Abstract
A new type of sediment microbial fuel cell (SMFC) with floating macrophyte Limnobium laevigatum, Pistia stratiotes, or Lemna minor L. biocathode was constructed and assessed in three phases at different hydraulic retention time (HRT) for electrical current generation during the degradation of urban river sediment. The results showed a highest voltage output of 0.88 ± 0.1 V, maximum power density of 80.22 mW m−3, highest columbic efficiency of 15.3%, normalized energy recovery of 0.030 kWh m−3, and normalized energy production of 0.005 kWh m−3 in the Lemna minor L. SMFC during phase 3 at HRT of 48 h, respectively. Highest removal efficiencies of total chemical oxygen demand of 80%, nitrite of 99%, ammonia of 93%, and phosphorus of 94% were achieved in Lemna minor L. system, and 99% of nitrate removal and 99% of sulfate removal were achieved in Pistia stratiotes and Limnobium laevigatum system during the SMFC operation, respectively. Pistia stratiotes exhibited the highest growth in terms of biomass and tap root system of 29.35 g and 12.2 cm to produce the maximum dissolved oxygen of 16.85 ± 0.2 mg L−1 compared with other macrophytes. The predominant bacterial phylum Proteobacteria of 62.86% and genus Exiguobacterium of 17.48% were identified in Limnobium laevigatum system, while the class Gammaproteobacteria of 28.77% was observed in the control SMFC. The integration of technologies with the continuous flow operation shows promising prospect in the remediation of polluted urban river sediments along with the generation of electrical current.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy resource scarcity and the concern of the harmful effects of sediment pollution as the human population increase have become a challenge, to which research has been focused on the development of renewable energy technologies to solve both issues (Noori et al. 2018a, b). A sediment microbial fuel cell (SMFC) is a bioelectrochemical system that can convert the chemical energy in sediments and wastewater rich in organic matter (OM) and sulfides to sustainable and electrical current via the metabolic activities of electrochemically active bacteria (EAB) (Domínguez-Garay and Esteve-Núñez 2018). SMFC consists of an anode embedded in anaerobic sediment and a cathode held in aerobic surface water above the anode, using indigenous anode-dwelling microorganisms in the sediment, to transfer electrons produced during the oxidation of substrates and OM at the anode and to reduce oxygen by accepting electrons at the cathode (Azari et al. 2017). As a result, an electric current is generated between the electrodes and the external circuit for in situ electrical current generation. Studies have shown that SMFCs deployed in aquatic ecosystems can produce sufficient electrical current to operate low-power sensors for remote monitoring (Donovan et al. 2011), wireless temperature sensors (Zhang et al. 2011), and submersible ultrasonic receivers (Donovan et al. 2013). Meanwhile, SMFC has also been explored as a new technology for the removal of organic pollutants from freshwater sediments (Yang et al. 2015).
However, there are several bottlenecks to in situ SMFC applications, viz., shortage of dissolved oxygen (DO) to serve as the final electron acceptor in the surface water for cathodic catalysis, which leads to activation, ohmic, and mass transfer losses. Organic matter depletion (only 0.4 ~ 2.2 wt%) in the sediment at the anode may lead to low electric potential with discontinuous power generation (Donovan et al. 2008). Additionally, the slow mass transfer of oxygen in surface water, as well as fouling, blocks dissolved oxygen from the aquatic ecosystem from diffusion to the cathode electrode during in situ SMFC operation (Debuy et al. 2015). Research efforts to solve cathodic problems via interventions such as chemically catalyzed cathodes (Clauwaert et al. 2007), algae-assisted cathodes (Wang et al. 2014), and photosynthetic cathodes (Gajda et al. 2013) to enhance SMFC have been performed. Nonetheless, the addition of chemical catalyst or substrate at the cathode is harmful to biota and will alter the aquatic ecosystem, leading to poisoning and unsustainable conditions during long-term SMFC operation (De Schamphelaire et al. 2010). Studies have shown that oxygen is the ideal terminal electron acceptor (TEA) for the cathodic reaction during SMFC applications because it is ubiquitous and has a relatively high reduction potential without any chemical waste product (water is the final end product) (Santoro et al. 2017).
In SMFC operation, there are three ways by which DO can be increased at the cathode, viz., (1) bubbling air into the catholyte; (2) exposing the cathode to the atmosphere (air cathode); and (3) as presented in this study, via photosynthesis, leading to the production of pure oxygen directly at the cathode (Juang et al. 2012; Commault et al. 2014). Bubbling air into the catholyte is an inefficient method because pumping requires energy. Moreover, the concentration of oxygen in the air is low and so, the amount of oxygen that can dissolve into the water through aeration is limited to c. 9 mg/L (Weiss 1970). This limitation can be overcome by using biocathodes, which use living organisms to assist in cathodic reactions, as they are more environmentally friendly, cost-effective, and self-sustained (Du et al. 2014). Also, the use of biocathode is advantageous because SMFC operation is open to the aquatic ecosystem to avoid catalyst poisoning or secondary pollution (De Schamphelaire et al. 2010).
Recently, the use of plant biocathode has become a more feasible and economical way to achieve both wastewater treatment and energy generation in plant-sediment microbial fuel cells (PSMFCs) (Li et al. 2019). PSMFCs have attracted much attention for sediment and surface water remediation with electrical current generation, as bioenergy is generated from the degradation of OM, plant exudates, and rhizodeposits by EABs (Habibul et al. 2016). Zhao et al. (2013) stated that the radial oxygen excreted by macrophyte can be used to construct efficient biocathode in MFC, and the presence of macrophytes between the top and middle layers of a CW-MFC had a positive effect on the redox conditions (Doherty et al. 2015). Furthermore, macrophytes release (5 ~ 25%) organic compounds through the roots, which can be used as a carbon source in PSMFC operation (Brix 1997), and assimilate nutrients such as nitrogen (N) and phosphorus (P) during wastewater treatment (Brix 1994). The integration of macrophytes with MFCs has been proven to be feasible by the addition of floating, submerged, and emergent macrophytes in MFC for electrical current generation during wastewater treatment (Mohan et al. 2010).
Currently, the development of SMFC for scale-up is a challenge, as SMFC, which is economical, easy operation, high longevity, and good scalability should be developed for in situ applications. Therefore, inexpensive, environmentally friendly cathode designs and sustainable substrate additions are desired to provide a way to improve DO concentration, oxygen reduction, and bioelectrochemical reactions in SMFCs for long-term applications. Plant biocathode is the most promising way to improve SMFC performance as it can produce DO four times higher than the oxygen obtained by aeration (Gajda 2016). Furthermore, plant biocathode is a good alternative because they are environmentally friendly, cost-effective, and self-sustained, as the plant uses sunlight as source of energy via photosynthesis to produce oxygen, exudates, and rhizodeposit in PSMFC operation (He and Angenent 2006). The oxygen produced forms a micro-oxidizing environment in the rhizosphere, while the root exudates and rhizodeposits serve as additional OM for the EABs to generate electrical current (Helder et al. 2010).
Herein, we investigated the efficacy of three free-floating aquatic macrophytes, Limnobium laevigatum, Pistia stratiotes, or Lemna minor L. biocathode and indigenous EABs during the bioremediation of polluted urban river sediment with concurrent electrical current generation during a continuous flow single-chamber SMFC operation. To the best of our knowledge, macrophyte biocathode with these three plants, SMFC operations have not been previously reported, indicating the novelty of this research. The SMFC presented herewith is a macrophyte-integrated SMFC (mSMFC), which is capable of self-oxygen supply via the rhizosphere at the cathode, OM addition via exudates, and rhizodeposits at the anode without any additional nutrients or inoculum addition. The scope of the current study includes (1) the electrode potentials, power generation, and pollutant removal from the sediment interstitial water in the SMFCs under continuous flow mode in three phases at different hydraulic retention time (HRT); (2) the structure and functions of the indigenous microbial community involved in the degradation of pollutants within the sediments and surface water for in situ electrical current generation; and (3) the prospects and future application of mSMFC in the field.
Materials and methods
Sediment and macrophyte sample collection
The Majia Ditch is a tributary which drains through the city of Harbin emptying into the Songhua River in the Heilongjiang Province of the People’s Republic of China, and just like most urban aquatic ecosystems, the sediment is polluted with high humus content, OM, nutrients, and heavy metals (Sun et al. 2015). Sediment and water samples were collected from the Majia Ditch (45° 75′ 32″ N, 126° 64′ 26″ E). in the vicinity of the shallow water, poor water mobility, and high sediment humus content using a home-made sediment column sampler. The samples were filtered through a 0.5-cm sieve to remove grit, large gravel, and coarse debris, mechanically homogenized, and characterized as shown in Table 1. All the remaining samples were stored in a refrigerator at 4 °C prior to use in the operation of the SMFC. Three free-floating aquatic macrophytes, Limnobium laevigatum, Pistia stratiotes, and Lemna minor L., were chosen for this study (Fig. 1). These three macrophytes were selected based on their local availability as aquarium plants, and as plants that have been used in constructed wetlands for heavy metal removal and nutrient bioaccumulation (San Juan et al. 2018). Also, the macrophytes had different morphological characteristics, which suggested their different biochemical properties. Limnobium laevigatum is a floating or emergent aquatic macrophyte with subcircular, floating, glabrous, and glossy above, with a thick layer of air-filled spongy tissue beneath, base rounded or shallowly cordate leaves. Young plants grow in rosettes of floating leaves that lie prostrate on the surface water, while the mature may grow up to 50 cm tall (Fig. 1a) (Cook and Urmi-König 1983). Pistia stratiotes is a free-floating, stoloniferous plant with sessile pale green leaves in rosettes, growing up to 20 cm long and 10 cm wide, mostly spathulate to broadly obovate with a rounded to truncate apex, with 7–15 prominent veins radiating fanwise from the base on both surfaces, in particular, the lower surface, covered by a dense mat of white wooly hairs (Fig. 1b) (Jacobs and Pickard 1981). Lemna minor L. is one of the smallest of the Lemna species, reaching a diameter of 1.5–4.0 mm. The species have green scale-like fronds that are either solitary or connected in small groups. The frond is small and flat, with 1–3 veins, which are often indistinct, obliquely ovate-elliptic, and the body is reduced to a minute oval, oblong flat, or globose thallus, which is leafless, with purplish beneath. Mature fronds range in diameter of 2–5 mm and are 0.1–2 mm thick (Fig. 1c) (Simonsen 1968). Macrophytes of the same size were acquired from the Harbin flower market, kept and regularly irrigated using the Majia Ditch water for 2 weeks to keep them adapted to the laboratory conditions before starting the mSMFC operation.
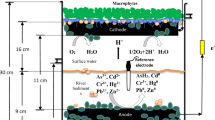
Construction of the SMFCs
Five plastic bioreactors (BRs) with inner dimensions of 37 × 26 × 30 cm and an effective volume of 28.86 L (Fig. S1) were constructed and used as described in our previous study (Kabutey et al. 2019). The bioreactors were parallel setup for continuous flow operation, as shown in Fig. S2. BR 1 had raw sediment with an electrode but was an opened-circuit, applied to mimic the natural degradation processes in aquatic ecosystems. The control (BR 2) contained raw sediment with a closed-circuit to imitate the SMFC system. The other three BRs, including BR 3, BR 4, and BR 5, contained raw sediment operated with Limnobium laevigatum, Pistia stratiotes as macrophyte, and Lemna minor L., at the cathode with closed-circuits. Each BR was filled with 9 cm height wet sediment, 16 cm height, raw ditch water, and the macrophyte were placed to cover the whole surface water to increase DO at the cathode as well as reduce evaporation. A peristaltic pump was attached to the inlet valve of each BR at a 12 cm height to pump the influent ditch water into the BRs and an outlet beneath the surface water at 26 cm height for the effluents. To create a dark root environment in the BRs, the sides of each BR were covered with aluminum foils. The electrodes were made of carbon fiber brushes, and each BR had three anodes (6 cm diameter × 22 cm length) inserted in the sediments, a reference electrode inserted in the middle and three cathodes (8 cm diameter × 22 cm length) held submerged 10 cm above the anode just below the surface water. The electrodes were connected to a rubber-sealed copper wire to 1000 Ω external resistors, and then to a data logger attached to a desktop computer for data acquisition. Each BR was attached with two micro aquarium aeration pumps in the cathode chamber to avoid fouling in the surface water because the SMFCs were operated in the laboratory. The effective volume of the cathode chamber was 15.39 L, and the total working volume of each BR was 24.05 L respectively, during the SMFC operation.
Operation of the SMFCs
Five parallel-connected SMFCs (Fig. S2) were initially operated in phases, phase 1 at an HRT of 12 h for 40 days of acclimation using ditch water with an average influent TCOD concentration of 954.39 ± 1.8 mg L−1 and an organic loading rate of 19.09 kg COD m−3 day−1. Phase 2 was operated at HRT = 24 h, organic loading 9.54 kg COD m−3 day−1 and phase 3 was at HRT = 48 h, organic loading 4.77 kg COD m−3 day−1, where each phase lasted for 40 days, respectively. SMFCs were operated in phases at different HRT to mimic the different flow rates of aquatic ecosystems, as long HRT can result in higher electricity generation and COD removal (Metcalf and Eddy 1991) as well as compare the results. Sediment samples were collected every 5 days from the sides and middle of each BR using a soil sampler. Sediments at the surface were discarded, and those from depths near the anode were mechanically homogenized and filtered, and the sediment interstitial water samples were analyzed immediately. Sampling was confined to 9 a.m. because of the low relative humidity in the morning and water loss due to evapotranspiration was negligible. Therefore, the treatment efficiencies of BRs by concentration were not affected when there was low evapotranspiration (Białowiec et al. 2014).
Analytical methods
Electrode potentials were measured against a standard reference electrode (Ag/AgCl; + 200 mV vs. standard hydrogen electrode, SHE) inserted in the middle of the anode chamber. The voltage output (V) of the closed-circuit SMFCs across 1000 Ω external resistors was acquired every 30 min using the data acquisition system (PCI-DAS PISO-813) connected to a desktop computer. The electrode potentials and voltage represent the averages of each day over the 120-day operation period. Polarization studies were carried out in each phase of the operation by adjusting the external resistor box (99,999.9 ~ 10 Ω) to determine the maximum power density after allowing the circuit to re-equilibrate for 10 min at each resistance. The power density P (mW m−3) was calculated according to the equation P = U×I/V0, where U is the voltage (V), I is the current (A), and V0 is the volume of the anodic chamber. The current and power densities were calculated normalized to the effective volume of the anodic chamber (0.00962 m−3). The DO at the cathode was measured with a handheld JPBJ-608 portable oxygen meter, temperature and pH were measured using a digital pH meter (pHS-3C), and macrophyte growth were quantified in terms of wet biomass. The organic carbon content in the sediment was quantified as total organic carbon (TOC) using a TOC-5000 total organic carbon analyzer (Shimadzu Co., Tokyo, Japan). Chemical oxygen demand (COD) in the form of total COD (TCOD) and soluble COD (SCOD), total phosphorus (TP), nitrogen in the form of ammonia nitrogen (NH4+-N), nitrite (NO2−-N), and nitrate (NO3−-N), sulfate (SO42−), and phosphate (PO43−) concentrations were analyzed after filtration through a 0.45-μm membrane filter using standard methods (APHA-AWWA-WEF 2012).
Microbial community analysis and SEM of electrodes
Biomass samples were collected on the 120th operative day from the sediments and the electrode biofilm of the SMFCs. The microbial structure formed was measured using the V4 region of the 16S rRNA gene (Sangon Biotech Co., Ltd., Shanghai, China). The full length of the 16S rRNA gene fragment was amplified using polymerase chain reaction (PCR) with 341F and 805R primers (BIO-RAD, USA) and was then analyzed using a MiSeq sequencing platform. This result was subsequently analyzed using Microsoft Excel, and the community diversity and distribution at the electrodes of the SMFCs were drawn using OriginPro8.0 (USA). A scanning electron microscope (SEM, Quanta 200, FEI, USA) was used to study the morphology of the electrode surface at the end of the experiments, and the samples were prepared as described in our previous study (Kabutey et al. 2019).
Statistical analysis
The effects of time, DO, and the presence or absence of macrophytes in BRs on electrical current generation and pollutant removal were assessed. Statistically significant differences were determined according to the single t test in the Origin program (OriginPro8.0, USA), and each experiment was performed in duplicate. The error bars represent the standard deviation of the mean (SD). Power generation and bioremediation performance, the total energy consumption of the aeration, and peristaltic pumps in each BR were calculated as described in our previous study (Kabutey et al. 2019). The coulombic efficiency (CE) was calculated as:
CEflow = MI/FnQΔCODwhere I (C/s) is the current of the SMFC, F is the Faraday’s constant (96, C/mol), ZO2 is the number of electrons used in oxygen reduction (ZO2 = 4), ΔCOD (mg/L) is the mass of the COD removed from the sediment through SMFC treatment, MO2 (g/mol) is the molecular weight of oxygen (MO2 = 32 g/mol), and Q is the volumetric influent flow rate.
Net energy recovery (NERv) was quantified as the normalized power production over the unit volume of the treated Majia water and the net energy production (NEPv) based on the volume of the treated water in the BRs at each phase of the operation according to a previous study (Zou and He 2017).
Results and discussion
Electrode potentials and voltage output
The electrode potential vs. the Ag/AgCl reference electrode was recorded daily to show the difference in the bioenergy generation performance of the SMFCs (Fig. 2a). The potentials represent the daily averages of each electrode over the operation period. The initial electrode potentials were − 0.06/0.11 V (BR 2), − 0.29/0.43 V (BR 3), − 0.39/0.19 V (BR 4), and − 0.07/0.11 V (BR 5), respectively. The maximum electrode potential was − 0.01/0.89 V (BR 5) and the minimum − 0.22/0.36 V (BR 2) showing varied electrode potentials. In all the BRs, the electrode potential increased gradually to a peak and then decreased, becoming stable subsequently with the anode potential of BR 4 which decreased. On the whole, the potentials exhibited similar patterns to the electrode potentials in the floating microbial fuel cell for harvesting energy for signal transmission from natural water bodies (Schievano et al. 2017). The variations in electrode potentials can be attributed to the presence of macrophytes and the varied conditions in the SMFCs. This shows that the electrode potential is a limiting factor in power generation in the SMFCs under a continuous flow regime, which is consistent with Song et al. (2012) who obtained varied electrode potentials in an SMFC operation to treat the river sediments.
The voltage output exhibited in the closed-circuit SMFCs is shown in Fig. 2b. Generally, there was an increase in voltage during the operation with some fluctuations. BR 3 and BR 5 exhibited higher voltage output than BR 4 or BR 2 SMFC. During the startup phase, the voltage output in descending order was 0.72 V (BR 3), 0.58 V (BR 4), 0.19 V (BR 5), and 0.17 V (BR 2), while the open-circuit voltage was 0.49 ~ 0.89 V in BR 1. However, in phase 1, the voltage output of 0.42 ~ 0.86 V was observed in BR 2 and BR 4, but it was reduced to 0.23 ~ 0.82 V in phase 2. In the final phase 3, there was an increased voltage output of 0.46 ~ 0.88 V in BR 3 and BR 5, indicating a higher voltage output at the longer HRT of 48 h in the L. minor biocathode, respectively. At the end of the SMFC operations, the mean voltage of BR 3 (0.74 ± 0.1 V), BR 4 (0.54 ± 0.2 V), and BR 5 (0.63 ± 0.2 V) were significantly greater than the control BR 2 (0.47 ± 0.13 V, p > 0.05). Compared with the control BR 2, there was an increase of 57.4%, 15%, and 34% of voltage in the presence of Limnobium laevigatum, Pistia stratiotes, and Lemna minor L. as macrophytes in BR 3, BR 4, and BR 5, respectively. The maximum voltage reported in this study (0.88 ± 0.1 V in BR 5) was greater than that of 0.821 V in Xu et al. (2015), which used a similar freshwater sediment SMFC with surfactant for the degradation of TOC and polychlorinated biphenyls (PCBs). Also, it was much higher than that of Wang et al. (2017) which achieved 0.18 ± 0.01 V in a CW-MFC with macrophytes for domestic sewage treatment. Therefore, the macrophyte biocathode, carbon fiber electrodes, and oxygen, a strong TEA, which were used in the present study, led to a higher voltage output in the mSMFCs compared with the unplanted cathode SMFC, as oxygen reduction at the cathode was carried out by the biofilm attached to the biocathode using bioelectrokinetic analysis (Srikanth and Mohan 2012).
Current and power density curves
To evaluate the electrical current generation performance at each phase of the experiments, polarization studies were performed at each stage of the study (Fig. 3). The current and power density curves of the SMFCs in phase 1 are shown in Fig. 3a. A maximum power density of 64.48 mW m−3 was achieved in BR 4 implanted with P. stratiotes under an external electrical resistor and a current density of 200 Ω and 238.48 A m−3. During phase 2, a maximum power density of 77.83 mW m−3 was obtained in BR 3 planted with L. laevigatum under an external circuit of 200 Ω and a current density of 265.39 A m−3 (Fig. 3b). In the final phase 3, a maximum power density of 80.22 mW m−3 was attained in BR 5 implanted with L. minor L. under an external electrical resistor and a current density of 200 Ω and 274.51 A m−3 (Fig. 3c). The highest maximum current and power density were observed in BR 5 (implanted with L. minor L.) in phase 3 at an HRT = 48 h which showed the highest performance during the SMFC operations. This indicated that the microorganisms had enough contact time with the sediment interstitial water at the longer HRT to degrade substrates and OM present in the sediment to yield high power density (Yadav et al. 2012).
Furthermore, different peaks of power density were exhibited at each phase of the experiment. This phenomenon is akin to freshwater SMFCs, due to the changes in OM in the sediment and surface water from the reduced to oxidized forms, as current production is directly linked to the ability of EABs to oxidize substrates and subsequently transfer electrons to the anode (Hong et al. 2009). Also, the maximum power density varied according to the type of macrophyte inserted at the cathode, and was higher than that of 21 mW m−2 in Acorus tatarinowii (Liu et al. 2018), 14.0 mW m−2 in Aconitum nagarum Stapf var (Wu et al. 2013), and 6.12 ± 2.53 mW m−2 in Typha domingensis (Cervantes-Alcalá et al. 2012), respectively. These results indicated that continuous mSMFC operation could be an alternative approach for power generation while treating polluted river sediments.
Columbic efficiency and normalized energy recovery
The columbic efficiency (CE), a key parameter for the evaluation of the energy recovery efficiency of an MFC system, was calculated for the BRs at the different phases of the SMFC operation. A CE of 13.5% was recorded in BR 4 and 12.3% in BR 2 in phase 1, while CE of 12.0% in BR 3 and 10.4% in BR 2 in phase 2. In comparison, the highest CE of 15.3% was recorded in BR 5 and the lowest of 10.9% in BR 2 during the final phase 3 at HRT = 48, which indicated that the high current and power density in BR 5 was due to the transfer of electrons from substrates and OM breakdown to the anode rather than into other processes (e.g., biomass synthesis or methanogenesis). This collaborated the accession that hybrid systems are more efficient in the generation of bioenergy at a low organic loading rate (Oon et al. 2017). The highest CE exhibited in the mSMFCs was lower than that reported by Ren et al. (2013) of 19.6%, probably because of the use of a polyaniline-graphene nanosheets modified cathode in their SMFC. However, the CE reported for the SMFCs was comparable with other CE of SMFCs reported in the literature (Prasad and Tripathi 2018).
NERv is a new parameter used to express the energy recovery performance of an MFC system. The NERv (kWh m−3) based on the volume of the treated Majia wastewater was calculated for each BR at the end of each phase to assess the energy recovered and consumed by the system. In phase 1, the NERv value ranged from a high of 0.0014 kWh m−3 in BR 4 to a low of 0.008 kWh m−3 in BR 2, while during phase 2 was higher 0.028 kWh m−3 in BR 3 and lower of 0.002 kWh m−3 in BR 2, respectively. In comparison, during phase 3, the highest NERv value was 0.030 kWh m−3 in BR 5 and the lowest was 0.020 kWh m−3 in BR 2, which was comparable with Zhang and He (2012) who recorded 0.032 kWh m−3 in a tubular dual-cathode MFC system for organics and nitrogen removal with electricity generation from a synthetic wastewater treatment. In addition, the NEPv showing the energy balance in the BRs was also quantified. The NEPv values were − 0.011 kWh m−3 in BR 4 and − 0.017 kWh m−3 in BR 2 at phase 1, 0.004 kWh m−3 in BR 3, and − 0.023 kWh m−3 in BR 2 during phase 2, respectively. At the final phase 3 of operation, the highest NEPv of 0.005 kWh m−3 was exhibited in BR 5, while the low of − 0.003 kWh m−3 was in BR 2 at HRT = 48 h which indicated that the long HRT = 48 h influenced the energy recovery during the degradation of pollutants from the sediment and surface water during the SMFC operation.
Despite the negative NEPv recorded during the BR operation, the highest NEPv and NERv values were enough compared with the consumed energy of the aeration and recirculation pumps of 0.025 kWh m−3 per BR. Because mSMFC system will be applied in e.g., rivers, lakes, lagoons, ponds, and ditches that are naturally rich in substrates and OM, human interventions such as artificial aeration and influent circulation are not required to consume additional energy. This indicates that the mSMFC is a useful approach for net energy recovery from sediments of polluted aquatic ecosystems.
Organic degradation
Organic removal
The organic pollutant removal performance of the SMFCs is shown in Fig. 4. The influent TCOD and SCOD concentrations at startup were 954.39 ± 1.8 mg L−1 and 269.97 ± 3.1 mg L−1, respectively. At the end of the experiment, the mean TCOD of BR 3 (623.79 ± 47.9 mg L−1), BR 4 (602.38 ± 48.1 mg L−1), and BR 5 (575.69 ± 48.7 mg L−1) were not significantly greater than the control BR 2 (644.06 ± 46.6 mg L−1, p > 0.05), which indicate that the TCOD removals in the SMFCs were not significantly greater than the control BR 2 (Fig. 4a). However, the highest TCOD removal was 80% in BR 5 and the lowest was 72% in BR 2, while the TCOD removal in BR 1 (natural degradation process) was 69%. The TCOD removal in BR 5 was higher than that of BR 1 because of the presence of EABs on the anode to oxidize the substrates to generate electrical energy in the closed-circuit of BR 5 as compared with the open-circuit degradation process without macrophyte in BR 1. The mean SCOD of BR 2 at the end of the experiment was 453.86 ± 31.3 mg L−1 (p > 0.05), while the mean SCOD in BR 3 (435.65 ± 31.1 mg L−1), BR 4 (416.79 ± 31.1 mg L−1), and BR 5 (391.89 ± 28.9 mg L−1) was not significantly greater than the control (Fig. 4b). This was consistent with Zhao et al. (2013) who recorded the highest COD removal of 76.5% in a continuous flow mode operation of CW-MFCs with reeds (Glyceria maxima) as biota in the treatment of swine wastewater. To sum up, all of the five SMFCs exhibited stable performance for sediment organic treatment, although BR 1 showed low treatment performance. The mSMFC system holds great promise in terms of COD removal, considering the influent TCOD varied from an initial concentration of 954.39 ± 1.8 to a minimum of 183.04 ± 4.4 mg L−1 in BR 5.
The initial TOC concentration in sediment was 1.74 ± 0.2 mg C g−1 sediment, and the mean TOC of BR 2 was 2.04 ± 0.1 mg C g−1 sediment (p > 0.05); however, the TOCs of BR 3 (1.99 ± 0.1 mg C g−1 sediment), BR 4 (2.03 ± 0.1 mg C g−1 sediment), and BR 5 (2.09 ± 0.1 mg C g−1 sediment) were not significantly greater than the mean of the control (Fig. 4c), which indicated that the mean TOC in the mSMFCs was not greater than the mean of the control (BR 2). The increase in TOC without exhaustion during the operation may be attributed to the macrophyte rhizodeposits, root exudations, and additional OM that accompanied the influent as it flowed from the Majia Ditch water storage tank into the SMFCs.
Nitrogen species removal
The removal of nitrogen species from the sediment interstitial water during the operation of the SMFC is illustrated in Fig. 5. The concentration of NH4+-N was 0.79 ± 0.1 mg L−1 at the beginning of the experiment. However, the NH4+-N in BR 3 (0.31 ± 0.2 mg L−1), BR 4 (0.28 ± 0.3 mg L−1), and BR 5 (0.26 ± 0.1 mg L−1) was not significantly greater than the mean of BR 2 (0.35 ± 0.2 mg L−1, p > 0.05) at the end of the SMFC operation. Conversely, BR 5 exhibited the highest NH4+-N removal of (93%) while BR 3 was the lowest (75%) (Fig. 5a). The initial NO2−-N concentration was 3.81 ± 0.5 mg L−1; however, the NO2−-N in BR 3 (1.36 ± 0.2 mg L−1), BR 4 (1.27 ± 0.2 mg L−1), and BR 5 (1.22 ± 0.2 mg L−1) was not significantly greater than the mean of BR 2 (1.38 ± 0.2 mg L−1, p > 0.05) at the end of the experiment. The highest NO2−-N removal was in BR 5 (99%) while the lowest was in BR 2 (98.1%), with all the BRs showing a high percentage removal of NO2−-N (Fig. 5b). The initial influent NO3−-N concentration was 5.30 ± 0.6 mg L−1; however, the NO3−-N in BR 3 (1.89 ± 0.2 mg L−1), BR 4 (1.61 ± 0.2 mg L−1), and BR 5 (1.76 ± 0.2 mg L−1) was not significantly greater than the test mean of BR 2 (1.98 ± 0.2 mg L−1, p > 0.05). The highest percentage removal of NO3−-N was in BR 4 (99%) and the lowest in BR 2 (88%) (Fig. 5c). Although the mSMFC treatments were not significant as compared with the control, a higher percentage removal of nitrogen species in the mSMFCs was observed. This can be attributed to the influent flow rate, macrophyte nutrient assimilation, and the high DO availability in the surface water, revealing that mSMFC may be a suitable approach for in situ nitrogen species remediation (Oon et al. 2017).
Phosphorus and sulfate removal
Phosphorus is a water pollutant of global concern because it limits the productivity of freshwater systems, and high P concentrations may lead to eutrophication (Wu et al. 2014). The P concentration at the startup was 0.361 ± 0.1 mg L−1, while the removals at the end of the SMFC operation in descending order were BR 5 (94%), BR 3 (92%), BR 4 (91%), BR 2 (87%), and BR 1 (84%) as shown in Fig. 6. The highest percentage of P removal was 94% (0.003 ± 0.3 mg L−1) in BR 5 during phase 3 (HRT = 48 h), while the lowest was 84% (0.033 ± 0.4 mg L−1) in BR 1 during phase 1 (HRT = 12 h) as shown in Fig. 6a. The high P removal in the mSMFCs compared with the control BR 2 can be attributed to macrophyte uptake by absorption and adsorption or via direct plant uptake (Sooknah and Wilkie 2004).
The initial SO42− concentration was 226.92 ± 35.5 mg L−1, while the percentage removal of SO42− at the end of the experiment in descending order was BR 3 (99%), BR 5 (98%), BR 4 (98%), BR 2 (95%), and BR 1 (92%) as shown in Fig. 6b, which indicated a high removal of sulfate from the sediments during the mSMFC operation. This showed that the microbes present in the sediments oxidized phosphorus and sulfate to produce reduced end products such as phosphate, sulfide, and sulfur, which donated electrons to the anode of the SMFC for the generation of electricity in situ with synchronized remediation of the sediment (Sajana et al. 2016).
Macrophyte growth and microbial aspects of SMFC
Macrophyte growth and DO concentration at the cathode
Macrophytes are integrated into biological systems because they play important roles such as the roots providing filtering effects and increasing surface availability for the growth of microbial biofilm, and radial oxygen produced influences the redox potential at the catholyte. The root exudates serve as an additional carbon source for denitrifiers to improve the removal of nitrate, increase the microbial communities, or as part of the substrate in plant microbial fuel cell as well (Oon et al. 2017). Plants have a major effect on the removal efficiency of pollutants such as N, P, and heavy metals in plant-integrated systems (Brix 1994).
The growth of macrophytes in terms of wet biomass (Fig. 7a) and root length was regularly sampled and measured for P. stratiotes and L. laevigatum, while the biomass only was measured for L. minor L. due to the lack of roots. All the macrophytes in the SMFCs and the control exhibited increases in biomass, but became static and did not show any increase after 30 days, 60 days, and 70 days in L. minor L., L. laevigatum, and P. stratiotes SMFCs, respectively. However, there were no withering or dying off of a whole plant, while dead leaves which turned brownish were quickly removed to prevent decaying into the system. The initial biomass of L. laevigatum, P. stratiotes, and L. minor L. were 5.25 ± 0.1 g, 6.69 ± 0.1 g, and 4.88 ± 0.2 g, respectively. The plants in the SMFC and the control under laboratory conditions exhibited positive growth as they increased in biomass to cover the entire water surface. Generally, P. stratiotes exhibited the highest growth compared with L. laevigatum and L. minor L in both the SMFC and the control. Pistia stratiotes in the SMFC and control showed similar growth patterns of 29.35 g and 29.26 g in the SMFC and control, respectively. Limnobium laevigatum showed comparable growth patterns of 22.79 g in the mSMFC, while the control was 21.17 g. Lemna minor L. exhibited the lowest growth of 16.08 g in both the mSMFC and the control, with greenish clumps covering the entire water surface at the end of the experiment. Pistia stratiotes had the longest tap root system of 12.2 cm and 11.6 cm compared with the 8.9 cm and 8.2 cm tap root system with several branches formed in the L. laevigatum in the SMFC and the control, respectively. This indicates that the operation of the mSMFC had no significant effect on the macrophyte growth rate/patterns and that the plants could successfully be integrated into SMFCs for pollutant removal with concurrent electrical current generation. The macrophyte biocathode took up pollutants by absorption and hyperaccumulation, and aerobic bacteria in association with plant rhizosphere oxidized nutrients and OM present to donate electrons to the anode for sustainable SMFC operation (Muratova et al. 2003).
The DO at the catholyte during SMFC operation serves as TEA to enhance the reduction reaction, increase electrical energy generation and aerobic microbial activity, and accelerate the degradation of pollutants in the surface water (Noori et al. 2018a, b). The average DO concentration in the catholyte at the beginning of the experiment was 3.99 ± 0.2 mg L−1. However, the mean DO of BR 3 (12.77 ± 0.6 mg L−1), BR 4 (13.01 ± 0.7 mg L−1), and BR 5 (12.10 ± 0.6 mg L−1) was significantly greater than the mean of the control BR 2 (10.19 ± 0.4 mg L−1, p > 0.05) at the end of the experiment (Fig. 7b). This showed that the macrophytes in the SMFC significantly increased the DO concentration at the cathode through radial oxygen loss compared with the control (Wang et al. 2018). The maximum DO of 16.85 ± 0.2 mg L−1 exhibited in BR 4 was higher than that reported for Elodea nuttallii planted CW-MFC system with supplementary aeration for wastewater treatment and electricity generation (Oon et al. 2017).
The high DO at the biocathode can be attributed to the presence of floating macrophytes, biofilms on the cathode, algae, or rise in temperature which increased the DO in the surface water. During the day, macrophytes and algae used energy from the sun to convert water and carbon dioxide into chemical energy to yield oxygen as a by-product (Chen et al. 2012). The oxygen was released via the plant biomass and roots into the catholyte to increase the DO concentration at the cathode. The increased DO at the catholyte which is a strong electron acceptor was utilized as a TEA at the cathode to enhance electrical energy generation with simultaneous pollutant removal during the SMFC operation.
Morphological characteristics of the electrode
The electrode surface in the SMFCs was evaluated at the end of the experiment (Fig. S3 and S4). Scanning electron microscopy of the electrodes showed difference in biofilm formation on the anode (Fig. S4) as compared with the cathode (Fig. S3), as well as differences in biofilm formation on the electrodes in the mSMFC compared with the control SMFC. Generally, there were visible biofilm formations on the electrodes of the mSMFCs than control (BR 2) and on the anode than on the cathode electrodes, respectively. SEM images of the cathode electrodes showed sparse bacterial formation (Fig. S3). Particularly, Fig. S3a and b exhibited less biomass formation compared with the cathode surface as in Fig. S3c, d, and e with visible uniform biomass formation. This showed that BR1 (the open-circuit SMFC) had less microbial activity as compared with the closed-circuit SMFC and mSMFCs. Conversely, the anode SEM images reviled visible biomass attachments with distinct morphologically rod-shaped cell types on the anode electrodes (Fig. S4c, d, and f). However, the anode surfaces of BR 1 and BR 2 were smooth with less biofilm formation, possibly because they were less suitable for microbial growth and biofilm formation (Liu and Logan 2004). The low electrical energy production in BR 2 seemed to be due to increased charge transfer resistance with less developed bacteria on the electrodes (Table S1). The SEM images of the biofilm attached to the electrodes were consistent with the results on electrical energy generation and pollutant removal behavior in the SMFCs as discussed before. The presence of macrophytes and root exudates led to differences in DO and OM content at the electrodes which in turn influenced the microbial biofilm formation on the electrodes. This may be considered the factor that led to variations in electricity generation within the SMFCs (Wang et al. 2017). Therefore, the number of bacteria on the electrodes in the mSMFCs could be one of the factors determining pollutant removal, electron transfer, and electrical current generation in the mSMFC.
Diversity of bacterial and archaeal communities
Species diversity indices of the bacterial and archaeal communities of the electrodes suggested that the macrophyte biocathodes (BR 3, BR 4, and BR 5) were diverse and richer than the unplanted cathode SMFCs (BR 1 and BR 2) as indicated in Table 2. Shannon and Simpson’s diversity indices are used to evaluate the abundance and uniformity of microbial species in a community (Xu et al. 2018). The Shannon and Simpson’s diversity indices of the bacterial and archaeal communities in the mSMFCs were slightly higher in BR 4 (6.50, 0.03; 3.85, 0.08) than in BR 2 (6.19, 0.01; 3.73, 0.06). The high abundance and uniformity of bacterial and archaeal communities in mSMFCs can be attributed to the macrophyte biocathode. The macrophytes enhanced the proliferation of microorganisms for the degradation of pollutants and electrical current generation, which is consistent with the planting of Phragmites australis in CW-MFC for the treatment of synthetic wastewater with electricity generation (Xu et al. 2018).
The taxonomic classification of the bacterial and archaeal communities in the sediments and on the electrodes of the SMFC at three taxonomic hierarchy levels (phylum, class, and genus) is shown in Fig. 8. At the phyla level, the most predominant bacteria were Proteobacteria in BR 3 (62.86%), the second was Firmicutes in BR 3 (10.62%) (Fig. 8a), and the phylum Hydrogenedentes was the least abundant in all the BRs (0.23% in BR 1). These findings are consistent with previous studies which revealed that Proteobacteria and Firmicutes were the distinct EABs found in MFC systems (Logan and Regan 2006). The dominant classes were Gammaproteobacteria in BR 2 (28.77%), Betaproteobacteria in BR 5 (21.16%), Alphaproteobacteria in BR1 (19.32%), and the least Anaerolineae in BR 1 (0.42%) (Fig. 8b). The class Gammaproteobacteria, Alphaproteobacteria, and Betaproteobacteria identified in the BRs are known to contribute to the reduction of nitrate and nitrite (He et al. 2016), while Gammaproteobacteria are known to improve denitrification and phosphorus removal (Yoshie et al. 2006). This may explain why the mSMFCs exhibited high nitrogen species and phosphorus removal from the sediment interstitial water. The microbial community compositions at the genus level are illustrated in Fig. 8c. Exiguobacterium was the most abundant genus in BR 3 (17.48%) and Pseudomonas in BR 2 (17.24%) while the rest showed low abundance with the least abundant genus Thiohalomonas in BR 1 (1.04%).
Archaeal phylum Euryarchaeota was the most abundant in BR 3 (61.02%), the second was Thaumarchaeota in BR 4 (29.58%), and the least was Verrucomicrobia in BR 4 (0.01%) (Fig. 8d). The class Methanomicrobia was the most dominant species in BR 3 (80.88%) and Thermoprotei in BR 4 (18.47%) while Thermoplasmata formed the least dominant in BR 1 (0.34%) (Fig. 8e). The Methanoregula genus was the most abundant in BR 3 (35.68%) and Nitrososphaera was the second most abundant in BR 4 (29.54%), while the least abundant genus was Pacearchaeota Incertae Sedis AR13 in BR 4 (0.03%) (Fig. 8f). The predominant class Methanomicrobia identified here was reported as the archaeal class of species in a phenanthrene-degrading culture initiated with harbor sediment (Chang et al. 2005) and also as a major phylogenetic archaeal group in the anoxic PAH-contaminated bay sediment treatment (Kim et al. 2008). The presence of class Methanomicrobia in the mSMFCs contributed to carbon cycling through methanogenesis in anoxic sediment and sulfate oxidation for in situ electrical current generation (Bhattacharyya et al. 2015).
Prospects and future application of mSMFC in the field
It has been demonstrated that SMFCs can be used in situ to harvest electricity from marine sediments to operate low-power devices, including remote sensing devices, monitoring devices, and telemetry systems in remote locations (Donovan et al. 2011), wireless sensors for environmental monitoring, and oceanographic study for real-time data acquisition (Donovan et al. 2008). Recently, SMFCs have been used for the generation of electrical energy from polluted sediments of freshwater habitats through the degradation of organic pollutants by electroactive bacteria (Habibul et al. 2016). However, the shortfall of DO in surface water which could be low or even zero (Debuy et al. 2015) and the depletion of OM in the sediments during long-term SMFC operation did not make it feasible for commercial scale-up. Therefore, placing macrophytes (L. minor L., P. stratiotes, and L. laevigatum) at the cathode of the SMFC in this study showed that plant biomass could increase and was not affected by the SMFC operations, DO concentration significantly increased at the cathode of the mSMFCs as compared with the control SMFC, and OM at the anode of the mSMFCs was not depleted at the end of the 120-day operation (Fig.5c). The organic pollutant was removed from the sediment interstitial water with concurrent electrical current generation at the varying HRT of 12 h in phase 1, 24 h in phase 2, and 48 h during phase 3, respectively. This confirms the assertion that oxygen excreted by macrophytes can be useful in the construction of efficient biocathodes in MFCs and the root exudates can be useful in the provision of substrate OM during long-term SMFC operation. Remediation of polluted river sediments and surface water was simultaneously realized. This shows that mSMFC is a potential approach for in situ sediment electrical current generation for aquatic ecosystem restoration, beneficial use, and esthetics. The electrical current generated by insertion of the anode in lakes, rivers, lagoons, and moving aquatic ecosystem and the cathode held in the water above with the macrophytes placed on the surface water could become a source for low-power consuming devices to light up the water body, gardens, or lawns for esthetics during the night. This result offered a new, alternative technology to generate electrical energy during the remediation of free-flowing aquatic ecosystems. This advancement may allow the development of more sustainable and cost-efficient SMFCs capable of in situ electrical current generation during free-flowing polluted aquatic ecosystem bioremediation.
Conclusions
This study showed the significance of floating aquatic macrophyte biocathode SMFC operation to achieve in situ electrical current generation and river sediment remediation under the continuous flow mode. Electrical energy production was evaluated in terms of voltage output, power density, normalized energy recovery (NER), and production. The HRT of 48 h was suitable for acclimatization of the wastewater for stable electrode potential and voltage output during the operation. The removal of organic pollutants from the sediment interstitial water was not significantly greater than that of the control BR 2. The organic matter was not exhausted at the end of the 120-day operation. Highest percentage removals of ammonia, nitrite, and phosphorus were observed in BR 5 (L. minor L), when the highest removal of nitrate and sulfate was achieved in BR 4 (Pistia stratiotes) and BR 3 (L. laevigatum), respectively. Pistia stratiotes exhibited the highest growth rate compared with the other macrophytes and it yielded the highest DO. SEM image of the cathode electrodes showed sparse bacterial formation, while the anode electrode revealed visible biomass attachments with distinct morphologically rod-shaped cell types. The bacteria were more predominant than the archaeal communities in the sediments and on the electrodes of the SMFCs. The predominant bacterial phyla were Proteobacteria, the dominate class was Gammaproteobacteria, and the abundant genus was Exiguobacterium while the Archaeal exhibited the dominate phylum of Euryarchaeota, class of Methanomicrobia, and the abundant genus of Methanoregula. The macrophyte L. minor L. biocathode mSMFC exhibited highest efficiencies in terms of electrical current generation, TCOD, ammonia, nitrite, and phosphorus removal when compared with the other SMFCs investigated. Therefore, in the design of mSMFC, it would be more suitable to use L. minor L. (with respect to macrophyte considered) to treat polluted aquatic ecosystems and produce electric current simultaneously.
References
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. In: Rice EW, Baird RB, Eaton AD, Clesceri LS (eds) American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF), Washington, D.C., USA
Azari MAG, Gheshlaghi R, Mahdavi MA, Abazarian E (2017) Electricity generation from river sediments using a partitioned open channel sediment microbial fuel cell. Int J Hydrog Energy 42(8):5252–5260
Bhattacharyya A, Majumder NS, Basak P, Mukherji S, Roy D, Nag S, Haldar A, Chattopadhyay D, Mitra S, Bhattacharyya M (2015) Diversity and distribution of Archaea in the mangrove sediment of Sundarbans. Archaea 2015:1–14
Białowiec A, Albuquerque A, Randerson PF (2014) The influence of evapotranspiration on vertical flow subsurface constructed wetland performance. Ecol Eng 67:89–94
Brix H (1994) Functions of macrophytes in constructed wetlands. Water Sci Technol 29(4):71–78
Brix H (1997) Do macrophytes play a role in constructed treatment wetlands? Water Sci Technol 35(5):11–17
Cervantes-Alcalá R, Arrocha-Arcos A, Peralta-Peláez L, Ortega-Clemente L (2012) Electricity generation in sediment plant microbial fuel cells (SPMFC) in warm climates using Typha domingensis Pers. Int Res J Biotechnol 3(9):166–173
Chang W, Um Y, Hoffman B, Holoman TRP (2005) Molecular characterization of polycyclic aromatic hydrocarbon (PAH)-degrading methanogenic communities. Biotechnol Prog 21(3):682–688
Chen Z, Huang YC, Liang JH, Zhao F, Zhu YG (2012) A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresour Technol 108:55–59
Clauwaert P, Van der Ha D, Boon N, Verbeken K, Verhaege M, Rabaey K, Verstraete W (2007) Open air biocathode enables effective electricity generation with microbial fuel cells. Environ Sci Technol 41(21):7564–7569
Commault A, Lear G, Novis P, Weld R (2014) Photosynthetic biocathode enhances the power output of a sediment-type microbial fuel cell. N Z J Bot 52(1):48–59
Cook CD, Urmi-König K (1983) A revision of the genus Limnobium including Hydromystria (Hydrocharitaceae). Aquat Bot 17(1):1–27
De Schamphelaire L, Boeckx P, Verstraete W (2010) Evaluation of biocathodes in freshwater and brackish sediment microbial fuel cells. Appl Microbiol Biotechnol 87(5):1675–1687
Debuy S, Pecastaings S, Bergel A, Erable B (2015) Oxygen-reducing biocathodes designed with pure cultures of microbial strains isolated from seawater biofilms. Int Biodeterior Biodegradation 103:16–22
Doherty L, Zhao Y, Zhao X, Hu Y, Hao X, Xu L, Liu R (2015) A review of a recently emerged technology: constructed wetland–microbial fuel cells. Water Res 85:38–45
Domínguez-Garay A, Esteve-Núñez A (2018) Designing strategies for operating microbial electrochemical systems to clean up polluted soils under non-flooded conditions. Bioelectrochemistry 124:142–148
Donovan C, Dewan A, Heo D, Beyenal H (2008) Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ Sci Technol 42(22):8591–8596
Donovan C, Dewan A, Heo D, Lewandowski Z, Beyenal H (2013) Sediment microbial fuel cell powering a submersible ultrasonic receiver: new approach to remote monitoring. J Power Sources 233:79–85
Donovan C, Dewan A, Peng H, Heo D, Beyenal H (2011) Power management system for a 2.5 W remote sensor powered by a sediment microbial fuel cell. J Power Sources 196(3):1171–1177
Du Y, Feng Y, Qu Y, Liu J, Ren N, Liu H (2014) Electricity generation and pollutant degradation using a novel biocathode coupled photoelectrochemical cell. Environ Sci Technol 48(13):7634–7641
Gajda I (2016) Self-sustainable cathodes for microbial fuel cells (Thesis). University of the West of England, Bristol, UK
Gajda I, Greenman J, Melhuish C, Ieropoulos I (2013) Photosynthetic cathodes for microbial fuel cells. Int J Hydrog Energy 38(26):11559–11564
Habibul N, Hu Y, Wang Y-K, Chen W, Yu H-Q, Sheng G-P (2016) Bioelectrochemical chromium (VI) removal in plant-microbial fuel cells. Environ Sci Technol 50(7):3882–3889
He Q, Zhou J, Wang H, Zhang J, Wei L (2016) Microbial population dynamics during sludge granulation in an A/O/A sequencing batch reactor. Bioresour Technol 214:1–8
He Z, Angenent LT (2006) Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18(19–20):2009–2015
Helder M, Strik D, Hamelers H, Kuhn A, Blok C, Buisman C (2010) Concurrent bio-electricity and biomass production in three plant-microbial fuel cells using Spartina anglica, Arundinella anomala and Arundo donax. Bioresour Technol 101(10):3541–3547
Hong SW, Chang IS, Choi YS, Kim BH, Chung TH (2009) Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioprocess Biosyst Eng 32(3):389–395
Jacobs SW, Pickard J (1981) Plants of New South Wales: a census of the cycads, conifers and angiosperms. Royal Botanic Gardens, Sydney, Australia, 226 pp
Juang D-F, Lee C-H, Hsueh S-C, Chou H-Y (2012) Power generation capabilities of microbial fuel cells with different oxygen supplies in the cathodic chamber. Appl Biochem Biotechnol 167(4):714–731
Kabutey FT, Antwi P, Ding J, Zhao Q-l, Quashie FK (2019) Enhanced bioremediation of heavy metals and bioelectricity generation in a macrophyte-integrated cathode sediment microbial fuel cell (mSMFC). Environ Sci Pollut Res 26(26):26829–26843
Kim M, Bae SS, Seol M, Lee J-H, Oh Y-S (2008) Monitoring nutrient impact on bacterial community composition during bioremediation of anoxic PAH-contaminated sediment. J Microbiol 46(6):615–623
Li H, Qu Y, Tian Y, Feng Y (2019) The plant-enhanced bio-cathode: root exudates and microbial community for nitrogen removal. J Environ Sci 77:97–103
Liu B, Ji M, Zhai H (2018) Anodic potentials, electricity generation and bacterial community as affected by plant roots in sediment microbial fuel cell: effects of anode locations. Chemosphere 209:739–747
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38(14):4040–4046
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518
Metcalf & Eddy Inc. (1991) Wastewater engineering: treatment disposal reuse (3rd Ed.). McGraw-Hill Inc.
Mohan SV, Mohanakrishna G, Chiranjeevi P, Peri D, Sarma P (2010) Ecologically engineered system (EES) designed to integrate floating, emergent and submerged macrophytes for the treatment of domestic sewage and acid rich fermented-distillery wastewater: evaluation of long term performance. Bioresour Technol 101(10):3363–3370
Muratova A, Hübner T, Tischer S, Turkovskaya O, Möder M, Kuschk P (2003) Plant–rhizosphere-microflora association during phytoremediation of PAH-contaminated soil. Int J Phytoremed 5(2):137–151
Noori MT, Ghangrekar M, Mukherjee C (2018a) Sediment microbial fuel cell and constructed wetland assisted with it: challenges and future prospects. In: microbial fuel cell: a bioelectrochemical system that converts waste to watts, Springer International Publishing, 335–352
Noori MT, Paul D, Ghangrekar M, Mukherjee C (2018b) Enhancing the performance of sediment microbial fuel cell using graphene oxide–zeolite modified anode and V2O5 catalyzed cathode. J Clean Energy Technol 6(2):150–154
Oon Y-L, Ong S-A, Ho L-N, Wong Y-S, Dahalan FA, Oon Y-S, Lehl HK, Thung W-E, Nordin N (2017) Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour Technol 224:265–275
Prasad J, Tripathi RK (2018) Scale up sediment microbial fuel cell for powering led lighting. Int J Renew Energy Develop 7(1):53
Ren Y, Pan D, Li X, Fu F, Zhao Y, Wang X (2013) Effect of polyaniline-graphene nanosheets modified cathode on the performance of sediment microbial fuel cell. J Chem Technol Biotechnol 88(10):1946–1950
Sajana T, Ghangrekar M, Mitra A (2016) In situ bioremediation using sediment microbial fuel cell. J Hazard Toxic Radioact Waste 21(2):04016022
San Juan MRF, Albornoz CB, Larsen K, Najle R (2018) Bioaccumulation of heavy metals in Limnobium laevigatum and Ludwigia peploides: their phytoremediation potential in water contaminated with heavy metals. Environ Earth Sci 77(11):404
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244
Schievano A, Colombo A, Grattieri M, Trasatti SP, Liberale A, Tremolada P, Pino C, Cristiani P (2017) Floating microbial fuel cells as energy harvesters for signal transmission from natural water bodies. J Power Sources 340:80–88
Simonsen R (1968) SCULTHORPE, C. D.: the biology of aquatic vascular plants. 610 S. London: Edward Arnold Ltd. 1967, £ 66 s. net. Internationale Revue der gesamten Hydrobiologie und Hydrographie 53(2):353–354
Song TS, Tan WM, Wu XY, Zhou CC (2012) Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. J Chem Technol Biotechnol 87(10):1436–1440
Sooknah RD, Wilkie AC (2004) Nutrient removal by floating aquatic macrophytes cultured in anaerobically digested flushed dairy manure wastewater. Ecol Eng 22(1):27–42
Srikanth S, Mohan SV (2012) Change in electrogenic activity of the microbial fuel cell (MFC) with the function of biocathode microenvironment as terminal electron accepting condition: influence on over potentials and bio-electro kinetics. Bioresour Technol 119:241–251
Sun H, Hou Y, Huang M (2015). Ecological strategy of water landscape planning: Harbin as a case study. 5th International Conference on Information Engineering for Mechanics and Materials, Atlantis Press.
Wang D-B, Song T-S, Guo T, Zeng Q, Xie J (2014) Electricity generation from sediment microbial fuel cells with algae-assisted cathodes. Int J Hydrog Energy 39(25):13224–13230
Wang J, Song X, Wang Y, Bai J, Li M, Dong G, Lin F, Lv Y, Yan D (2017) Bioenergy generation and rhizodegradation as affected by microbial community distribution in a coupled constructed wetland-microbial fuel cell system associated with three macrophytes. Sci Total Environ 607:53–62
Wang Q, Hu Y, Xie H, Yang Z (2018) Constructed wetlands: a review on the role of radial oxygen loss in the rhizosphere by macrophytes. Water 10(6):678
Weiss RF (1970) The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Research 17:721–735
Wu X, Song T, Zhu X, Zhou C, Wei P (2013) Research on different wetland plants to construct the plant-sediment microbial fuel cell. Kezaisheng Nengyuan/Renew Energy Resourc 31(9):78–82
Wu Y, Wen Y, Zhou J, Wu Y (2014) Phosphorus release from lake sediments: effects of pH, temperature and dissolved oxygen. KSCE J Civ Eng 18(1):323–329
Xu F, Cao F-q, Kong Q, Zhou L-l, Yuan Q, Zhu Y-j, Wang Q (2018) Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem Eng J 339:479–486
Xu X, Zhao Q, Wu M (2015) Improved biodegradation of total organic carbon and polychlorinated biphenyls for electricity generation by sediment microbial fuel cell and surfactant addition. RSC Adv 5(77):62534–62538
Yadav AK, Dash P, Mohanty A, Abbassi R, Mishra BK (2012) Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol Eng 47:126–131
Yang Y, Lu Z, Lin X, Xia C, Sun G, Lian Y, Xu M (2015) Enhancing the bioremediation by harvesting electricity from the heavily contaminated sediments. Bioresour Technol 179:615–618
Yoshie S, Makino H, Hirosawa H, Shirotani K, Tsuneda S, Hirata A (2006) Molecular analysis of halophilic bacterial community for high-rate denitrification of saline industrial wastewater. Appl Microbiol Biotechnol 72(1):182–189
Zhang F, He Z (2012) Integrated organic and nitrogen removal with electricity generation in a tubular dual-cathode microbial fuel cell. Process Biochem 47(12):2146–2151
Zhang F, Tian L, He Z (2011) Powering a wireless temperature sensor using sediment microbial fuel cells with vertical arrangement of electrodes. J Power Sources 196(22):9568–9573
Zhao Y, Collum S, Phelan M, Goodbody T, Doherty L, Hu Y (2013) Preliminary investigation of constructed wetland incorporating microbial fuel cell: batch and continuous flow trials. Chem Eng J 229:364–370
Zou S and He Z (2017) Efficiently "pumping out" value-added resources from wastewater by bioelectrochemical systems: a review from energy perspectives, Water Res 131:62–73
Funding
This research was financially supported by the National Nature Science Foundation of China (Project 51778176) and the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (2019DX07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2666 kb)
Rights and permissions
About this article
Cite this article
Kabutey, F.T., Ding, J., Zhao, Q. et al. Electrical current generation from a continuous flow macrophyte biocathode sediment microbial fuel cell (mSMFC) during the degradation of pollutants in urban river sediment. Environ Sci Pollut Res 27, 35364–35380 (2020). https://doi.org/10.1007/s11356-020-09812-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09812-y