Abstract
Phosphorus reuse by application of biochar is a recent concept that needs to be supported by long-term field data. To monitor biochar’s long-term effects on P turnover, one-off biochar was applied in 2013 with mineral NPK fertilizers being applied every year since then. Biochar application rates included 0 t ha−1 (CK), 15.75 t ha−1 (BC1), 31.5 t ha−1 (BC2), and 47.25 t ha−1 (BC3). Over the 5 years’ field experiment, P distribution in soil profile, inorganic and organic P fractions in bulk, and rhizosphere soil and maize P uptake were determined. The results showed that biochar reduced the inorganic P fractions (Ca2-P, Ca8-P, Al-P, Fe-P and O-P by 4.8–33.7%, 8.8–59.0%, 13.7–28.6%, 8.4–17.6%, and 3.3–25.5%, respectively), and increased organic P fractions (MLOP and HROP by 67.2–11.6% and 18.8–87.7%, respectively) in bulk soil, while in rhizosphere soil, Fe-P and MLOP were decreased by 13.4–34.5% and 67.2–111.6%, respectively, in 2017. After the application of biochar for 5 years, moderately labile organic phosphorus (MLOP), moderately resistant organic phosphorus (MROP), and highly resistant organic phosphorus (HROP) with different biochar treatments were enhanced by 12.8–42.7%, 20.1–48.0%, and 5.5–66.6%, respectively, but Ca8-P, Al-P, O-P, and Ca10-P were all decreased by 18.6–24.9%, 16.4–21.4%, and 3.3–23.48%, respectively. Total P storage in 0–100 cm was declined by biochar. Increases in maize P uptake in the stover (38.6–71.3%) and grain (20.9–25.5%) were occurred after 31.5 t ha−1 and 47.25 t ha−1 biochar addition. To sum up, biochar is found to regulate the distribution, storage, and transformation of soil P, which lead to increase in maize P uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key limiting nutrient factor for plant growth and development. P exists as organic and inorganic phosphorus fractions (Rubio et al. 2012), and their stability in the soil is controlled by varying reactions such as solubilization, leaching, sorption, desorption, immobilization, mineralization, and precipitation (Zhu et al. 2018). Potential shortages in the supply of P rock reserves necessitate an efficient utilization of P fertilizer and recovery P in agro-ecosystem. Another effective solution of reducing the dependency on phosphate rock is to recycle P-rich agricultural residues (Kim et al. 2018). The conversion of agricultural residues into biochar by pyrolysis may be an alternative solution of reducing consumption of phosphate (Wesenbeeck et al. 2014).
The application of biochar can not only decrease P fixation on soil solid phases but also improve the efficiency of P uptake by plant from soils (Arif et al. 2017; Bornø et al. 2018). Biochar application could decrease the leaching loss of soil P, which can also reduce serious environmental problems such as eutrophication of surface waters (Chen et al. 2019). Biochar itself contains various forms of P (Xu et al. 2016). As the related study suggested, total P in biochar ranges from 0.2 to 73.0 g kg−1 and available P ranges from 15 to 23,000 mg kg−1 (Sun et al. 2018; Uzoma et al. 2011). Total P in soils usually changes from 0.2 to 1.2 g kg−1 (Davis 2006). Therefore, biochar is a promising agent to directly increase the total phosphorus content of soil when applied to soils.
The mobility of P is very small and it is not easy to leach down from the upper part of the profile (Baumann et al. 2020). Plant roots absorb P from the lower soil. There are a lot of organic colloids and weathering complexes in the upper layer of profile soil, which enhance the adsorption of phosphate ions (Glæsner et al. 2019). Therefore, P contents were more concentrated in the topsoil. The downward migration of P in the soil profile could be attributed to the desorption or dissolution of P as well as wormholes, macropores, or cracks in the soil (Liu et al. 2019). Greater proportion of P transferred through the soil profile was mainly present in organic P compared with the inorganic P due to the greater mobility of monoesters and diesters, while inorganic P could also be susceptible to leaching in circumstances where the P sorption capacity is relatively more saturated (Siemens et al. 2004). Biochar has a high adsorption capacity, because of its characteristics of porosity and large surface area, which also has the ability of absorbing soluble substances in soil, such as phosphate, dissolve organic phosphorus, which could enhance the phosphorus retention in soil and prevent phosphorus from moving downward along the soil profiles (Eduah et al. 2019). Biochar can change soil P characteristics by means of its anion or cation ions on biochar surface (Zhang et al. 2016). Biochar has a lot of positive charges on its surface, combining with the negative charges in soil (e.g., HPO42−, H2PO4−), thus which can reduce the phosphorus leaching (Riddle et al. 2019). However, rare studies have addressed how the long-term biochar application affects P content and storage in the deeper soil layers.
The difference of pH value between biochar and the attached soil also affected the characteristics of soil P. P interacting with Ca2+ could form insoluble Ca-P in alkaline soils, while insoluble Al-P and Fe-P minerals would be formed in acidic soils. The pH was enhanced in the soil applied with biochar which resulted in the dissolution of the phosphates bounding to the free cations (e.g., Ca2+, Mg2+, Fe3+, and Al3+) and released available P for plant uptake (Hong and Lu 2018). As some studies also indicated that the alkaline metals (Ca2+, Mg2+, and K+) in biochar would increase the soil pH, which favored to reduce the formation of insoluble Al-P minerals (Sun et al. 2018). On the contrary, the acid biochar added in acid soils decreased soil pH which favored the formation of insoluble Al-P and Fe-P minerals (Cheng et al. 2006).
Biochar can also affect soil phosphorus transformation by changing soil phosphatase activity, microbial biomass, or microbial community structure. Phosphatase secreted by microorganisms and plant roots can hydrolyze organic P species and release bioavailable P. (Zhu et al. 2017). Biochar stimulated the phosphorus solubilizing microorganism activities, and the addition of biochar was related to the relative increase of soil microbial abundance, which can dissolve tricalcium phosphate (Fox et al. 2016; Mendes Gde et al. 2014).
Plant perception of the environment can initiate a series of signal transductions, which can change the exudation of various compounds related to the phosphate bounding to soil minerals and the hydrolysis of organic P species (Raghothama and Karthikeyan 2005). The application of biochar can alter the composition of plant root exudates of especially glucose and fructose secreted into the rhizosphere, and these exudates would affect the P form in the rhizosphere soil (Bornø et al. 2018). Therefore, the P fractions in rhizosphere soil may be quite different from that in bulk soil.
In spite of the effects of biochar on soil P properties have been extensively studied, little research has been proceeded on the long-term regulation of phosphorus transformation and availability by biochar. In addition, P obtained directly from biochar was considered to be short-lived, and the indirect value of P can be retained in the long-term experiments (Wang et al. 2015). Most of all, the impact of biochar amendment on characteristics of P pools and the usefulness of biochar as sustainable P need to be clarified in the long term of agricultural field (Zhai et al. 2015). In order to find out whether biochar can affect soil P status effectively and continuously for a long time, a long-term biochar trial was established in 2013 and used to investigate the effect of biochar on P fractions in both bulk soil and rhizosphere soil, P storage, and maize P uptake. We hypothesized that (1) biochar would affect P fractions in both bulk soil and rhizosphere soil, but the effect degree between bulk soil and rhizosphere soil is quite different; (2) soil P fractions and P storage can be changed greatly after 5 years of application of biochar; (3) biochar can decline P leaching in soil profile and increase soil P storage, and the higher the amount of application of biochar, the stronger the effect; and (4) thereby increasing P uptake of maize even though biochar has been applied for 5 years.
Materials and methods
Experimental materials and field experiments
The field experiment was conducted from May 2013 to October 2017 at Shenyang Agricultural University (41° 49′ N, 123° 33′ E). The soil of this study is Hapli-Udic Cambisol as qualified by FAO classification (for a detailed description of the experimental site, see Yang et al. (2017)). The experiment consisted of four biochar application rates—0 t ha−1 (CK), 15.75 t ha−1 (BC1), 31.5 t ha−1 (BC2), and 47.25 t ha−1 (BC3). Randomized block design was adopted and repeated three times with an area of 36 m2 (3.6 m × 10 m); all plots were separated with 1-m interval on each side as buffer zone. The biochar adopted in this experiment was pyrolysized from maize stover with a vertical kiln at a temperature of 550 °C for 2 h. Biochar was screened through a 2-mm sieve, then thoroughly mixed and spread evenly on the treated plots and mixed into the topsoil (0–20 cm) by a rotary cultivator only in May 2013. All plots received urea, calcium superphosphate, and potassium sulfate at the rate of N fertilizer at 120 kg N ha−1, P fertilizer at 26 kg P ha−1, and K fertilizer at 50 kg K ha−1; the fertilizers were artificially broadcasted on surface of the soil and mixed into the soil (0–20 cm) by rotary tillage before sowing in each year. The properties of the biochar and the top soil (0–20 cm) in 2013 were shown in Table 1.
In this experiment, continuous cropping of maize was adopted. The hybrid of maize used in this experiment was Danyu 405. Mechanical sowing was done at the end of April and manual harvest at the end of September every year. The planting density of maize was 60,000 stalks per hectare.
Sampling and analysis
The initial soil was collected before sowing in 2013. The soil of different biochar treatments and plant samples were collected after maize harvest in 2017. Maize grain and stover dry biomass were measured by harvesting all the plants in each plot at the end of the growing season in 2017. The aboveground of five consecutive plants were collected from each plot and divided into stover and grain. All maize samples were oven-dried at 80 °C until the weight stays the same.
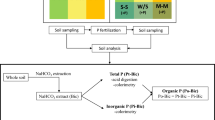
The method of rhizosphere soil collection refers to the shake-off method; three maize plants were randomly collected from each plot, and the rhizosphere soil was extracted from the soil at a depth of 0–20 cm. At first, shake off large pieces of soil without roots, and then sweep all the soil attached to the surface of the root with a brush to obtain the rhizosphere soil. The bulk soil (0–20 cm, 20–40 cm, 40–60 cm, 60–80 cm, and 80–100 cm) was collected by a soil sampler with three replicates in each plot.
The pH of the soil and the biochar was measured in 1:2.5 (w:v) and 1:10 (w:v) ratio, respectively (HANNA HI2221, Italy). The electrical conductivity (EC) was tested in 1:5 soil to water suspension. The cation exchange capacity (CEC) was measured by the ammonium acetate method (Abdel-Fattah et al. 2015). Total C and total N were tested by an Elementar Vario max Analyzer (Elementer Macro Cube, Germany). Specific surface area and average pore size were determined by the Brunauer–Emmett–Teller (BET) method (Surface Area and Pore Porosimetry Analyzer V-sorb 4800P, Gold APP Instrument Corporation, China). Volatile matter, ash contents, and fixed carbon of biochar were tested followed the method of Antunes et al. (2017). Olsen-P was measured followed the method of Olsen (1954). The soil and the biochar were digested by H2SO4 and HClO4 at 280 °C for 2 h and then analyzed total P concentration through the molybdate-ascorbic acid procedure (Adesanwo et al. 2013). The maize stover and grain were digested with H2SO4 and H2O2, and the P concentration was tested following Murphy and Riley (2014).
The inorganic P fractions of the soil were measured following the methods of Jiang and Gu (1989). P in 1.0 g soil was sequentially extracted inorganic P with the following extractants: (1) 50 ml 0.25 M NaHCO3 was added, which extracted Ca2-P; (2) 50 ml 0.5 M NH3Ac was added, which extracted Ca8-P; (3) 50 ml 0.5 M NH4F, which extracted Al-P; (4) 50 ml 0.1 M NaOH-0.1 M NaCO3, and 1 ml 18.4 M H2SO4, which extracted Fe-P; (5) 40 ml 0.3 M Na3Cit and 1 g Na2S2O3 and 10 ml 0.5 M NaOH, which extracted O-P; and (6) 50 ml 1 M H2SO4, which extracted Ca10-P. The organic P fractions of the soil are described in Bowman and Cole (1978). A total of 5 g soil samples were used to extract LOP with 0.5 M NaHCO3, and 2 g soil samples were used to extract MLOP with 1 M H2SO4; then, the residue was extracted with 1 M NaOH to measure MROP and HROP; the content of each organic P fraction is determined by the difference between total P and inorganic P in the extractions. The concentrations of P in the extracts were immediately tested by the ascorbic acid-molybdenum blue method (Murphy and Riley 2014).
Data analysis
The storage of total P in soil profile was calculated using the following equation (Lemercier et al. 2009):
where total P is the concentration of P (g kg−1); BD is the soil bulk density (g cm−3); i is the number of soil layers; and H is the thickness of per soil layer (20 cm).
The P accumulation in grain (stover) samples was determined by the following formula (Arif et al. 2017):
SPSS version 21.0 (IBM, New York, USA) was used to perform statistical analysis. One-way ANOVA was used to evaluate the differences. The differences were compared by least significant difference (LSD) tests at p < 0.05.
Results
P in bulk soil
Olsen-P fluctuated from 20.0 to 37.6 mg kg−1, and Olsen-P was significantly reduced by different levels of application of biochar. Total inorganic P in BC3 treatment was 262.3 mg kg−1, which reached the lowest point among all the treatments. Compared with the CK treatment, total inorganic P under BC1, BC2, and BC3 was decreased by 15.3%, 12.2%, 17.0%, respectively. Total organic P was significantly enhanced by different levels of application of biochar. Total organic P in BC3 treatment was 167.5 mg kg−1, which reached a maximum among the treatments.
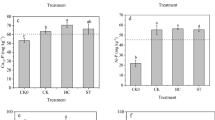
Organic P fractions of different biochar treatments were shown in Fig. 1a. In bulk soil, LOP of BC3 treatment reached the highest point and 42.3% higher than CK treatment, while LOP of BC2 treatment was significantly reduced by 36.8%. Relative to CK treatment, MLOP of BC1, BC2, and BC3 treatment was all significantly enhanced. MLOP of BC3 treatment reached the highest point and 111.6% higher than CK treatment. MROP of BC2 treatment was 42.45 mg kg−1 and significantly higher than CK treatment, while MROP of BC3 treatment was significantly lower than CK treatment. The addition of biochar increased HROP significantly, HROP of BC2 treatment was 38.1 mg kg−1 and significantly higher than other three treatments.
Organic P fractionation (a) and inorganic P fractions (b) of bulk soil of different biochar treatment in 2017. The difference of organic P fractions (c) and inorganic P fractions (d) of each biochar treatment in 2017 compared with initial soil in 2013. Different lowercase letters indicate the difference across treatments (p < 0.05)
Inorganic P fractions of different biochar treatments were shown in Fig. 1b. In bulk soil, Ca2-P fluctuated within a range from 14.65 to 22.10 mg kg−1, Compared with the CK treatment, Ca2-P of BC1 and BC3 treatments was all significantly decreased by 25.1% and 33.7%, but Ca2-P of BC2 treatment was not significantly different from that of CK treatment. Ca8-P of BC1, BC2 ,and BC3 treatments was all significantly decreased relative to CK treatment. Ca8-P of BC1 treatment was 5.2 mg kg−1 and 59.0% lower than that of CK treatment. Al-P of different treatment followed the trend CK > BC2 > BC3 > BC1; compared with CK treatment, Al-P of BC1, BC2, and BC3 treatments was all significantly decreased by 28.6%, 13.7%, and 20.9%. Fe-P was reduced by the addition of biochar. Fe-P of BC3 treatment was 96.4 mg kg−1 and 17.5% lower than CK treatment. O-P was reduced by the application of biochar. O-P of BC2 treatment was 67.8 mg kg−1 and 25.5% lower than CK treatment. Ca10-P of BC1 treatment was 44.2 mg kg−1 and 11.6% higher than CK treatment, while Ca10-P of BC2 treatment was 27.5 mg kg−1 and 30.4% lower than CK treatment.
Changes of P fractions
Different biochar treatments in 2017 can increase every organic phosphorus fraction of soil in varying degrees compared with the original soil in 2013 (Fig.1c). MLOP and HROP of the CK treatment were all decreased in 2017 compared with the that of the initial soil in 2013, while LOP and MLOP were all increased.
Ca2-P of CK and BC2 treatment were all increased compared with the initial soil in 2013 as well as Fe-P of all the treatments in 2017(Fig.1d). Ca8-P, Al-P, O-P, and Ca10-P of different treatments in 2017 were all decreased compared with the initial soil in 2013.
P in rhizosphere soil
Olsen-P, organic P, and inorganic P in rhizosphere soil are shown in Table 2. Olsen-P of BC3 treatment was 23.0 mg kg−1 and significantly lower than CK treatment. Total inorganic P of BC3 treatment reached the highest point among all the treatments and total inorganic P of BC3 treatment was 17.6% higher than CK treatment. Total organic P of BC1 treatment was 236.0 mg kg−1 and significantly higher than that of CK treatment.
The organic P fractions under different biochar treatments are shown in Fig. 2a. In rhizosphere soil, LOP was increased by the application of biochar; LOP of BC3 treatment was 5.1 mg kg−1 and higher than other three treatments. MLOP was reduced by the application of biochar, relative to CK treatment, MLOP of BC2 and BC3 treatments were significantly decreased by 2.5% and 10.8%, respectively. MROP was enhanced by the application of biochar; MROP of BC1 treatment was 40.36 mg kg−1 and significantly higher than that of the CK treatment. HROP of BC1 treatment reached the highest point among all the treatments, and HROP of BC1 treatment was 12.5% higher than CK treatment.
In rhizosphere soil, Ca2-P of BC2 and BC3 treatments was significantly decreased relative to CK treatment. Ca2-P of BC2 treatment reached the lowest point among all the treatments, and Ca2-P in BC2 treatment was 29.7% lower than that of CK treatment. Ca8-P of BC2 and BC3 treatments was 7.4 mg kg−1 and 7.2 mg kg−1 as well as 10.7% and 13.2% higher than that of CK treatment, respectively. Al-P of BC2 treatment was 26.3 mg kg−1 and 18.1% lower than CK treatment. Fe-P of the different treatments followed the trend BC2 > BC3 > BC1 > CK, and Fe-P of different biochar treatments was all significantly higher than CK treatment. O-P fluctuated within a range from 73.4 to 99.6 mg kg−1, O-P of the BC2 treatment reached the lowest point among all the treatments. Ca10-P of the BC1 treatment was 35.0 mg kg−1 and 12.2% lower than that of CK treatment.
Total P content and storage in soil profile (0–100 cm)
Total P content firstly decreased and then increased with the increasing soil depth in all treatments (Fig. 3c). In 20–40 cm, 40–60 cm, and 60–80 cm layer, total P content was reduced by the application of biochar, and total P content in BC2 treatment reached the lowest point among all the treatments.
The content of total P (a) and storage of total P (b) of initial soil profile (0–100 cm) in 2013; the content of total P (c) and storage of total P (d) of different biochar treatments in 2017.The ratios in a bar are the percentage of total phosphorus storage in each layer (0–20 cm) of profile soil (0–100 cm). Error bars indicate standard deviations (n = 3)
Total P storage in every soil layer were all declined after biochar application in 2017. The total P storage in soil profile followed the trend CK > BC3 > BC1 > BC2. Total P storage in BC2 treatment in the 20–40 cm, 40–60 cm, and 60–80-cm layers was all reached the lowest point among all the treatments.
Changes of total P content and storage
Total P content of CK, BC1, BC2, and BC3 in 0 ~ 20-cm layer in 2017 was all higher than the initial soil in 2013. Total P of CK, BC1, BC2, and BC3 in 20 ~ 40-cm and 40 ~ 60-cm layer in 2017 was lower than the initial soil in 2013. The application of biochar decreased the total P content in 60 ~ 80-cm layer compared with the initial soil in 2013.
Total P storage of CK, BC1, BC2, and BC3 in 20 ~ 40-cm and 40 ~ 60-cm layer was all lower than the initial soil in 2013. Total P storage of BC1, BC2, and BC3 in 60–80-cm layer was all decreased compared with the initial soil in 2013.
Maize biomass and their P uptake
P concentration and uptake in maize were shown in Fig. 4. P concentration in grain of BC2 and BC3 treatments was significantly increased by 16.1% and 17.3%, compared with the CK treatment. P concentration in the stover of BC2 treatment was the highest among the treatments, BC1and BC3 were not significantly different from CK treatment. P uptake in grain of BC2 and BC3 treatments was significantly higher than CK treatment. P accumulation in maize stover followed the trend BC2 > BC3 > BC1 > CK, with significant differences among the treatments.
There was no significant effect on maize grain dry biomass under different treatments, but the grain dry biomass of BC3 treatment was higher than the CK treatment by 6.8% and reached the highest point among the treatments. Maize stover dry biomass and total maize dry biomass were all increased with the increase of biochar application. Maize stover biomass and total maize dry biomass of BC3 treatment reached the highest point among the treatments.
Pearson’s correlation analysis
The Pearson correlation coefficients among P concentration in stover, P concentration in grain, P uptake in stover, P uptake in grain, P fractions in bulk soil (a), and P fractions in rhizosphere soil (b) are shown in Fig. 5.
The P fractions in the bulk soil were mainly related to total phosphorus and Olsen-P in soil. Ca2-P, Ca8-P, Al-P, and Fe-P were significantly (p < 0.05) and positively correlated with total P; Ca2-P, Ca8-P, Al-P, and Fe-P were significantly (p < 0.05) and positively correlated with Olsen-P .
The P fractions in rhizosphere soil were mainly related to the related indexes of maize phosphorus uptake. Ca2-P, Ca8-P, Ca10-P, and MLOP were significantly and negatively correlated with P concentration in grain and P uptake in grain; LOP was significantly and positively correlated with P concentration in grain; and Ca2-P and Ca10-P were significantly and negatively correlated with P uptake in stover.
Discussion
The fate of P in soils can be affected by soil properties, rhizosphere reaction processes, plant growth, and other soil management practices (Nishigaki et al. 2019). Some studies indicated that biochar can affect soil chemistry, plant growth, and soil biota, which in turn can influence the characteristics and transformation of P in soils (Chen et al. 2018b; Farrell et al. 2014; Gul and Whalen 2016).
Effect of biochar on P fractions
The characterization of inorganic P and organic P pools in rhizosphere soil and bulk soil was the foundation to enhance the understanding of P cycling in plant-soil system (Li et al. 2007). Organic P was an important part of total P in some soils. As many previous investigations have showed that organic P could be divided into LOP, MLOP, MROP, and HROP fractions, which is conducive to understand the dynamic change of organic P (Zhu et al. 2017). The organic phosphorus in bulk soil was increased with the addition of biochar. LOP is mainly composed of phospholipid and nucleic acid (Steffens et al. 2010). Plants can absorb LOP to meet the nutritional needs of plant growth and proliferation. In this study, the content of LOP in the biochar-amended rhizosphere soil was increased, and the content of MLOP in the biochar-amended bulk soil was also increased significantly, which can be interpreted the reasons why the biochar application could promote the growth of maize and increase the P accumulation of maize. It is reported that MROP and HROP are either sources or sinks of labile and moderately labile P. Accumulation of soil organic matter (e.g., plant roots and exudates and secondary microbial biomass) is the main reason for the increase of MROP and HROP. Many studies found that biochar addition results in the soil organic matter increasing significantly (Chen et al. 2019). Therefore, the increase of MROP and HROP in this research is mainly because the increase of organic matter content in soil after the application of biochar.
Relative to the accumulation of organic P, P fertilization enriched inorganic P to a larger extent. Soil organic P remained unchanged with phosphate fertilizer application in other researches (Ahlgren et al. 2013). The input and output of P, land use, and environmental conditions (such as temperature and humidity) may all lead to the discrepancy of organic P dynamics caused by the application of phosphate fertilizer among studies (Liu et al. 2019). The accumulation of organic P and mineral P usually takes place at the carbon accumulation site, because the changes of organic P and organic carbon are usually interrelated and related, which is due to the predominant C-O-P bonds (Keller et al. 2012). According to previous studies, biochar application can significantly improve the C accumulation of soil (Yang et al. 2017). Therefore, biochar addition could enhance the soil organic P content. Biochar was also demonstrated to enhance the activity of P-solubilizing bacteria and microbial secretions of P-solubilizing acids. P-solubilizing bacteria have been shown to enhance the amount of organic P changed into inorganic P through the release of organic acids and phosphatase enzymes (Liu et al. 2017, 2019).
Fe-P and Al-P are generally considered to be moderately available for plants and have been proved to be available P buffers, especially under strong weathered (Guo et al. 2000) and sandy soils (Wang et al. 2014). Ca-P is not easy to be used by plants, but can act as a buffer for available P. (Cross and Schlesinger 1995; Guo et al. 2000). While Ca2-P, Ca8-P, Fe-P, Al-P, O-P, and Ca10-P (except for Ca10-P in BC1 treatment) were all reduced in different degrees in biochar-amended bulk soil, the adsorption of biochar can decrease the presence of free Al3+, Fe3+, and Ca2+ ions in soil solution, thus releasing P trapped with metal oxides or hydroxides. (Xu et al. 2014; Zhang et al. 2016). These results are consistent with several other researches (Arif et al. 2017; Gao et al. 2019),in which the soil of biochar amendment could decline P fixation of soil solid phases and increase soil P availability. A lot of studies have reported that biochar addition increased soil pH and changed the activity or availability of Al3+, Fe3+, and Ca2+, resulting in changed Al-P, Fe-P, and Ca-P content (Hong and Lu 2018). Generally, the initial reaction products of mineral phosphate fertilizer are relatively soluble forms of Ca-P (such as brushite), which are transformed into stable forms of Ca-P (such as apatite) in alkaline soil over time (Lindsay 1979).
Root exudation decomposes Fe/Al oxides of phosphorus by changing the surface charge of root (Bornø et al. 2018). The net protons from the roots enhance the solubility of P related to Ca/Mg, or improve the desorption of P by soil minerals (Richardson et al. 2009). The response of the plant to biochar application can change the composition of root exudates secreting into the rhizosphere (Foster et al. 2016). This may be one of the important reasons why the P fractions were various in the rhizosphere soil of different treatments. The concentrations of NaHCO3-Po and NaOH-Po in the rhizosphere soil of pigeon pea were higher than those in the bulk soil; this was mainly due to the existence of root exudates which generated the change of rhizosphere microorganisms, which resulted in the transformation of inorganic P into organic P (Rubio et al. 2012; Sugihara et al. 2016). Similarly, LOP, MLOP, and HROP in the rhizosphere were higher than those in bulk soil in our study.
Effect of biochar on soil total P and Olsen-P (0–20 cm)
It is distinct that biochar has a much larger amount of total P than the soil in this study; therefore, the application of biochar could directly enhance the total P (in 0–20 cm) in the biochar-amended soils (Gao et al. 2019). In this research, the biochar had been applied for 5 years when sampling the soil in 2017, according to other researches which indicated that the direct P supplied from biochar was short-lived, which was one of the reasons why the addition of biochar did not increase the total P content in this study. Many researchers reported that application of biochar can increase the content of Olsen-P from 3 to 46 mg kg−1 in red earth and from 13 to 137 mg kg−1 in fluvo-aquic soil (Sun et al. 2018; Zhai et al. 2015). Jin et al. (2019) found that the addition of biochar (biochar application rate of 0, 2.5, 5, 10, 20, 30, and 40 t ha−1, respectively) after 5 years had no significant effect on the content of available P in the soil. The research of Xu et al. (2016) indicated that the application of biochar reduced available P in soil (0–20 cm), because not only more P was carried away by maize in the biochar-amended soil but also the negatively charged P could attach onto the surface of Fe/Al oxides making large proportions of P turn into unavailable forms. The P availability highly depended on pH in the soil, and biochar which can enhance soil pH was widely reported in previous researches (Biederman and Harpole 2013). The increase of pH values enhances the precipitation of phosphate to less soluble forms (Schneider and Haderlein 2016).
Effect of biochar on soil total P storage (0–100 cm)
The biochar application could decrease the leaching loss of P fertilizer, and then decrease the risk of water eutrophication caused by soil phosphorus. (Zhou et al. 2018). The research of Madiba et al. (2016) reported that applying chicken manure or wheat chaff biochar enhanced absolute P leaching with increasing application rates of P fertilizer. In contrast to this find, Major et al. (2012) reported that the application of biochar enhanced nutrient retention and then declined leaching. Phosphorus extracted from agricultural soils is a complex process, which depends on soil properties, such as acidity and alkalinity, clay minerals, iron/aluminum oxides, and organic matter. (Chen et al. 2018a). Biochar may affect the retention and migration of phosphorus adsorbed on iron-aluminum oxides or soil organic carbon complexes, thus reducing the release of phosphorus and the leaching of phosphorus in soil (Cui et al. 2011).
In this experiment, the application of biochar did not significantly increased the P storage of the soil but slightly reduced the total P storage of each biochar treatment. This was mainly because the biochar has been applied for 5 years and the soil bulk density of biochar treatments was reduced. In addition, biochar can produce a large number of beneficial effects on other aspects of soil besides phosphorus, so as to promote the growth of maize and the absorption of phosphorus by maize (Romualdo et al. 2018). The application of biochar to improve the growth of maize and the absorption of P by maize have also been verified in this experiment.
Effect of biochar on maize P uptake
The positive effects of biochar or combined application of biochar and mineral P fertilizer on yield in alkaline soils were reported. Limited or direct negative influences of biochar in alkaline soils on yield and P uptake have also been observed (Hussain et al. 2017). Asai et al. (2009) found no significant effect of biochar on maize yield in acidic soil, and we also found no statistically significant positive influence of biochar on maize grain productivity, and here we applied much higher rates (31.5 t ha−1 and 47.25 t ha−1). While maize stover dry biomass was increased by the application of biochar. Maize plants were able to assimilate P originating from biochars (Bornø et al. 2018), which was reflected by the shoot biomass and total maize P uptake in this study. This also provides a reason why such a large amount of biochar was applied at one time without significantly increasing the total P content of the soil in this study. The amount of biochar applied 31.5 t ha−1 and 47.25 t ha−1 significantly increased the amount of plant P uptake, which may be also one of the reasons why the Olsen-P (0 ~ 20 cm) of biochar treatments was decreased.
Conclusion
P fractions were affected by biochar in both bulk soil and rhizosphere soil, and the effect degrees between bulk and rhizosphere soil were different. Biochar reduced the inorganic P fractions (Ca2-P, Ca8-P, Al-P, Fe-P, and O-P), while organic P fractions (MLOP and HROP) were increased by biochar in bulk soil. In rhizosphere soil, Fe-P and MLOP were exactly opposite to those in bulk soil. Soil P fractions changed greatly after 5 years of application of biochar. Different biochar treatments in 2017 increased every organic P fraction of soil in varying degrees compared with the original soil in 2013, while Ca8-P, Al-P, O-P, and Ca10-P were decreased. Total P storage in 0–100 cm was declined by the application of biochar. The application of biochar decreased the total P content and total P storage in 20 ~ 40-cm, 40 ~ 60-scm, and 60 ~ 80-cm layers in 2017 compared with the initial soil in 2013. The P uptake of maize stover was greatly improved with biochar of 31.5 t ha−1, and maize grain P uptake was highest with 47.25 t ha−1 biochar after biochar being applied for 5 years. Altogether, biochar could be a potential agent to regulate soil P fractions, possibly by reducing the leaching of soil P and promote the uptake of P by crops.
Abbreviations
- Ca2-P:
-
Dicalcium phosphate dehydrate
- Ca8-P:
-
Octocalcium phosphate
- Ca10-P:
-
Hydroxyapatite
- Al-P:
-
Aluminum phosphate
- Fe-P:
-
Iron phosphate
- O-P:
-
Occluded phosphate
- LOP:
-
Labile organic phosphorus
- MLOP:
-
Moderately labile organic phosphorus
- MROP:
-
Moderately resistant organic phosphorus
- HROP:
-
Highly resistant organic phosphorus
References
Abdel-Fattah TM, Mahmoud ME, Ahmed SB, Huff MD, Lee JW, Kumar S (2015) Biochar from woody biomass for removing metal contaminants and carbon sequestration. J Ind Eng Chem 22:103–109. https://doi.org/10.1016/j.jiec.2014.06.030
Adesanwo OO, Ige DV, Thibault L, Flaten D, Akinremi W (2013) Comparison of colorimetric and ICP methods of phosphorus determination in soil extracts. Commun Soil Sci Plan 44:3061–3075. https://doi.org/10.1080/00103624.2013.832771
Ahlgren J, Djodjic F, Börjesson G, Mattsson L (2013) Identification and quantification of organic phosphorus forms in soils from fertility experiments. Soil Use Manag 29:24–35. https://doi.org/10.1111/sum.12014
Antunes E, Schumann J, Brodie G, Jacob MV, Schneider PA (2017) Biochar produced from biosolids using a single-mode microwave: characterisation and its potential for phosphorus removal. J Environ Manag 196:119–126. https://doi.org/10.1016/j.jenvman.2017.02.080
Arif M, Ilyas M, Riaz M, Ali K, Shan K, Haq IU, Fahad S (2017) Biochar improves phosphorus use efficiency of organic-inorganic fertilizers, maize-wheat productivity and soil quality in a low fertility alkaline soil. Field Crop Res 214:25–37. https://doi.org/10.1016/j.fcr.2017.08.018
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res 111:81–84. https://doi.org/10.1016/j.fcr.2008.10.008
Baumann K, Shaheen SM, Hu Y, Gros P, Heilmann E, Morshedizad M, Wang J, Wang SL, Rinklebe J, Leinweber P (2020) Speciation and sorption of phosphorus in agricultural soil profiles of redoximorphic character. Environ Geochem Hlth. https://doi.org/10.1007/s10653-020-00561-y
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214. https://doi.org/10.1111/gcbb.12037
Bornø ML, Eduah JO, Müller-Stöver DS, Liu F (2018) Effect of different biochars on phosphorus (P) dynamics in the rhizosphere of Zea mays L. (maize). Plant Soil 431:257–272. https://doi.org/10.1007/s11104-018-3762-y
Bowman RA, Cole CV (1978) An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci 125(2):95–101. https://doi.org/10.1097/00010694-197802000-00006
Chen M, Alim N, Zhang YT, Xu N, Cao XD (2018a) Contrasting effects of biochar nanoparticles on the retention and transport of phosphorus in acidic and alkaline soils. Environ Pollut 239:562–570. https://doi.org/10.1016/j.envpol.2018.04.050
Chen Q, Qin J, Cheng Z, Huang L, Sun P, Chen L, Shen G (2018b) Synthesis of a stable magnesium-impregnated biochar and its reduction of phosphorus leaching from soil. Chemosphere 199:402–408. https://doi.org/10.1016/j.chemosphere.2018.02.058
Chen W, Meng J, Han X, Lan Y, Zhang W (2019) Past, present, and future of biochar. Biochar 1:75–87. https://doi.org/10.1007/s42773-019-00008-3
Cheng C-H, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488. https://doi.org/10.1016/j.orggeochem.2006.06.022
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214. https://doi.org/10.1016/0016-7061(94)00023-4
Cui HJ, Wang MK, Fu ML, Ci E (2011) Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soils Sediments 11:1135–1141. https://doi.org/10.1007/s11368-011-0405-9
Davis TR (2006) Soil phosphorus levels in residential lawns comparisons among four land uses in Springfield, Missouri. Doctral dissertation. Missouri State University
Eduah JO, Nartey EK, Abekoe MK, Breuning-Madsen H, Andersen MN (2019) Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 341:10–17. https://doi.org/10.1016/j.geoderma.2019.01.016
Farrell M, Macdonald LM, Butler G, Chirino-Valle I, Condron LM (2014) Biochar and fertiliser applications influence phosphorus fractionation and wheat yield. Biol Fertil Soils 50:169–178. https://doi.org/10.1007/s00374-013-0845-z
Foster EJ, Hansen N, Wallenstein M, Cotrufo MF (2016) Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric Ecosyst Environ 233:404–414. https://doi.org/10.1016/j.agee.2016.09.029
Fox A, Gahan J, Ikoyi I, Kwapinski W, O’Sullivan O, Cotter PD, Schmalenberger A (2016) Miscanthus biochar promotes growth of spring barley and shifts bacterial community structures including phosphorus and sulfur mobilizing bacteria. Pedobiologia 59:195–202. https://doi.org/10.1016/j.pedobi.2016.07.003
Gao S, DeLuca TH, Cleveland CC (2019) Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Sci Total Environ 654:463–472. https://doi.org/10.1016/j.scitotenv.2018.11.124
Glæsner N, van der Bom F, Bruun S, McLaren T, Larsen FH, Magid J (2019) Phosphorus characterization and plant availability in soil profiles after long-term urban waste application. Geoderma 338:136–144. https://doi.org/10.1016/j.geoderma.2018.11.046
Gul S, Whalen JK (2016) Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol Biochem 103:1–15. https://doi.org/10.1016/j.soilbio.2016.08.001
Guo F, Yost RS, Hue NV, Evensen CI, Silva JA (2000) Changes in phosphorus fractions in soils under intensive plant growth. Soil Sci Soc Am J 64:1681–1689. https://doi.org/10.2136/sssaj2000.6451681x
Hong C, Lu S (2018) Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ Sci Pollut R Int 25:8725–8734. https://doi.org/10.1007/s11356-018-1219-8
Hussain M, Farooq M, Nawaz A, Al-Sadi AM, Solaiman ZM, Alghamdi SS, Ammara U, Ok YS, Siddique KHM (2017) Biochar for crop production: potential benefits and risks. J Soils Sediments 17:685–716. https://doi.org/10.1007/s11368-016-1360-2
Jiang BF, Gu YC (1989) A suggested fractionation scheme of inorganic phosphorus in calcareous soils. Fert Res 20:159–165. https://doi.org/10.1007/bf01054551
Jin ZW, Chen C, Chen XM, Jiang F, Hopkins I, Zhang XL, Han ZQ, Billy G, Benavides J (2019) Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: a five-year field trial in upland red soil, China. Field Crop Res 232:77–87. https://doi.org/10.1016/j.fcr.2018.12.013
Keller M, Oberson A, Annaheim KE, Tamburini F, Mäder P, Mayer J, Frossard E, Bünemann EK (2012) Phosphorus forms and enzymatic hydrolyzability of organic phosphorus in soils after 30 years of organic and conventional farming. J Plant Nutr Soil Sci 175:385–393. https://doi.org/10.1002/jpln.201100177
Kim JA, Vijayaraghavan K, Reddy DHK, Yun YS (2018) A phosphorus-enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers. J Clean Prod 196:163–171. https://doi.org/10.1016/j.jclepro.2018.06.004
Lemercier B, Walter C, Arrouays D, Aurousseau P, Follain S, Michaud A, Saby NPA, Sarr JB, Schvartz C, Vertes F (2009) Phosphorus storage in soil: monitoring and analysis at national and regional scales. Oceanis S D 33:103–122
Li H, Shen J, Zhang F, Clairotte M, Drevon JJ, Le Cadre E, Hinsinger P (2007) Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant Soil 312:139–150. https://doi.org/10.1007/s11104-007-9512-1
Lindsay WL (1979) In: Lindsay WL (ed) Chemical equilibria in soils. Wiley-Interscience, New York
Liu J, Sui P, Cade-Menun BJ, Hu Y, Yang J, Huang S, Ma Y (2019) Molecular-level understanding of phosphorus transformation with long-term phosphorus addition and depletion in an alkaline soil. Geoderma 353:116–124. https://doi.org/10.1016/j.geoderma.2019.06.024
Liu SN, Meng J, Jiang LL, Yang X, Lan Y, Cheng XY, Chen WF (2017) Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl Soil Ecol 116:12–22. https://doi.org/10.1016/j.apsoil.2017.03.020
Madiba OF, Solaiman ZM, Carson JK, Murphy DV (2016) Biochar increases availability and uptake of phosphorus to wheat under leaching conditions. Biol Fertil Soils 52:439–446. https://doi.org/10.1007/s00374-016-1099-3
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2012) Nutrient leaching in a Colombian savanna Oxisol amended with biochar. J Environ Qual 41:1076–1086. https://doi.org/10.2134/jeq2011.0128
Mendes Gde O, Zafra DL, Vassilev NB, Silva IR, Ribeiro JI Jr, Costa MD (2014) Biochar enhances Aspergillus niger rock phosphate solubilization by increasing organic acid production and alleviating fluoride toxicity. Appl Environ Microbiol 80:3081–3085. https://doi.org/10.1128/AEM.00241-14
Murphy J, Riley JP (2014) A modified single solution method for the determination of phosphate in natural waters. J Colloid Interface Sci 425:91–95. https://doi.org/10.1016/s0003-2670(00)88444-5
Nishigaki T, Tsujimoto Y, Rinasoa S, Rakotoson T, Andriamananjara A, Razafimbelo T (2019) Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 435:27–38. https://doi.org/10.1007/s11104-018-3869-1
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture Circular 939. US Department of Agriculture, Washington DC
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49. https://doi.org/10.1007/s11104-004-2005-6
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Riddle M, Bergstrom L, Schmieder F, Lundberg D, Condron L, Cederlund H (2019) Impact of biochar coated with magnesium (hydr)oxide on phosphorus leaching from organic and mineral soils. J Soils Sediments 19:1875–1889. https://doi.org/10.1007/s11368-018-2197-7
Romualdo J, Lima S, Silva WDM, Medeiros EVD, Duda GP, Corrêa MM, Pereira A, Filho M, Clermont-Dauphin C, Celso A (2018) Effect of biochar on physicocshemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma 319:14–23. https://doi.org/10.1016/j.geoderma.2017.12.033
Rubio G, Faggioli V, Scheiner JD, Gutierrez-Boem FH (2012) Rhizosphere phosphorus depletion by three crops differing in their phosphorus critical levels. J Plant Nutr Soil Sci 175(6):810–871. https://doi.org/10.1002/jpln.201200307
Schneider F, Haderlein SB (2016) Potential effects of biochar on the availability of phosphorus —mechanistic insights. Geoderma 277:83–90. https://doi.org/10.1016/j.geoderma.2016.05.007
Siemens J, Ilg K, Lang F, Kaupenjohann M (2004) Adsorption controls mobilization of colloids and leaching of dissolved phosphorus. Eur J Soil Sci 55:253–263. https://doi.org/10.1046/j.1365-2389.2004.00596.x
Steffens D, Leppin T, Luschin-Ebengreuth N, Min Yang Z, Schubert S (2010) Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods. J Plant Nutr Soil Sci 173:765–771. https://doi.org/10.1002/jpln.201000079
Sugihara S, Tomita Y, Nishigaki T, Kilasara M, Wasaki J, Funakawa S (2016) Effects of different phosphorus-efficient legumes and soil texture on fractionated rhizosphere soil phosphorus of strongly weathered soils. Biol Fertil Soils 52:367–376. https://doi.org/10.1007/s00374-015-1082-4
Sun D, Hale L, Kar G, Soolanayakanahally R, Adl S (2018) Phosphorus recovery and reuse by pyrolysis: applications for agriculture and environment. Chemosphere 194:682–691. https://doi.org/10.1016/j.chemosphere.2017.12.035
Uzoma KC, Inoue M, Andry H, Zahoor A, Nishihara E (2011) Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J Food Agric Environ 9:1137–1143. https://doi.org/10.1016/j.foodchem.2011.01.033
Wang T, Camps-Arbestain M, Hedley M (2014) The fate of phosphorus of ash-rich biochars in a soil-plant system. Plant Soil 375:61–74. https://doi.org/10.1007/s11104-013-1938-z
Wang Y, Lin YX, Chiu PC, Imhoff PT, Guo MX (2015) Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci Total Environ 512:454–463. https://doi.org/10.1016/j.scitotenv.2015.01.093
Wesenbeeck SV, Prins W, Ronsse F, Antal MJ Jr (2014) Sewage sludge carbonization for biochar applications. Fate of heavy metals. Energy Fuel 28:5318–5326. https://doi.org/10.1021/ef500875c
Xu G, Sun JN, Shao HB, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol Eng 62:54–60. https://doi.org/10.1016/j.ecoleng.2013.10.027
Xu G, Zhang Y, Sun JN, Shao HB (2016) Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci Total Environ 568:910–915. https://doi.org/10.1016/j.scitotenv.2016.06.079
Yang X, Meng J, Lan Y, Chen W, Yang T, Yuan J, Liu S, Han J (2017) Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric Ecosyst Environ 240:24–31. https://doi.org/10.1016/j.agee.2017.02.001
Zhai LM, Caiji ZM, Liu J, Wang HY, Ren TZ, Gai XP, Xi B, Liu HB (2015) Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol Fertil Soils 51:113–122. https://doi.org/10.1007/s00374-014-0954-3
Zhang H, Chen C, Gray EM, Boyd SE, Yang H, Zhang D (2016) Roles of biochar in improving phosphorus availability in soils: a phosphate adsorbent and a source of available phosphorus. Geoderma 276:1–6. https://doi.org/10.1016/j.geoderma.2016.04.020
Zhou K, Sui Y-y XX, J-y Z, Y-m C, Hou M, X-g J (2018) The effects of biochar addition on phosphorus transfer and water utilization efficiency in a vegetable field in Northeast China. Agr Water Manage 210:324–329. https://doi.org/10.1016/j.agwat.2018.08.007
Zhu J, Qu B, Li M (2017) Phosphorus mobilization in the Yeyahu wetland: phosphatase enzyme activities and organic phosphorus fractions in the rhizosphere soils. Int Biodeterior Biodegradation 124:304–313. https://doi.org/10.1016/j.ibiod.2017.05.010
Zhu J, Li M, Whelan M (2018) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537. https://doi.org/10.1016/j.scitotenv.2017.08.095
Funding
This work was supported by the National Natural Science Foundation of China (No. 41401325; No. 31901449), the Natural Science Foundation of Liaoning Province (No. 20180550247; No.2019-BS-212), and the 13th Five-Year Plan National Key R&D Program of China (No. 2018YFD0300300; No. 2018YFD0300309).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, D., Chen, W., Yang, P. et al. Spatio-temporal variabilities of soil phosphorus pool and phosphorus uptake with maize stover biochar amendment for 5 years of maize. Environ Sci Pollut Res 27, 36350–36361 (2020). https://doi.org/10.1007/s11356-020-09716-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09716-x