCR reaction. Fuel Process Technol 169:112–121
Wang Q, Xu H, Huang W, Pan Z, Zhou H (2019a) Metal organic frameworks-assisted fabrication of CuO/Cu2O for enhanced selective catalytic reduction of NOx by NH3 at low temperatures. J Hazard Mater 364:499–508
Wang D, Yao Q, Hui S, Niu Y (2019b) Source of N and O in N2O formation during selective catalytic reduction of NO with NH3 over MnOx/TiO2. Fuel 2019:23–29
Wu Z, Jiang B, Liu Y, Zhao W, Guan B (2007) Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by sol-gel method. J Hazard Mater 145:488–494
Wu Z, Jiang B, Liu Y (2008) Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl Catal B-Environ 79:347–355
Wu S, Zhang L, Wang X, Zou W, Cao Y, Sun J, Dong L (2015) Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl Catal A-Gen 505:235–242
Wu X, Feng Y, Du Y, Liu X, Zou C, Li Z (2019) Enhancing deNOx performance of CoMnAl mixed metal oxides in low-temperature NH3-SCR by optimizing layered double hydroxides (LDHs) precursor template. Appl Surf Sci 467:802–810
Xiong Y, Tang C, Yao X, Zhang L, Li L, Wang X, Deng Y, Gao F, Dong L (2015) Effect of metal ions doping (M=Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl Catal A-Gen 495:206–216
Xu H, Liu S, Wang Y, Lin Q, Lin C, Lan L, Chen Y (2018a) Promotional effect of Al2O3 on WO3/CeO2-ZrO2 monolithic catalyst for selective catalytic reduction of nitrogen oxides with ammonia after hydrothermal aging treatment. Appl Surf Sci 427:656–669
Xu W, Zhang G, Chen H, Zhang G, Han Y, Chang Y, Gong P (2018b) Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: effect of manganese precursors. Chin J Catal 39:118–127
Yang N, Guo R, Pan W, Chen Q, Wang Q, Lu C (2016) The promotion effect of Sb on the Na resistance of Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3. Fuel 169:87–92
Yang G, Zhao H, Luo X, Shi K, Zhao H, Wang W, Wu T (2019) Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts. Appl Catal B-Environ 245:743–752
Zhang Z, Yu H, Liao B, Huang H, Chen Y (2012) Influence of iron precursors on NH3-SCR behavior of Fe/β catalyst. Chin J Catal 33:576–580
Zhang S, Zhao Y, Yang J, Zhang J, Zheng C (2018) Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas. Chem Eng J 348:618–629
Zhu Y, Zhang Y, Xiao R, Huang T, Shen K (2017) Novel holmium-modified Fe-Mn/TiO2 catalysts with a broad temperature window and high sulfur dioxide tolerance for low-temperature SCR. Catal Commun 88:64–67
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 51779024) and the Fundamental Research Funds for the Central Universities (Grants 3132019032 and 3132019330).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The effects of Fe and Mn precursors on NH3-SCR performance were investigated.
• The catalyst prepared using ferric nitrate and manganese nitrate precursors exhibited the best catalytic activity at low temperature, while the catalysts prepared with chloride precursors exhibited much lower catalytic activities.
• The choice of Fe and Mn precursors was crucial for preparing Fe-Mn/TiO2 catalysts, which would probably affect the redox property, Mn4+ content, the surface chemisorbed labile oxygen content, and the dispersion of active species over support.
Electronic supplementary material
ESM 1
(DOCX 877 kb)
Rights and permissions
About this article
Cite this article
Du, H., Han, Z., Wang, Q. et al. Effects of ferric and manganese precursors on catalytic activity of Fe-Mn/TiO2 catalysts for selective reduction of NO with ammonia at low temperature. Environ Sci Pollut Res 27, 40870–40881 (2020). https://doi.org/10.1007/s11356-020-10073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10073-y