Abstract
Obesity and physical inactivity threaten human health, and both could be solved with exercise. However, a higher amount of pollutants is inhaled during exercise. Exposure to air pollution increases the incidence and progression of diseases. Therefore, the aim of the study was to investigate the rate of pollution inhalation of lean, overweight, and obese individuals in a low and high-intensity hypothetical exercise session. Healthy sedentary men (n = 135) classified as lean, overweight, or obese were enrolled in our study. All participants performed a cardiopulmonary exercise testing (CPX) to collect ventilation rate (VE) data, which was used to predict total ventilation and pollutant inhalation of a 5-km running session. Air pollutant concentration of São Paulo City, Brazil was evaluated and the toxicological risk was estimated based on the potential intake dose. The concentrations of PM2.5 were 29.57 μg/m3 and 51.71 μg/m3, PM10 were 45.85 μg/m3 and 74 μg/m3, NO2 were 63.71 μg/m3 and 66.65 μg/m3, and O3 were 69 μg/m3 and 37 μg/m3, respectively in the summer and winter. In the hypothetical exercise session, total VE and time in both the first and second threshold were increased in the obese group (p < 0.001) (p < 0.001). The inhalation of PM2.5, PM10, NO2, and O3, during the hypothetical session, was increased in obese individuals (p < 0.001). Obese individuals should be considered a susceptible population, once they are more exposed to air pollution during exercise.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, obesity, physical inactivity, and environmental pollution are among the ten threats to global health, and the ambient air pollution has been recognized as the greatest environmental risk to human health. According to the World Health Organization, nine out of ten people breathe polluted air every day (WHO 2019). Obesity, physical inactivity, and pollution are important risk factors for the development of many chronic diseases, inducing morbidity and early mortality (Kampa and Castanas 2008; Burnett et al. 2014; Checa Vizcaíno et al. 2016; Pope et al. 2016). Worryingly, 91% of the world’s population lives in places where air quality exceeds the WHO guideline limits. Regarding the main pollutants, particulate matter (PM), nitrogen dioxide (NO2), and ozone (O3) exposure cause serious risks to health (WHO 2016).
Physical activity promotes benefits on health and quality of life. Exercise training performs an immunoregulatory role responsible for the maintenance of low levels of inflammatory mediators and induces immunometabolic adaptations (Da Silva et al. 2019; Dorneles et al. 2019; Dorneles et al. 2020). In this sense, endurance exercise emerges as a low-cost non-pharmacological therapy for the treatment and prevention of obesity and related diseases (Kodama et al. 2009; Lee et al. 2012; Fiuza-Luces et al. 2013). The primary recommendations from American College of Sports Medicine (ACSM) include the practice of at least 30 min continuous moderate-intensity endurance exercise (in an intensity of ~ 65% of the maximal oxygen uptake, VO2max) or 20 min of vigorous exercises (intensity up to 75–80% VO2max) 3 days per week (Haskell et al. 2007).
However, environmental characteristics, such as air pollution, may modulate the exercise adaptations. The increased metabolic demand during exercise is related to higher oxygen uptake (VO2) and carbon dioxide production (VCO2), which elevates the minute ventilation (VE) and the amount of inhaled gas and particles during the bout. Furthermore, a transition of nasal respiratory pattern to an oronasal pattern is observed during the increase of exercise intensity (Niinimaa et al. 1980; Chlif et al. 2007; Sood 2009). Volume and intensity of the bout have a pivotal role on VE increasing, yet, some individual characteristics, as body composition might impact on respiratory mechanisms in response to acute exercise. In fact, several respiratory factors accompany obesity, such as decreased thoracic compliance and increased airway resistance (Sood 2009; Shoukri 2015).
Although regular physical activity is associated with reduced mortality rates by all causes, the benefits of exercise can be negated in polluted environments. Obese individuals may be more susceptible to the harmful effects of air pollution, as hyperventilation during exercise can lead to excessive inhalation of air pollutants and, consequently, additional health risk, overcoming benefits of exercise. To the author’s knowledge, there is a lack in the literature regarding the impact of different intensities of endurance exercise on VE and the estimation of air pollutant inhalation by individuals with different body mass index (BMI). Therefore, the aim of this study was to investigate the pollution inhalation of lean, overweight, and obese individuals in a moderate and high-intensity exercise session.
Materials and methods
Subjects
This cross-sectional study was approved by the Ethic’s Research Committee of the UFCSPA (63282416.6.0000.5345), and all experimental procedures were performed according to the Declaration of Helsinki. All participants were informed about the study and signed the informed consent. A total of 135 apparently healthy sedentary young men were recruited from local universities via advertisements and oral communications.
According to the criteria established by the World Health Organization, participants were assigned to three experimental groups according to their body mass index (BMI): lean (n = 45), overweight (n = 45), and obesity grade 1 (n = 45). Participants were healthy, no smokers, age ranging from 20 to 40 years, and free of illness and injury, and they were not engaged in physical training programs for a period of 6 months prior to the experimental trial. Exclusion criteria included excessive alcohol intake (> 10 drinks/week), orthopedic limitations, neuromuscular or joint injury, autoimmune or cardiac diseases, or type 1 and type 2 diabetes, and acute and chronic infections. Individuals who presented very low VO2peak (< 25.00 mL kg min) or high values of VO2peak (> 50.0 mL kg min) were also excluded. Body mass (kg) and height (m) were determined by a semi-analytical scale with a stadiometer attached (Welmy, Santa Barbara D’Oeste, Brazil) before the cardiopulmonary exercise testing (CPX).
Cardiopulmonary exercise testing
The CPX was performed on an electric treadmill (Centurion 300) using a ramp protocol (Balady et al. 2010). The laboratory temperature control (18–22 °C) and relative humidity (40–60%) were continuously monitored. Briefly, exercise test started at 4.5 km/h and no slope for all study participants. Then, the load (speed and slope) was individually increased for each participant considering their physical condition and tolerance. The test was terminated after verification of at least two of the following circumstances: (a) request of the participant due to extreme tiredness and/or perception of the intense dyspnea; (b) reached the maximum heart rate (HR) predicted by age (HRmax) which was ≥ 85%; (c) peak respiratory exchange ratio RER > 1.1; (d) VO2 plateau which was reached even with increasing workload.
Ventilatory and metabolic parameters were collected by respiration using Metalyzer 3B (Germany) and were analyzed after the mean of the data in eight respiratory cycles. The CPX system was calibrated before each test concerning both airflow and O2 and CO2 analyzers. The average of the last test 30 s was used to determine the VO2peak (Balady et al. 2010). The first ventilatory threshold (VT1) was identified as the minimum workload at which the ventilatory equivalent ratio for oxygen (VE/VO2) systematically increased without an increase in the ventilatory equivalent ratio for carbon dioxide (VE/VCO2), and the second ventilatory threshold (VT2) as the lowest workload where both VE/VO2 and VE/VCO2 increased (Balady et al. 2010). VT1, VT2, and VO2max were obtained by visual inspection of graphs by 3 independent observers.
Air pollution concentration
The megacity São Paulo, with a population of 21 million, is the biggest city of Brazil, representing more than 11% of the total population of the country and covering an area of 7947 km2 (UN 2014; IBGE 2016). The concentration of air pollutants was provided by the Environment Company of São Paulo State (CETESB - Companhia Ambiental do Estado de São Paulo). The pollutants selected for analyses were particulate matter 2.5 and 10 (PM2.5 and PM10), nitrogen dioxide (NO2), and ozone (O3).
We evaluate air pollution concentration in the hottest day of the summer and coldest day of the winter in the year 2019, in order to have a panorama of air pollution exposure during seasonal variations. The meteorological data of temperature, humidity, precipitation, and solar radiation were accessed in the National Institute of Meteorology (INMET).
Prediction of total ventilation and pollutant inhalation during exercise
The minute ventilation (VE, L/min) was extracted at VT1 and VT2 identified in CPX each participant and used to predict VEtotal in two hypothetical 5-km endurance exercise sessions. The intensities were used based on moderate-intensity and high-intensity endurance exercise recommendations (Garber et al. 2011) but with equalized volume (5 km). The volume of exercise was equalized considering the time required to complete a 5-km exercise with the speed (km/h) identified in each VT1 and VT2. Thus, the VE corresponding to VT1 and VT2 of the VO2max was extracted from incremental test and used in Eq. 1:
Further, the VEtotal values were used to calculate the inhaled dose of PM2.5, PM10, NO2, and O3 during the two hypothetical exercise sessions, according to Eq. 2:
In which, pollutant inhaled is the total amount of pollutant inhaled during the exercise, VEtotal is the total ventilation during the two hypothetical endurance exercise sessions, and pollutant concentration is the concentration of air pollutant measured in São Paulo City.
Toxicological risk
The toxicological risk was estimated based on the potential intake dose of the pollutants and the reference dose. We considered the calculation of toxicological risk of performing 30 min of running, 5 days/week with the second threshold intensity and the risk of incremental time of exercise in an environment with PM2.5 concentration equal to the city of São Paulo in our study. Equation 3 was used to calculate the potential intake dose for both situations (I, μg/kg day) considering:
CA = average PM2.5 concentrations of monitored day (μg/m3)
IP = inhalation rate during exercise, representing total ventilation of the second threshold (m3)
FR = retention factor—we assumed a retention factor of FR = 1, which represents the highest exposure and the highest potential impact on subjects’ health.
FA = absorption factor—we assumed an absorption factor of FA = 1, which represents the highest exposure and the highest potential impact on subjects’ health.
ET = exposure time (h/day)—exercise’s duration
EF = exposure frequency (day/year): 5 times/week—20 time/month—260 days/year
ED = duration of exposure (year): 260 days of exposure/365 days = 0.71
BW = body weight (kg)
AT = average time—period of exposure in which the dose was measured (day): 260
Toxicological risk:
In which:
RQ = risk quotient;
Risk quotients are classified as follows: RQ ≤ 1: unlikely risk, even in population groups that are sensitive to adverse health effects; RQ > 1: there is a risk of non-carcinogenic adverse effects on human health.
I = potential intake dose (μg/kg day);
RfD: reference dose for PM2.5 = 1.14, according to Silva et al. (2016).
Statistical analysis
The normality of the data was measured using Kolmogorov-Smirnov test. Unpaired Student t test was used to compare variation of the air pollutant concentration and seasonal variation. A 1-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was applied to compare age, BMI, waist circumference, ventilation rate, VO2, respiratory exchange ratio, speed, and time during different exercise conditions and air pollutant concentrations. A 2-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was applied to compare the inhaled dose of PM2.5, PM10, NO2, and O3 between exercise intensity and pollution concentration. All analyses were performed using SPSS 20.0 (SPSS Inc., EUA). The significance level was set at p ≥ 0.05.
Results
Sample characterization
Sample characterization showed no difference in age among the groups (p = 0.0529), and BMI and waist circumference were increased in obese and overweight compared with lean individuals, as well as in obese compared with overweight men (p < 0.001). In the first ventilatory threshold, the VE and VO2 of the obese group were higher than in the lean participants (p = 0.0114) (p = 0.0462). Also, the obese group showed decreased speed (p < 0.001) compared with the lean and overweight groups. In the second ventilatory threshold, no difference in VE was observed among groups (p = 0.5250). While, VO2 and speed were increased in the lean and overweight subjects compared with the obese group (p < 0.001). In the maximal oxygen capacity (VO2max), VE of the lean group was increased compared with obese subjects (p = 0.0490). The lean and overweight groups demonstrated increased VO2 and speed compare with the obese group (p < 0.001) and respiratory exchange ratio was not different among groups (p = 0.9120). Regarding the hypothetical exercise session, the total VE and duration of session in both the first and second threshold were increased in the obese group compared with the lean and overweight groups (p < 0.001) (Table 1).
Pollutant concentration
PM2.5 and PM10 concentrations were higher in the winter compared with the summer (p < 0.001). NO2 concentrations demonstrated no difference (p > 0.05). O3 levels in the summer demonstrated increased concentration when compared with the winter (p < 0.001) (Table 2).
Meteorological measures
The summer day showed increased temperature and UV radiation compared with the winter day (p < 0.05). There was no significant difference in humidity, wind speed, and precipitation between summer and winter days (p > 0.05) (Table 3).
Inhalation of air pollutants
The inhalation of PM2.5, PM10, NO2, and O3, during the hypothetical session, was increased in the obese group compared with lean and overweight groups (p < 0.001) (Fig. 1). In the same way, all pollutants had higher inhalation levels in high-intensity than in moderate-intensity exercise in all groups (p < 0.001). Regarding the pollutant inhalation in each season, PM2.5 (p < 0.001), PM10 (p < 0.001), and NO2 (p < 0.049) inhalation were increased in the winter compared with the summer, and O3 inhalation was increased in the summer in relation to the winter (p < 0.001).
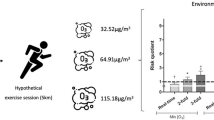
Air pollutant inhalation during a hypothetical session of exercise of lean, overweight, and obese individuals. a PM2.5 inhalation. b PM10 inhalation. c NO2 inhalation. d O3 inhalation. Data are presented as mean ± SD. Between groups, differences were verified by one-way ANOVA followed by Bonferroni’s post hoc (p < 0.05). Statistical difference compared with the obese group (p < 0.05)
Inhalation of PM2.5, PM10, and O3 showed an interaction between group and season (p < 0.007) (p = 0.023) (p = 0.002), in which obese individuals inhaled more PM2.5 and PM10 in the winter compared with all groups in both seasons. Also, obese individuals inhaled more O3 in the summer compared with all groups in both seasons (Fig. 2). The obese group had increased inhalation of PM2.5 (p < 0.001), PM10 (p = 0.004), and O3 (p < 0.001), in high-intensity exercise compared with all groups in the winter for PM2.5 and PM10, and during the summer for O3. The inhalation of NO2 showed no difference in the interaction of group with intensity (p = 0.708), group with season (p = 0.966), intensity with season (p = 0.781), and group with intensity and season (p = 1.00). No difference was observed between group with intensity in inhalation of PM2.5 (p = 0.725), PM10 (p = 0.721), and O3 (p = 0.729), and among group, intensity and season in inhalation of PM2.5 (p = 0.976), PM10 (p = 0.982), and O3 (p = 0.972).
Interaction of air pollutant inhalation during a hypothetical session of exercise of lean, overweight, and obese individuals in the summer and winter seasons. a PM2.5 inhalation. b PM10 inhalation. c NO2 inhalation. d O3 inhalation. Data are presented as mean ± SD. Between groups, differences were verified by two-way ANOVA followed by Bonferroni’s post hoc (p < 0.05). a Denotes statistical difference compared with the lean and overweight groups (p < 0.05). b Denotes statistical difference compared with all groups in the summer season (p < 0.05)
Toxicological risk
A session of aerobic exercise performed for 30 min with the intensity detected in the second threshold in individuals from our study would cause toxicological risk with exposure to PM2.5 at 115 μg/m3 for lean individuals and 125 μg/m3 for overweight and obese individuals. Considering the PM2.5 concentration used in our study, the time to achieve the risk by performing a running was 1 h and 25 min in the lean group and 1 h and 30 min in the overweight and obese groups, as illustrated in Fig. 3.
Toxicological risk of lean, overweight, and obese individuals during exercise. a Toxicological risk of considering incremental time of exposure in a PM2.5 concentration of 40.64 μg/m3 during high-intensity exercise. b Toxicological risk considering incremental concentration of PM2.5 during 30 min of high-intensity exercise
Discussion
Our study points out an increased ventilation and VO2, and reduced speed of obese participants in the first threshold of the cardiopulmonary exercise testing, while in the second threshold, VO2 and speed were decreased in this group. Considering the hypothetical aerobic exercise session, obese individuals would had increased total ventilation during the session and spend more time to perform the exercise, consequently inhaling more air pollutants.
In our study, obese individuals showed increased total ventilation during exercise, which might occur due to alterations on respiratory physiology caused by obesity, including changes in lung volumes, ventilatory behavior, and decreased respiratory compliance. In the same way, obesity could cause a fatigue of inspiratory muscle causing inefficiency of respiratory muscles (Chlif et al. 2005; Chlif et al. 2007). So, obese subjects demonstrate a pattern of breathing fast and shallow, increasing the oxygen cost of breathing (Rochester 1998; Kress et al. 1999; Chlif et al. 2009). Also, during a peak exercise, obese individuals may hyperinflate and increase ventilation rate to avoid significant levels of expiratory flow limitation, contributing to our result of increased respiratory rate during exercise (Sakamoto et al. 1993).
The concentrations of PM2.5, PM10, and NO2 pollutants were higher in the winter, while the O3 concentration was higher in the summer, which were expected results once levels of particulate matter and NO2 are elevated in the winter due to thermal inversion phenomenon, and increased production of O3 is detected in the summer due to higher solar radiation (Cichowicz et al. 2017; Trinh et al. 2018; Marmett et al. 2019). Consequently, the inhalation of particulates and NO2 was higher in the winter and O3 in the summer in lean, overweight, and obese individuals. As well as, the inhalation of pollutants was augmented in high-intensity exercise compared with moderate intensity, since the VE was increased, and the inhalation of pollutants increased as well. Similarly, Pasqua et al. (2018) hypothesized an aerobic exercise with 65% intensity for 30 min in the ten cities with the highest and lowest levels of PM2.5 and PM10, and demonstrated that the inhalation of pollution was higher during exercise and in the ten more polluted cities.
Obese individuals inhaled more PM2.5, PM10, NO2, and O3. The inhalation of PM2.5, PM10, and O3 demonstrated interaction of group with season, and intensity with season, indicating that a higher inhalation could depend on BMI and intensity of exercise. Obese subjects that perform exercise in the winter would inhale higher amount of PM2.5 and PM10, while in the summer, it would result in higher inhalation of O3. Besides, it was already demonstrated that the deposition of particles in airways increases 6 to 10-fold during high-intensity exercise due to an increase on that ventilation switch nasal breathing to oral overcoming the nasal filtration and increasing pollution dose inhaled (Niinimaa et al. 1980; Chalupa et al. 2004; Giles and Koehle 2014).
Regarding the influence of body mass on pollutant inhalation, obese participants may face additional health risks by being exposed to higher levels of pollutants, as Mazidi and Speakman (2018) demonstrated by indicating that the effects of PM2.5 were more deleterious in areas with higher rates of obesity, especially on cardiovascular diseases and stroke mortality. The increase vulnerability of obese individuals was also observed in studies demonstrating that obesity may enhance the effects of air pollution on the prevalence of cardiovascular diseases and stroke (Chen et al. 2007; Qin et al. 2015).
In our study, high-intensity exercise led to higher inhalation of air pollutants, a result more prominent in obese individuals. Despite benefits of exercise, even performed in a polluted environment, obese individuals might have increased susceptibility to pulmonary infections (Misumi et al. 2019; Marmett et al. 2020). It is well-known that air pollution exposure enhances the severity of respiratory infections and exercise mobilizes higher volumes of air that increase the contact of the respiratory tract with pollutants (Kurt et al. 2016). Air pollutants trigger oxidative and proinflammatory processes and, together with intense exercise, increase the vulnerability to respiratory infection (Puett et al. 2014; Hart et al. 2015). An experimental study demonstrated that obesity predisposes to a unique form of pathogenesis during systemic viral infection (Misumi et al. 2019). Furthermore, obese individuals had compromised pulmonary function, impairing ventilation, decreased immune responses to infections, increased inflammatory cytokines, and prolonged inflammation, which may contribute to the morbidity associated with infections (O’brien et al. 2012; Dietz and Santos-Burgoa 2020).
A study of SAPALDIA cohort demonstrated that the improvement of air quality attenuates lung function decline in normal weight and underweight participants; however, in obese individuals, no improvement was observed, highlighting the importance of contemplate health effects of air pollution exposure and obesity together (Schikowski et al. 2013). An increased susceptibility of obese people to air pollution exposure may be justified by oxidative stress and chronic low-grade inflammation that follows this condition; therefore, both air pollution and obesity are associated with increased incidence of disease and all-cause mortality (Galili et al. 2007; Pope et al. 2016; Iliodromiti et al. 2018; Khan et al. 2018).
Regarding the toxicological risk of exposure to PM2.5, a session of aerobic exercise performed for 30 min would cause risk when pollution concentration overcome 115 μg/m3 for lean individuals, and 125 μg/m3 for overweight and obese individuals. However, when considering the pollution levels of São Paulo, from 1 h, 25 min of running would already represent a risk. Pasqua et al. (2018) estimated a relative risk of all-cause mortality for running, indicating that 15 min of running with moderate intensity in cities with high levels of PM2.5 would no longer be beneficial and after 75 min, the risk of air pollution would overcome the benefits of exercise. Additionally, Tainio et al. (2016) established that in 30 min of cycling, the maximal benefits of the exercise (tipping point) would reach a PM2.5 concentration of 95 μg/m3 and the risk from air pollution would overweight the benefits of physical activity (break-even point) at 160 μg/m3. Considering these studies together with our results, toxicological risk and relative risk of all-cause mortality demonstrated similar point in which exercising in a polluted environment could overcome the benefits of exercise.
It is important to highlight that even though obese individuals did not show additional risk compared with lean and overweight individuals, it does not mean that they are not a sensitive population. A great number of evidence cited above reported the susceptibility of the obese group to incidence and prevalence of diseases, as well as mortality, so the same dose of PM2.5 could be more detrimental to health of obese than lean subjects (Schikowski et al. 2013; Iliodromiti et al. 2018; Khan et al. 2018).
The present study had some limitations. First, we only evaluated São Paulo air pollution, so our results may not be generalized to other world’s cities. Second, our study was performed with healthy sedentary individuals; therefore, it is not possible to assume the data to another population. Third, the equation used to calculate the toxicological risk showed a result relativized to body mass, and it is possible that if body mass was not included, a difference would be detected. As strengths, we highlight the comparison of lean, overweight, and obese individuals, as well as the equalization of the volume of exercise considering the time required to complete a 5-km exercise to estimate the air pollution inhalation. Nevertheless, we observed the inhalation of four different pollutants, which are all included in the last global update of the WHO Air Quality Guidelines, demonstrating a complete scenario of air pollution.
Conclusion
Obese individuals had higher total ventilation and time during the hypothetical running leading to an augment in pollutant inhalation. So, this group should be considered a susceptible population, once they are more exposed to air pollution during exercise. Hence, exercise recommendations must consider the personal characteristics of individuals and environment particularities, and it may be necessary to modulate the exercise prescription according to the body mass index and the daily air pollution levels.
References
Balady GJ, Arena R, Sietsema K, Myers J et al (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2):191–225
Burnett RT, Pope CA, Ezzati M, Olives C et al (2014) An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect 122(4):397–403
Chalupa DC, Morrow PE, Oberdörster G, Utell MJ et al (2004) Ultrafine particle deposition in subjects with asthma. Environ Health Perspect 112(8):879–882
Checa Vizcaíno MA, González-Comadran M, Jacquemin B (2016) Outdoor air pollution and human infertility: a systematic review. Fertil Steril 106(4):897–904.e891
Chen JC, Cavallari JM, Stone PH, Christiani DC (2007) Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect 115(7):1002–1006
Chlif M, Keochkerian D, Mourlhon C, Choquet D et al (2005) Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int J Obes (Lond) 29(12):1478–1483
Chlif M, Keochkerian D, Feki Y, Vaidie A et al (2007) Inspiratory muscle activity during incremental exercise in obese men. Int J Obes (Lond) 31(9):1456–1463
Chlif M, Keochkerian D, Choquet D, Vaidie A et al (2009) Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol 168(3):198–202
Cichowicz R, Wielgosiński G, Fetter W (2017) Dispersion of atmospheric air pollution in summer and winter season. Environ Monit Assess 189(12):605
DA Silva IM, Santos MA, Galvão SL, Dorneles GP et al (2019) Blood flow restriction impairs the inflammatory adaptations of strength training in overweight men: a clinical randomized trial. Appl Physiol Nutr Metab
Dietz W, Santos-Burgoa C (2020) Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 28(6):1005
Dorneles GP, DA Silva I, Boeira MC, Valentini D et al (2019) Cardiorespiratory fitness modulates the proportions of monocytes and T helper subsets in lean and obese men. Scand J Med Sci Sports 29(11):1755–1765
Dorneles GP, DOS Passos AAZ, Romão PRT, Peres A (2020) New insights about regulatory T cells distribution and function with exercise: the role of immunometabolism. Curr Pharm Des
Fiuza-Luces C, Garatachea N, Berger NA, Lucia A (2013) Exercise is the real polypill. Physiology (Bethesda) 28(5):330–358
Galili O, Versari D, Sattler KJ, Olson ML et al (2007) Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol 292(2):H904–H911
Garber CE, Blissmer B, Deschenes MR, Franklin BA et al (2011) American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc, 43(7):1334–1359
Giles LV, Koehle MS (2014) The health effects of exercising in air pollution. Sports Med 44(2):223–249
Hart JE, Spiegelman D, Beelen R, Hoek G et al (2015) Long-term ambient residential traffic-related exposures and measurement error-adjusted risk of incident lung cancer in the Netherlands cohort study on diet and cancer. Environ Health Perspect 123(9):860–866
Haskell WL, Lee IM, Pate RR, Powell KE et al (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116(9):1081–1093
IBGE (2016) Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics). http://cod.ibge.gov.br/QHF (accessed 03.02.2016)
Iliodromiti S, Celis-Morales CA, Lyall DM, Anderson J et al (2018) The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J 39(17):1514–1520
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151(2):362–367
Khan SS, Ning H, Wilkins JT, Allen N et al (2018) Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol 3(4):280–287
Kodama S, Saito K, Tanaka S, Maki M et al (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301(19):2024–2035
Kress JP, Pohlman AS, Alverdy J, Hall JB (1999) The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med 160(3):883–886
Kurt OK, Zhang J, Pinkerton KE (2016) Pulmonary health effects of air pollution. Curr Opin Pulm Med 22(2):138–143
Lee IM, Shiroma EJ, Lobelo F, Puska P et al (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380(9838):219–229
Marmett B, Carvalho RB, Rhoden FR, Rhoden CR (2019) Seasonal influence in traffic-related air pollutants concentrations in urban parks from Porto Alegre, Brazil. Open Journal of Air Pollution 8:96–107
Marmett B, Carvalho RB, Dorneles GP, Nunes RB et al (2020) Should I stay or should I go: can air pollution reduce the health benefits of physical exercise? Med Hypotheses 144:109993
Mazidi M, Speakman JR (2018) Impact of obesity and ozone on the association between particulate air pollution and cardiovascular disease and stroke mortality among US adults. J Am Heart Assoc 7(11)
Misumi I, Starmer J, Uchimura T, Beck MA et al (2019) Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep 27(2):514–524.e515
Niinimaa V, Cole P, Mintz S, Shephard RJ (1980) The switching point from nasal to oronasal breathing. Respir Physiol 42(1):61–71
O’brien KB, Vogel P, Duan S, Govorkova EA et al (2012) Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis 205(2):252–261
Pasqua LA, Damasceno MV, Cruz R, Matsuda M et al (2018) Exercising in air pollution: the cleanest versus dirtiest cities challenge. Int J Environ Res Public Health 15(7)
Pope CA, Bhatnagar A, Mccracken J, Abplanalp WT et al (2016) Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res
Puett RC, Hart JE, Yanosky JD, Spiegelman D et al (2014) Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort. Environ Health Perspect 122(9):926–932
Qin XD, Qian Z, Vaughn MG, Trevathan E et al (2015) Gender-specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: a large population based cross sectional study. Sci Total Environ 529:243–248
Rochester D (1998) Obesity and pulmonary function. NY, USA: In: MAJ Alpert, Futura Publishing Company: 108–132
Sakamoto S, Ishikawa K, Senda S, Nakajima S et al (1993) The effect of obesity on ventilatory response and anaerobic threshold during exercise. J Med Syst 17(3–4):227–231
Schikowski T, Schaffner E, Meier F, Phuleria HC et al (2013) Improved air quality and attenuated lung function decline: modification by obesity in the SAPALDIA cohort. Environ Health Perspect 121(9):1034–1039
Shoukri A (2015) Effects of obesity on respiratory mechanics at rest and during exercise. Egypt J Bronchol 9:224–226
Silva RA, Adelman Z, Fry MM, West JJ (2016) The impact of individual anthropogenic emissions sectors on the global burden of human mortality due to ambient air pollution. Environ Health Perspect 124(11):1776–1784
Sood A (2009) Altered resting and exercise respiratory physiology in obesity. Clin Chest Med 30(3):445–454
Tainio M, DE Nazelle AJ, Götschi T, Kahlmeier S et al (2016) Can air pollution negate the health benefits of cycling and walking? Prev Med 87:233–236
Trinh TT, Le TT, Nguyen TDH, Tu BM (2018) Temperature inversion and air pollution relationship, and its effects on human health in Hanoi City, Vietnam. Environ Geochem Health
UN (2014) United Nations, Department of Economic and Social Affairs, Population Division. http://esa.un.org/unpd/wup/highlights/wup2014-highlights.pdf (accessed 04.02.2016)
WHO (2016) WHO releases country estimates on air pollution exposure and health impact: World Health Organization
WHO (2019) Ten threats to global health in 2019. World Health Organization
Funding
BM and RBC are supported by doctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), GPD is supported by postdoctoral fellowship from (CAPES), and PRTR is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the PQ productivity scholarship.
Author information
Authors and Affiliations
Contributions
Bruna Marmett and Gilson Pires Dorneles drafted the first version of the manuscript. Bruna Marmett, Roseana Boek Carvalho, and Gilson Pires Dorneles designed the scope of the study. Bruna Marmett, Roseana Boek Carvalho, Gilson Pires Dorneles, and Igor Martins da Silva developed the methodology of this study. All authors contributed to the writing of this paper. All authors approved the submission of this version for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marmett, B., Carvalho, R.B., Dorneles, G.P. et al. Air pollution inhalation during acute exercise is dependent of the body mass index and ventilation of young men. Environ Sci Pollut Res 27, 39019–39028 (2020). https://doi.org/10.1007/s11356-020-10019-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10019-4