Abstract
After the accidental release of crude oil in marine environment, dispersants are applied on sea surface transferring oil into the water column where it can be broken down by biodegradation, thereby reducing potential pollution to coastal areas. Before they can be used in the wild, the ecotoxicity of such dispersants is usually evaluated with toxicity assays using algae, crustacean and fishes. Nowadays, there is a need to find alternative species to reduce the use of vertebrates both for ethical considerations and for reducing the cost of bioassays. Ciona robusta is a solitary ascidian that inhabits shallow waters and marine coastal areas. This species has been recently adopted as valuable biological model for ecotoxicity studies, thanks to its rapid embryonic and larval development, resemblance to vertebrates, and low risk of ethical issues. On this ground, the lethal and sublethal toxicity of two dispersants has been evaluated on Ciona juveniles. At this stage, the organisms become filter-feeders and the morphological alterations of the organs can be easily observed. The median lethal concentrations at 96 h (96hLC50) for Dispersant 1 (non-ionic surfactant) and for Dispersant 2 (mixture of non-ionic surfactants and anionic surfactants) are 41.6 mg/L (38.6–44.9) and 92.5 mg/L (87.7–97.5), respectively. The Ciona juvenile model was compared to Dicentrarchus labrax fish juveniles test, and it showed increased sensitivity for Ciona to these compounds. These results suggest that 96 h mortality test bioassay could be a good alternative method to 96 h mortality assay with D. labrax, limiting the use of vertebrates for dispersant toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dispersant products mainly used during oil spill response in marine environment are a combination of surfactants and solvents. The surfactants are characterized by a mix of hydrophilic and oleophilic components that facilitates the separation between oil and water in case of oil spill events (Wise and Wise 2011). The solvents (water, water miscible hydroxy compounds or hydrocarbons) dissolve surfactants and favor their solubility in the oil (IMO/UNEP 2011). Then, the oil droplets, formed by the dispersant action, are naturally dispersed by the wave and/or wind and degraded by bacteria.
Dispersants, alone or in combination with hydrocarbons, may induce toxic effects on marine organisms. For this reason, it is important to know the chemical and ecotoxicological characteristics of these products to assess their environmental suitability.
Despite few exceptions, in many European countries, the dispersant use is regulated by national policies. In particular, in Italy, dispersant ecotoxicity has to be assessed during approval procedure before their use at sea. Three toxicity assays are required: algal growth inhibition test and mortality tests with crustaceans and fish.
The need for alternative approaches to the use of vertebrates has become of growing significance for ethical considerations and for reducing the cost of ecotoxicological bioassays. As a matter of fact, considerable advances have been made in this field over the last few decades, as reviewed in Lillicrap et al. (2016). However, fish tests are still required in some countries for regulatory purposes, including dispersant approval procedures. However, there is a need for new and alternative protocols to Reduce, Replace, and Refine fish tests according to 3R strategy (Halder et al. 2014), which have to be also Reproducible, ecologically Relevant, and Regulatory acceptable (additional “3Rs”).
In this regard, ascidians such as Ciona robusta, could be taken into consideration as biological models for alternative methods in dispersant ecotoxicity testing because (a) they are a reliable and sensitive model system for ecotoxicology studies, (b) they are invertebrates, (c) their genome seems to lack genes related to pain (Okamura et al. 2005), and (d) the small size of Ciona larvae and/or juveniles (stage used for fish tests, OECD/203 1992) requires only small volumes of test water.
The ascidian Ciona is a marine sessile invertebrate that belongs to the Subphylum of Urochordates, which have been recognized as the closest living relatives of vertebrates (Delsuc et al. 2006). The Ciona lifespan is relatively short and includes embryonic, larval, juvenile, and adult phases (Satoh 1994). Fertilized eggs develop, in 20–24 h, into swimming tadpole larvae showing typical chordate characteristics, like a rigid notochord and a dorsal neural tube (Sasakura et al. 2012). The larval stage of this species is thus very useful for testing the effects of environmental stressors on the embryonic development of a “simplified chordate ancestor.” After swimming for a few hours, the non-feeding larva attaches to a substrate and starts the metamorphosis process to become a juvenile. According to Chiba et al. (2004), 4 days after fertilization, Ciona juveniles reach the stage 4 in which all organs are formed: the heart, which is specified by known gene regulatory networks (GRNs) (Anderson and Christiaen 2016), the digestive tract (esophagus, stomach and intestine), the nervous system, the gonad rudiment, the gill slits, and the oral and atrial siphons. At this stage, the juveniles become filter-feeders and they can accumulate any toxicant present in the water. Notably, the juveniles have a transparent tunic, thus permitting to see all the internal organs, under the microscope, and visualize any eventual morphological alteration under stress conditions (Chiba et al. 2004; Sato et al. 1997; Willey 1893a, 1893b; Yamamoto and Okada 1999). Juvenile development takes about 10 days, then the young adults become reproductive, grow isometrically, and die in 12–18 months (Berrill 1947; Dybern 1965; Millar 1953; Petersen et al. 1995). Here too, Ciona adults, as filter-feeders, represent an important sentinel for marine environmental monitoring because they tend to accumulate and therefore sequester trace elements.
After being used for more a century for embryological and more recently in evo-devo studies, ascidians such as Ciona robusta are currently attracting growing interest for toxicological analyses. This model system allows evaluating different endpoints besides the death rate of juveniles. A brief survey of ecotoxicological studies using Ciona robusta has been reported in the Supplementary section. Although there are still few studies, the filter-feeding Ciona juveniles have been used to monitor the potential toxicity of two endocrine disruptors (EDCs), bisphenol A (BPA) and tributyltin (TBT) (Mansueto et al. 2011), and to investigate the effect of polystyrene microplastics (Messinetti et al. 2017).
In this study, the lethal and sublethal toxicity of two dispersants has been tested on Ciona juveniles and compared to toxicity response of fish juveniles. The aim was to explore the possibility to use the ascidian C. robusta as alternative biological model to the vertebrate Dicentrarchus labrax in dispersant approval procedures.
Materials and methods
Animal collection
Adults of C. robusta were collected from natural habitat (Taranto, Italy) and transported within few hours into the aquarium of the Zoological Station Anton Dohrn of Naples (Italy). The animals were fed daily with a mixed algal diet and maintained for a week in flow-through circulating aquarium at 18 ± 1 °C under continuous light to promote gamete production and to avoid spawning (Lambert and Brandt 1967).
Juveniles of the marine fish D. labrax (size: 4.1 ± 0.1 cm and 0.7 ± 0.1 g) were obtained directly from a fish farm (Rovigo, Italy) and transported within few hours into the aquarium of the Regional Agency for Environmental Protection in Emilia-Romagna of Ferrara (Italy). The animals were fed with commercial marine fish food (2% of body weight), until 24 h before beginning toxicity testing. The fish were acclimated for 7 days, and no mortality was recorded in this period.
Gamete collection, in vitro fertilization, and juvenile collection of C. robusta
Gametes of C. robusta were obtained by dissecting the gonoducts with a scalpel. Different specimens were used to collect oocytes and sperm. Pooled oocytes were suspended in filtered natural sea water (0.2 μm) and washed twice. Fertilization was performed by adding a dilution (1:100 in FNSW) of pooled sperm to the egg suspension. After an incubation of 10 min on a rotating shaker, the fertilized eggs were washed, transferred in tissue culture plates, and grown until the desired stage of development. Stage 4 (4 days after hatching, size: 2 mm) was chosen for toxicity testing exposure, since at this stage, all the organs are present and the individuals can feed and contract their siphons (Chiba et al. 2004).
Toxicant exposures
Juveniles of C. robusta and D. labrax were exposed for 96 h at five concentrations (0–25–50–100–200 mg/L) of two dispersants, called D1 and D2. D1 is a non-ionic surfactant (10–20%) in alkaline aqueous solution; it is soluble in water and its bioaccumulation is very low in the environment (log Kow < 3). D2 is a mixture of non-ionic surfactants (> 24%) and anionic surfactants (12–24%); it contains hydrocarbons (C11–C14), n-alkanes, isoalkanes, cyclic, and aromatics. D2 is soluble in water, and its bioaccumulation is very low in the environment (log Kow < 3). Sodium dodecyl sulfate (SDS) is an anionic surfactant and contains sodium salt and dodecyl sulfate, with moderate solubility in water (15 g/100 mL at 20 °C) and low bioaccumulation (log Kow < 3). It was used as reference toxicant (positive control) and tested at five concentrations (0–1.56-3.12-6.25-12.5 mg/L). SDS was selected as a reference toxicant because (a) it is a surfactant as the dispersants; (b) it is the reference toxicant in dispersant fish tests according to Italian law (D.D. 02/25/2011), and (c) there are many marine toxicity data for it in literature as reported in Manfra et al. (2017, 2019). The toxicant concentrations were prepared by dissolving SDS or dispersant in seawater. All the assays were performed with daily change of toxicants, because SDS can be easily aerobically degraded in non-sterilized aqueous solution (Scott and Jones 2000), and dispersant degradability was unknown. The SDS concentrations were chosen based on literature data (Conti et al. 2015; Mariani et al. 2006), while a wider range of concentrations was preferred for dispersants, given the lack of information about their toxicity. A control sample (seawater without toxicant) was tested in all experiments, and 3 replicates were done for each concentration. Thirty and 7 individuals were exposed for each replicate for C. robusta and D. labrax, respectively. All bioassays were performed at the experimental conditions reported in Table 1. The experiments were carried out according to Messinetti et al. (2017) with some modifications (no feeding, 96 h exposure) and OECD/203 (1992.) for C. robusta and D. labrax, respectively. Morphological and behavioral endpoints were considered as qualitative sublethal endpoints. Tunic thickening, internal organ disorganization, and slowdown in growth were observed daily using the stereomicroscope Zeiss Stemi 2000 for C. robusta, while D. labrax swimming was recorded for 2 min.
Statistical analyses
After 96 h of exposure, the mortality rate of juveniles was evaluated and 96hLC50 values were calculated by using the GraphPad Prism 6 and ToxStat software for ascidians and fish, respectively. All bioassays were considered acceptable when the control mortality percentage was equal or lower than 10%.
Results and discussion
Effects on survival
In control treatments (seawater without toxicant), all juveniles were alive and healthy. The analysis of the data by nonlinear regression of data obtained from the 96 h acute toxicity tests revealed a dose-response effect for Ciona juveniles whereas an all-or-none response for Dicentrarchus juveniles for all the compounds tested (Fig. 1).
Mortality rate of Ciona robusta and Dicentrarchus labrax after 96 h of exposure to different concentrations of dispersant D1 (a, b), dispersant D2 (c, d) and sodium dodecyl sulfate SDS (e, f). Curves represent the nonlinear regression of mortality data (sigmoidal) with the best fit for LC50 values (dashed lines) as well as the relative 95% CIs (dotted lines). Error bars represent standard deviation
The 96hLC50 values for SDS are 7.0 (5.4–9.1) and 8.6 mg/L (8.0–9.1) for C. robusta and D. labrax, respectively (Table 2). These values are comparable or lower than the LC50 of SDS published in the literature for D. labrax (Conti et al. 2015; Mariani et al. 2006), other fish species (Ribelles et al. 1995; Rosety et al. 2001), and invertebrates (Mariani et al. 2006; Rotini et al. 2015).
In D1 exposure, 96hLC50 of 41.6 (38.6–44.9) and 86.2 mg/L (75.2–98.8) were recorded for C. robusta and D. labrax, respectively (Table 2). In D2 exposure, 96hLC50 of 92.5 (87.7–97.5) and 136.8 mg/L (128.3–145.9) were recorded for C. robusta and D. labrax, respectively (Table 2). Sensitivity of organisms to SDS is similar in both ascidians and fish whereas the 96hLC50 of fishes were 2-fold and 1.5-fold higher than 96hLC50 of ascidians for D1 and D2 respectively, resulting in a slight different sensitivity of Ciona juveniles compared to Dicentrarchus juveniles.
Effects on morphology of ascidians and swimming behavior of fishes juveniles
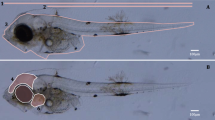
Morphological abnormalities and swimming behavior were scored as sublethal endpoints in C. robusta and D. labrax, respectively. In control specimens, all Ciona juveniles had a beating heart located between the endostyle and the stomach, a tunic around the body, detectable gill slits I and IV, and the oral siphon open, while D. labrax juveniles were highly motile (Fig. 2a, b).
Surviving fish juveniles did not show gross morphological abnormalities. However, slow and uncoordinated swimming behavior was observed in surviving fishes at the higher concentrations of tested toxicants (6.25 mg SDS/L and 100 mg D1–D2/L), while no alteration was observed at the lower concentrations (data not shown). Concerning ascidians, surviving Ciona juveniles did not show gross morphological abnormalities at the lowest SDS concentration (1.56 mg/L), while intermediate concentrations (3.12 and 6.25 mg SDS/L) induced tunic thickening and internal organs disorganization, besides a slowdown in growth (compare Fig. 3a with b and c). At the highest SDS concentration (12.5 mg/L), no individual survived (Fig. 3d). Death of juveniles was also induced by D1 and D2 treatments (Fig. 3e–n), with D1 showing the strongest lethality (D1 at 50 mg/L resulted in 18% surviving juveniles whereas D2 at the same dose resulted in 95% survival) (Fig. 3f). It is intriguing to note that a darkening of internal organs was observed in almost all the surviving D1 and D2 treated individuals, probably due to necrotic tissue of unhealthy juveniles. These data clearly indicate that both D1 and D2 dispersants, already at the lower concentrations, strongly compromise the wellness of Ciona juveniles and consequently the viability of the surviving specimens.
Morphological abnormalities in Ciona robusta exposed at increasing concentrations of sodium dodecyl sulfate SDS (a–d), dispersant D1 (e–h) and dispersant D2 (i, l, m, n). All the treatments (except a) caused a reduction in size, internal organ disorganization and presence of necrotic tissues (see b, c, e, f, i, l, m); the dead juveniles were shown in the images d, g, h and n. The percentage of surviving juveniles is depicted on the pictures
Conclusion
In this study, we showed that the ascidian Ciona robusta juvenile model is a reliable and sensitive model system for ecotoxicology studies. Ciona tadpole larva, indeed, represents the basic and most simplified chordate ancestor while its juvenile and adult stages share many organs with higher chordates like a beating heart specified by known gene regulatory networks (GRNs), a digestive system and an endostyle. This, coupled with a number of computational tools, techniques, and genomic resources, makes Ciona a foundation to reveal the cellular/molecular processes in developing organisms, which could provide valuable information potentially useful for higher and more complex chordates. Moreover, being an invertebrate chordate and lacking in its genome most genes responsible for pain sensation, Ciona is less restricted than fishes by ethical and legal issues. Ninety-six hours mortality bioassay with C. robusta juveniles could thus be proposed as alternative method to 96 h mortality bioassay with D. labrax to reduce, refine, and replace (the 3Rs rule, 86/609/CEE) the use of vertebrates for dispersant toxicity testing.
References
Anderson HE, Christiaen L (2016) Ciona as a simple chordate model for heart development and regeneration. J Cardiovasc Dev Dis 3(3):1–20
Berrill NJ (1947) The development and growth of Ciona. J Mar Biol Assoc U K 26:616–625. https://doi.org/10.1017/S0025315400013825
Chiba S, Sasaki A, Nakayama A, Takamura K, Satoh N (2004) Development of Ciona intestinalis juveniles (through 2nd ascidian stage). Zool Sci 21:285–298. https://doi.org/10.2108/zsj.21.285
Conti D, Balzamo S, Paina A, Martone C, Raso E, Cadoni F, Savorelli F, Croppo M, Bellaria V, Pati A (2015) European sea bass (Dicentrarchus labrax L. 1758) as a sentinel species in Europe to study the effects of contaminants. Annu Res Rev Biol 8(4):1–13
Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439(7079):965–968 https://hal.archives-ouvertes.fr/halsde-00315436/document
Dybern, B. I. (1965). The life cycle of Ciona intestinalis (L) f. typical; in relation to environmental temperature Oikos(16), 109–131
Halder ME, Kienzler A, Whelan M, Worth A (2014) EURL ECVAM Strategy to replace, reduce and refine the use of fish in aquatic toxicity and bioaccumulation testing. Publications Office of the European Union. https://doi.org/10.2788/084219
IMO/UNEP (2011) Guidelines for the use of dispersants for combating oil pollution at sea in the Mediterranean region. Part II: Basic information on dispersants and their application. www.rempec.org
Lambert CC, Brandt CL (1967) The effect of light on the spawning of Ciona intestinalis. Biol Bull 132:222–228
Lillicrap A, Belanger S, Burden N, Du Pasquier D, Embry MR, Halder M, Lampi MA, Lee L, Norberg-King T, Rattner BA, Schirmer K, Thomas P (2016) Alternative approaches to vertebrate ecotoxicity tests in the 21st century: a review of developments over the last 2 decades and current status. Environ Toxicol Chem 35(11):2637–2646. https://doi.org/10.1002/etc.3603
Manfra L, Tornambè A, Guyomarch J, Le Guerrogue P, Kerambrun L, Rotini A, Savorelli F, Onorati F, Magaletti E (2017) Dispersant approval procedures in France and Italy: a comparative ecotoxicity study. Ecotoxicol Environ Saf 143:180–185
Manfra L, Tornambè A, Guyomarch J, Duboscq K, Faraponova O, Sebbio C (2019) Could a harmonized tiered approach assess dispersant toxicity in Italy and France? Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06535-7
Mansueto V, Cangialosi MV, Faqi AS (2011) Postembryonic development effect of Bisphenol a and Tributyltin effects in Ciona intestinalis. Caryologia 64(4):478–484
Mariani L, De Pascale D, Faraponova O, Tornambè A, Sarni A, Giuliani S, Ruggiero G, Onorati F, Magaletti E (2006) The use of a test battery in marine ecotoxicology: the acute toxicity of sodium dodecyl sulfate. Environ Toxicol 21:373–379 https://onlinelibrary.wiley.com/doi/abs/10.1002/tox.20204
Messinetti S, Mercurio S, Parolini M, Sugni M, Pennati R (2017) Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ Pollut 237:1080–1087. https://doi.org/10.1016/j.envpol.2017.11.030
Millar RH (1953) Ciona. In: L.M.B.C. Memoirs on Typical British Marine Plants and Animals. Liverpool University Press, Liverpool, pp 1–123
OECD/203 (1992) Test no. 203: fish, acute toxicity test, OECD guidelines for the testing of chemicals, Section 2. OECD Publishing Paris
Okamura Y, Nishino A, Murata Y, Nakajo K, Iwasaki H, Ohtsuka Y, Tanaka-Kunishima M, Takahashi N, Hara Y, Yoshida T, Nishida M, Okado H, Watari H, Meinertzhagen IA, Satoh N, Takahashi K, Satou Y, Okada Y, Mori Y (2005) Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiol Genomics 22(3):269–282 Retrieved from https://www.physiology.org/doi/pdf/10.1152/physiolgenomics.00229.2004
Petersen JK, Schou O, Thor P (1995) Growth and energetics in the ascidian Ciona intestinalis. Mar Ecol Prog Ser 120:175–184. https://doi.org/10.3354/meps120175
Ribelles A, Carrasco C, Rosety M, Aldana M (1995) A histochemical study of the biological effects of sodium dodecyl sulfate on the intestine of gilthead seabream, Sparus aurata. Ecotoxicol Environ Saf 32:131–138 https://www.sciencedirect.com/science/article/pii/S0147651385710937?via%3Dihub
Rosety RM, Ordonez FJ, Rosety M, Rosety JM, Rosety I, Ribelles A, Carrasco C (2001) Morphohistochemical changes in the gills of turbot, Scophthalmus maximus L ., induced by sodium dodecyl sulfate. Ecotoxicol Environ Saf 3:223–228
Rotini A, Manfra L, Canepa S, Tornambè A, Migliore L (2015) Can Artemia hatching assay be a (sensitive) alternative tool to acute toxicity test. Bull Environ Contam Toxicol 95(6). https://doi.org/10.1007/s00128-015-1626-1
Sasakura Y, Mita K, Ogura Y, Horie T (2012) Ascidians as excellent chordate models for studying the development of the nervous system during embryogenesis and metamorphosis. Develop Growth Differ 54(3):420–437 https://onlinelibrary.wiley.com/doi/full/10.1111/j.1440-169X.2012.01343.x
Sato Y, Terakado K, Morisawa M (1997) Test cell migration and tunic formation during post-hatching development of the larva of the ascidian, Ciona intestinalis. Develop Growth Differ 39:117–126 https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1440-169X.1997.00013.x?sid=nlm%3Apubmed
Satoh N (1994) Developmental biology of ascidians. Cambridge University Press, New York
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochim Biophys Acta (BBA) 1508(1–2):235–251
Willey A (1893a) Studies on the Protochordata. I. on the origin of the branchial stigmata, praeoral lobe, endostyle, atrial cavities, etc. in Ciona intestinalis, Linn., with remarks on Clavelina lepadiformis. Q J Micr Sci 34:317–360
Willey A (1893b) Studies on the Protochordata. II. The development of the neuro-hypophyseal system in Ciona intestinalis and Clavelina lepadiformis, with an account of the origin of the sense organs in Ascidia mentula. Q J Micr Sci 35:295–316
Wise J, Wise JP (2011) A review of the toxicity of chemical dispersants. Rev Environ Health 26(4):281–300 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6730675/pdf/nihms-1048563.pdf
Yamamoto M, Okada T (1999) Origin of the gonad in the juvenile of a solitary ascidian, Ciona intestinalis. Dev Growth Differ 41:73–79 https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1440-169x.1999.00410.x?sid=nlm%3Apubmed
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28.2 kb)
Rights and permissions
About this article
Cite this article
Eliso, M.C., Manfra, L., Savorelli, F. et al. New approaches on the use of tunicates (Ciona robusta) for toxicity assessments. Environ Sci Pollut Res 27, 32132–32138 (2020). https://doi.org/10.1007/s11356-020-09781-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09781-2