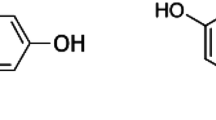Abstract
Bisphenol A (BPA) is a high production volume chemical that has wide industrial applications, especially as a color developer in thermal papers. The present study focused on the determination of levels of BPA in thermal receipts collected from different locations in Akure, Nigeria, and the estimation of daily intake of BPA through dermal absorption. Thermal receipts were collected from different locations, and the levels of extracted BPA were determined using fluorescence spectroscopy. The daily intake of BPA was estimated, and the amount was compared with the reference value. BPA was detected in all the samples analyzed with levels ranging from 1.50 to 3.16 mg/g. These values were lower than the values detected in thermal receipts obtained from other countries. The estimated mean daily intakes of BPA by dermal absorption due to handling of thermal receipts were 0.20 and 9.89 μg/day for the general population and the occupationally exposed individuals, respectively, and were much lower than the reference value of 50 μg/kg bw/day provided by the European Food Safety Authority. This indicates that dermal exposure to BPA is not a serious health risk to the population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ubiquitous occurrence of bisphenol A (BPA) and its less toxic analogues such as bisphenol S, bisphenol F, and bisphenol AF in the environment poses threats to both humans and wildlife. High levels of BPA have been detected in different matrices: up to 9730 μg/kg in dust (Geens et al. 2009), up to 39,000 μg/kg in thermal receipts (Rocha et al. 2015), and up to 380 μg/kg in meat (Goodson et al. 2002). Although, some countries like Japan, Canada, and the European Unions have banned the use of BPA in certain consumer products such as baby feeding bottles (Almeida et al. 2018), it is worrisome to note that its production continue to rise over the years. The global production of BPA was estimated to be 2.2 million tons in the year 2009 alone, and it has been projected that it would reach 7.3 million tons by the year 2023 (Research and Markets 2018). BPA is still being used in several consumer products like water bottles, sports equipment, CDs/DVDs, epoxy resins which are used as linings for food cans, dental resins, and thermal sales receipts. Aside its use in consumer products, BPA is being used in automobile and construction industries where it is being considered as alternative to glass and metals (Research and Markets 2018).
The toxic effects of BPA to organisms are widely reported in the literature including endocrine disruption, genotoxicity, cytotoxicity, developmental, and reproductive impairments. BPA has also been implicated in some human diseases and metabolic disorders such as obesity, type 2 diabetes, and cancer. As an endocrine disrupting agent, BPA mimics the activity of endogenous estrogens such as 17β-estradiol by binding to estrogen receptors and altering the gene expression (Eckstrum et al. 2016; Ohore and Zhang 2019). The exposure to BPA caused activation of CYP gene expression in the Leydig cells of mice and rats, consequently resulting in alteration of sex hormone ratio (Lan et al. 2017). In another study, George and Rupasinghe (2018) showed that the exposure of human bronchial cells to BPA caused cytotoxicity, oxidative stress, caspase-mediated apoptosis, and DNA damage. In addition, the presence of BPA in blood and urine has been shown to be a risk factor for human diseases like type 2 diabetes and obesity (Duan et al. 2018; Hwang et al. 2018; Amin et al. 2019).
Various exposure routes have been proposed for BPA exposure in man and other organisms; they include dust inhalation, dermal, and dietary (Lv et al. 2016; Ribeiro et al. 2017; Almeida et al. 2018). Of these possible routes, dietary route through ingestion accounts for up to 90% of BPA in man (Mikołajewska et al. 2015; Ribeiro et al. 2017). While quite a number of studies have focused on quantifying the levels of BPA in environmental samples such as in surface waters and sediments (Klecka et al. 2009; Wang et al. 2017; Staples et al. 2018), and also in food items consumed by man (Garcia-Corcoles et al. 2018; Shi et al. 2018), not much attention has been given to quantification of levels of BPA in thermal sales receipts notwithstanding its potential as a source of human exposure to BPA. Specifically, to the best of our knowledge, there have not been an attempt to determine the levels of BPA in thermal receipts in Nigeria although quite a number of studies have looked at quantification of BPA levels in various environmental samples and food products (Makinwa and Uadia 2015; Adeyi and Babalola 2019). The current study is therefore aimed at determining the levels of BPA in thermal sales receipts obtained from Nigeria to estimate the daily exposure to BPA through human contact with thermal receipts. To the best of our understanding, there have not been studies that determine the levels of BPA in thermal papers in most African countries, despite its importance as a major source of BPA exposure to man.
Materials and methods
Thermal receipt papers (n = 80) were collected from locations such as banks, supermarkets, gas stations, and lottery outlets within Akure metropolis, Ondo State, Nigeria. The samples were stored in sealed bisphenol-free bags until analysis. Sample extraction for BPA determination was carried out as previously described by Rocha et al. (2015). Briefly, a circular spot (diameter: 12 mm) was cut from the middle of each paper with the aid of paper punch, weighed, and transferred into a 15 ml Falcon tube. This was followed by addition of 5 ml methanol into the tube. BPA extraction was performed by one cycle of vortex (60 s) followed by ultrasonication (10 min). The mixture was centrifuged at 4000×g, and a 100 μl aliquot was transferred into another tube. The aliquot was diluted to 10 ml with methanol/H2O (1:1) and spiked with the internal standard (BPA-d16). The solutions were stored at − 20 °C until analysis. Before analysis, the aliquots were filtered through a 0.45-μm filter. Each sample was analyzed in duplicate. In addition to the samples, a reagent blank was prepared without the thermal receipt paper.
Sample analysis was done using fluorescence spectroscopy with the aid of a LS 55 (Perkin Elmer USA) following the procedures reported in Gallimberti et al. (2020). The equipment is capable of measuring fluorescence, phosphorescence, chemiluminescence, and bioluminescence. Its source is a xenon discharge lamp, with a power of 20 kW for 8 μs in duration. The detection occurs through a photomultiplier model R928 (Red-Sensitive) that can operate up to 900 nm. The selection of wavelengths is performed by Monk-Gillieson monochromators, which cover the range from 200 to 800 nm for excitation and from 200 to 900 nm for emission. Excitation (2.5–15.0 nm) and emission (2.5–20.0 nm) slits can be varied and selected in 0.1-nm increments. It has a scan speed of 10–1500 nm/min and can be selected in 1-nm increments. The sensitivity of the equipment is given through the signal/noise ratio (500:1 r.m.s.), using the Raman band of the water, with excitation at 350 nm and 10 nm emission and excitation crack. The specific conditions used in the determination of BPA were as follows: 5.0 nm excitation slits, 5.0 nm emission slits, 200 to 400 nm range, Δλ 85 nm, and scan rate 1500 nm/min. The BL Studio software version 1.04.02 (BioLight Luminescence Systems Ltd) was used for data acquisition, storage, and processing.
The efficiency of extraction procedure was confirmed by randomly selecting 10 paper samples and subjecting them to extraction procedure again, after which the extracts were analyzed for BPA levels. BPA was not detected in the samples, thus confirming that the extraction procedure was efficient. To each batch of 20 samples analyzed, a blank (methanol/water) and a spiked blank (50 ng/ml BPA) were processed and analyzed. BPA was not detected in the blanks, and recovery of BPA from spiked blanks was 98 ± 12% (mean ± SD). The limit of detection (LOD) and limit of quantification (LOQ) were calculated by the following equation: LOD = 3 SD/m and LOQ = 10 SD/m, where SD is the standard deviation of 10 blank determinations and m is the slope of the calibration curve. The LOD and LOQ were 3.61 ± 0.68 and 12.04 ± 2.26 μg/L, respectively.
The estimated daily intake (EDI; ng/d) of BPA was calculated using the following equation: (Liao and Kannan 2011)
where k is the paper-to-skin transfer coefficient of BPA (21,522.4 ng/s), C is the mean concentration of BPA determined in thermal receipts (μg g−1 of receipt), and HF is the handling frequency (times/day). This study assumed the frequency of handling to be 150 times/day for the occupationally exposed population and 3 times/day for the general population. HT is the handling time of receipts (5 s); and AF is the absorption fraction of BPA by skin and was taken 27%, on the basis of data from the literature. The estimated daily intake values were then compared with the reference value provided by the European Food Safety Authority (EFSA) for BPA. Data were presented as mean and range. The differences in mean values for the different categories of thermal receipts were determined statistically using a one-way analysis of variance (ANOVA), followed by Tukey’s tests in case of significant difference. Statistics were performed using Graphpad Prism 5, and significance was assumed at p < 0.05.
Results and discussion
Table 1 shows the levels of BPA in thermal receipts obtained from different locations within Akure metropolis, Southwest Nigeria. BPA was detected in all the samples analyzed (100% detection), with a concentration range of 1.50–3.16 mg/g. There was a significant difference in the mean levels of BPA in the different categories of the thermal receipts (p < 0.0001). The mean levels of BPA were higher in papers generated from supermarkets and gas stations in comparison with those from banks and lotteries.
The levels of BPA in the present study were compared with the levels obtained in thermal papers from other countries, and the results are shown in Table 2. The analyses revealed that the levels of BPA detected in thermal receipts in the present study were similar or sometimes lower compared with the levels detected in thermal papers from other countries. However, the lower limit of detection of BPA in the present study (1.50 mg/g) was several magnitudes higher than the levels obtained analyses from other countries in which the lower limits were most of the times below the limits of detection. The high level of the lower limit is an indication of ubiquitous presence of BPA in Nigerian thermal papers samples, which could predispose the population to health risks due to BPA exposure.
Aside oral ingestion, dermal exposure through handling of thermal receipts has been identified as one of the major exposure routes to BPA by human (Thayer et al. 2016; Lv et al. 2017). Hence, we estimated potential exposure to BPA through thermal receipts in this study. The estimated mean daily intake of BPA by dermal absorption due to handling of thermal receipts was 0.20 and 9.89 μg/day for the general population and the occupationally exposed individuals, respectively (or 2.86 ng/kg bw/day and 141.3 ng/kg bw/day, for the general population and the occupationally exposed, respectively, when adjusted for the body weight of 70 kg of a human adult). These values were quite low when compared with the EDI values obtained for the Belgian (0.45 μg/d; Geens et al. 2012) and Chinese (1.77 μg/d; Yang et al. 2019) populations. However, the mean EDI value in the present study was higher than the values estimated for the Italian population which was given as 0.06 μg/d (Russo et al. 2017). In addition, the estimated daily intake of BPA through dermal absorption in this study was several magnitudes lower than the reference value of 4 μg/kg bw/day (oral toxicity) provided by the European Food Safety Authority (2015). Currently, there have been no official reference values for BPA through dermal exposure. The differences in the levels of the EDIs in these studies were generally due to the differences in the levels of BPA detected in the paper samples. The low levels of BPA in Nigerian receipts could be an indication that alternatives to BPA such as bisphenol S, D-8, and D-90 are being used as color developers, as such the risk of exposure to high levels of BPA through dermal exposure was minimized. Future studies are therefore required to detect and quantify the levels of these BPA alternatives in Nigerian thermal receipt samples.
References
Adeyi AA, Babalola BA (2019) Bisphenol-A (BPA) in foods commonly consumed in Southwest Nigeria and its human health risk. Sci Rep 9:17458
Almeida S, Raposo A, Almeida-Gonzalez M, Carrascosa C (2018) Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf 17:1503–1517
Amin MM, Ebrahim K, Hashemi M, ShoshtariYeganeh B, Rafiei N, Mansourian M, Kelishadi R (2019) Association of exposure to bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int J Environ Health Res 29:94–106
Castro G, Rodriguez I, Ramil M, Cela R (2019) Direct analysis in real time accurate mass spectrometry determination of bisphenol A in thermal printing paper. Talanta 205:120086
Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, Chen L (2018) Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut 243:1719–1726
Eckardt M, Simat TJ (2017) Bisphenol A and alternatives in thermal paper receipts - a German market analysis from 2015 to 2017. Chemosphere 186:1016–1025
Eckstrum KS, Weis KE, Baur NG, Yoshihara Y, Raetzman LT (2016) Icam5 expression exhibits sex differences in the neonatal pituitary and is regulated by estradiol and bisphenol A. Endocrinology:article en 20151521. https://doi.org/10.1210/en.2015-1521
European Food Safety Authority (2015) Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in food stuffs: executive summary. EFSA J 13:3978
Gallimberti M, Rocha BA, Souza VCO, Campiglia AD, Barbosa F Jr (2020) Determination of Bisphenol A in paper products by synchronous fluorescence spectroscopy and estimation of daily exposure. J Braz Chem Soc. https://doi.org/10.21577/0103-5053
Garcia-Corcoles MT, Cipa M, Rodriguez-Gomez R, Rivas A, Olea-Serrano F, Vilchez JL, Zafra-Gomez A (2018) Determination of bisphenols with estrogenic activity in plastic packaged baby food samples using solid-liquid extraction and clean-up with dispersive sorbents followed by gas chromatography tandem mass spectrometry analysis. Talanta 178:441–448
Geens T, Roosens L, Neels H, Covaci A (2009) Assessment of human exposure to bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 76:755–760
Geens T, Goeyens L, Kannan K, Neels H, Covaci A (2012) Levels of bisphenol A in thermal paper receipts from Belgium and estimation of human exposure. Sci Total Environ 435-436:30–33
George VC, Rupasinghe HPV (2018) DNA damaging and apoptotic potentials of bisphenol A and bisphenol S in human bronchial epithelial cells. Environ Toxicol Pharmacol 60:52–57
Goodson A, Summerfield W, Cooper I (2002) Survey of bisphenol A and bisphenol F in canned foods. Food Addit Contam 19:796–802
Hwang S, Lim J, Choi Y, Jee SH (2018) Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord 18:81
Klecka GK, Staples CA, Clark KE, Van Der Hoeven N, Thomas DE (2009) Exposure analysis of bisphenol A in surface waters in North America and Europe. Environ Sci Technol 43:6145–6150
Lan H, Lin KW, Yang Z, Chang A, Hu M (2017) Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 185:237–246
Liao C, Kannan K (2011) Widespread occurrence of bisphenol a in paper and paper products: implications for human exposure. Environ Sci Technol 45:9372–9379
Lv Y, Rui C, Dai Y, Pang Q, Li Y, Fan R, Lu S (2016) Exposure of children to BPA through dust and the association of urinary BPA and triclosan with oxidative stress in Guangzhou, China. Environ Sci Process Impacts 18:1492–1499
Lv Y, Lu S, Dai Y, Rui C, Wang Y, Zhou Y, Li Y, Pang Q, Fan R (2017) Higher dermal exposure of cashiers to BPA and its association with DNA oxidative damage. Environ Int 98:69–74
Makinwa TT, Uadia PO (2015) A survey of the level of Bisphenol A (BPA) in effluents, soil leachates, food samples, drinking water and consumer products in South-Western Nigeria. World Environ 5:135–139
Mikołajewska K, Stragierowicz J, Gromadzińska J (2015) Bisphenol A – application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health 28:209–241
Molina-Molina JM, Jimez-Diaz I, Fernandez MF, Rodriguez-Carrillo A, Peinado FM, Mustieles V, Barouki V, Piccoli C, Olea N, Freire C (2019) Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France and Spain. Environ Res 170:406–415
Ohore OE, Zhang S (2019) Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci Afr 5:e00135
Research and Markets (2018) Global bisphenol A market report 2018: Analysis 2013–2017 & Forecasts 2018–2023. (Accessed at https://www.researchandmarkets.com/research/hl86rz/global_bisphenol?w=5 Oct, 2019
Ribeiro E, Ladeira C, Viegas S (2017) Occupational exposure to bisphenol A (BPA): a reality that still needs to be unveiled. Toxics 5:22
Rocha BA, Azevedo LR, Gallimberti M, Campiglia AD, Barbosa F Jr (2015) High levels of bisphenol A and bisphenol S in Brazilian thermal paper receipts and estimation of daily exposure. J Toxicol Environ Health A 78:1181–1188
Russo G, Barbato F, Grumetto L (2017) Monitoring bisphenol A and bisphenol S in thermal paper receipts from Italian markets and estimated transdermal human intake. A pilot study. Sci Total Environ 599-600:68–75
Shi R, Liang J, Zhao Z, Liu Y, Liu A (2018) In situ determination of bisphenol A in beverage using a molybdenum selenide/reduced graphene oxide nanoparticle composite modified glassy carbon electrode. Sensors 18:e1660. https://doi.org/10.3390/s18051660
Staples C, Van der Hoeven N, Clark K, Mihaich E, Woelz J, Hentges S (2018) Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 201:448–458
Thayer KA, Taylor KW, Garantziotis S, Churman SH, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI, Scheri RC, Birnbaum LS, Bucher JR (2016) Bisphenol A, Bisphenol S, and 4-hydro xyphenyl 4-Isopro oxyphenyl sulfone (BPSIP) in urine and blood of cashiers. Environ Health Perspect 124:437–444
Wang Q, Zhu L, Chen M, Ma X, Wang X, Xia J (2017) Simultaneously determination of bisphenol A and its alternatives in sediment by ultrasound-assisted and solid phase extractions followed by derivatization using GC-MS. Chemosphere 169:709–715
Yang Y, Yang Y, Zhang J, Shao B, Yin J (2019) Assessment of bisphenol A alternatives in paper products from the Chinese market and their dermal exposure in the general population. Environ Pollut 244:238–246
Funding
The authors acknowledge the financial supports by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grants 2015/20725-5 and 2018/24069-3), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior – Brasil (CAPES) – Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adeyemi, J.A., Gallimberti, M., Olise, C.C. et al. Evaluation of bisphenol A levels in Nigerian thermal receipts and estimation of daily dermal exposure. Environ Sci Pollut Res 27, 37645–37649 (2020). https://doi.org/10.1007/s11356-020-09898-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09898-4