Abstract
Camphor wood is welcomed by museums due to its insect-repelling effect but the smell indicates a potential risk to the collections. In order to judge the suitability of camphor wood as a museum storage material, typical camphor wood (Cinnamomum camphora) samples aged for different years were evaluated by conducting the Oddy test. Gas chromatography–mass spectrometry (GC-MS), X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), and time of flight–secondary ion mass spectrometry (ToF-SIMS) were applied to identifying the volatile organic compounds (VOCs) emitted by the materials and the corrosion products, respectively. The results showed that the camphor wood samples led to visible corrosion on copper and lead coupons. GC-MS indicated that the major VOCs emitted were terpenes and their derivatives, while XRD, EDS, and ToF-SIMS provided various clues to the corrosion mechanisms. Pb10(CO3)6(OH)6O and CuO were regarded as the major corrosion products of lead and copper coupons, respectively. The study provides the museum curators and the conservators with abundant information to reassess the application of camphor wood to museums as well as a different way to understand the mechanism of metallic corrosion caused by camphor wood.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environment inside museums has received more attention in recent years (Borrego and Molina 2019; Fabbri 2018; Lee et al. 2011). Museum exhibition hall and storage room are a special indoor environment in which both the health of human beings and the safety of cultural objects should be considered carefully. As a matter of fact, the latter is in many cases prior to the former, especially when priceless collections are exhibited or stored. Compared with the hall or the storage room, the showcases or the storage boxes are more attractive to conservators because they are a potential source of volatile compounds which may directly affect the objects placed in the micro environment. In fact, conservators try their best to take more factors into consideration and introduce effective techniques into conservation. Differing from natural disasters and human errors, daily environmental factors impact on cultural objects in a slow but irreversible way. Among various environmental factors, the volatile corrosive compounds emitted by storage or display materials have been investigated by the British Museum since the 1970s (Oddy 1973). Many collections are sensitive to the volatile compounds, especially when metallic objects are surrounded by acidic atmosphere, corrosion occurs significantly. A convenient method called as Oddy test was developed in order to judge the suitability of uncertain storage or display materials (Oddy 1973; Robinet and Thickett 2003; Thickett and Lee 1996). In this accelerated corrosion test, pure copper, silver, and lead coupons are employed to simulate the cases of metallic objects. Higher temperature (typically 60 °C) and relative humidity (approximately 100%) are set to accelerate the corrosion so that the phenomenon can be observed in a 28-day period. Corrosion is observed by naked-eye, which reflects the concentration of volatile corrosive compounds and the suitability level of the potential materials, while the specific contaminants and the corrosion mechanisms have to be given by other methods.
Wooden materials are welcomed by museums due to their advantages in availability, air permeability, mechanical property (high strength in spite of low density), and texture. Camphor wood is especially favorable in many cases because its smell is a natural insect repellant and a fumigant, which has been approved since the era of Ancient Egypt and Babylon, as well as in Europe in the Middle Ages (Chen et al. 2013). As a valuable material preferred by ancient Chinese intellectuals, camphor wood has also been applied to making storage boxes in China for many centuries. Chinese people believe that camphor wood is not only a sturdy material which can be used for decades, but also an uncommon natural material useful for repelling parasites and inhibiting microbes. This is also accepted by many traditional museums (Ding 2018; Li 2012). Camphor wood is thus made into packaging boxes, storage boxes, and showcases and it really offers protection against insects and microbes in some cases (Jia et al. 2017; Li 2012). Nevertheless, as a volatile mixture, the smell of camphor wood indicates a potential risk to the collections, which may lead to corrosion, degradation, deterioration, or other diseases on the objects (Fu 1955; Jia et al. 2017). It is necessary to evaluate the suitability of camphor wood as a museum storage material and provide conservators with useful information so that they can make correct decisions when camphor wood is listed as a potential candidate.
In this work, the Oddy test was employed to judge the reliability of camphor wood (Cinnamomum camphora) samples as museum storage materials. Cinnamomum camphora is a typical species of camphor wood which is widely distributed in the tropics and subtropics. The use of it is not just limited in the source area but common all over the world through trade. Modern instrumental methods including gas chromatography–mass spectrometry (GC-MS), X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), and time of flight–secondary ion mass spectrometry (ToF-SIMS) were applied to identifying the volatile components emitted by the camphor wood samples aged for different years and the corrosion products. The study gives the museum curators and staffs substantial information for making decisions on selecting storage materials, helps conservators understand the character of camphor wood more clearly, as well as provides environmentalists with a new perspective to museums.
Materials and methods
Materials
Camphor wood (Cinnamomum camphora) materials were collected from Jiangxi Province, China. They were respectively aged for about 30, 50, and 100 years and have not been processed through boiling or drying. The initial moisture contents were 15.8%, 15.6%, and 15.6% for the camphor wood materials aged for 30, 50, and 100 years, respectively. The raw wood materials were cut into the same shape for the Oddy test. Isooctane (analytical grade), and copper, silver, and lead foils (99.9%) were purchased from Sinopharm Chemical Reagent Company, China. Metallic coupons used in the present study were obtained by cutting the raw metallic foils into the same size (3.5 cm × 1.0 cm) (Thickett and Lee 1996). Isooctane was used without further purification. Deionized water was used throughout this work.
The Oddy test
The reliability of camphor wood samples was judged by conducting an improved 28-day Oddy test. The preparation of wood sample bricks and metallic coupons, the assembling of experimental devices, and the testing process were based on the reference (Shen et al. 2018). Typically, several customized glass bottles equipped with ground glass stoppers were employed. For each bottle, a thin iron wire was made into a hanger and fixed inside the stopper. Specifically, the wire was bent to the shape containing four sharp turnings, playing the role as holding three coupons. For each material, about 2.0 g of wood sample was put into the bottle first. Then, a 2-mL glass tube was filled with deionized water, plugged with a small ball of cotton wool, and then placed into the bottle. The small tube was kept standing during the test. Three cotton strings were threaded through the holes punched on a copper, a silver, and a lead coupon, respectively, and then tied in the sharp turnings of the hanger. After that, the bottle was plugged with the stopper. Coupons were kept away from each other, as well as from the sample and the small tube. The joint between bottle and stopper was coated by two layers of parafilm. Thus, a typical experimental vessel was assembled. Also, the control vessel was assembled like this but without adding material samples. The vessels were then moved into a Memmert HPP 750 thermo-hygrostatic chamber to conduct the Oddy test. The temperature and the relative humidity inside the chamber were respectively set as 60 °C and 40% in order to accelerate the corrosion and promote the evaporation of water stored in the small tube while prevent the metallic chamber from being affected by high relative humidity. The typical device is shown in Fig. 1.
Identification of the volatile compounds
Volatile compounds emitted by the wood samples were identified using an Agilent 7890B-5977B gas chromatography–mass spectrometer (GC-MS) through solid-phase micro-extraction (SPME). The SPME conditions are as follows: device: CTC 3-in-1 automatic sampler; fiber: 50/30 μm CAR/DVB on PDMS; temperature: 50 °C; time: shaking for 15 min and extracting for 30 min; shaking rate: 250 rpm; desorption time: 5 min; GC cycling time: 50 min. GC-MS conditions: column: DB-wax (30 m × 0.25 mm × 0.25 μm); inlet temperature: 260 °C; split mode: splitless; carrier gas: helium (99.999 %); flow: 1 mL min−1; column temperature: held at 40 °C for 5 min, then increased to 220 °C at 5 °C min−1, and then increased to 250 °C at 20 °C min−1 and held for 2.5 min; interface temperature: 260 °C; ionization temperature: 230 °C; quadrupole temperature: 150 °C; ionization mode: EI+, 70 eV; scanning mode: full scan; mass range: 20–400; spectra database: NIST 2014.
The total volatile organic compound (TVOC) emitted by each wood sample was measured using a RAE PGM-7340 portable TVOC analyzer in a 16-L closed desiccator with 2.0 g of wood sample.
Identification of the corrosion products
Coupons were analyzed using a Rigaku SmartLab X-ray diffractometer (target: Cu Kα; voltage: 40 kV; current: 100 mA) in situ to identify the corrosion products adhered to the coupon surface when the Oddy test was finished. An Oxford INCA energy dispersive X-ray spectrometer was employed to detect the relative contents of elements existed on the coupon surface (voltage: 10 kV, under which the elements can be detected effectively while the substrate of metallic coupons can be prevented from being scanned to the greatest extent possible). Species adhered to the coupon surface were identified using an ION-TOF GmbH ToF-SIMS 5 instrument (primary ion: Bi3++; voltage: 30 kV; raster area: 200 μm × 200 μm).
Results and discussion
Coupon appearances
Coupon appearances before and after the Oddy test are shown in Fig. 2. Corrosion was observed on lead and copper coupons, while the silver coupons maintained the initial conditions in general. It is easy to find that the corrosion level of lead coupons increased along with the aging of camphor wood, which was in accord with the monotonic increasing tendency of the corresponding TVOC values (see below); while in the case of copper, the corroded coupons showed partly discolored surfaces compared with the control. According to the reference (Thickett and Lee 1996), materials are classified based on the degree of corrosion of the corresponding metallic coupons. Specifically, materials are judged unusable if severe colorful corrosion is observed on the corresponding lead coupon, or clearly black/green corrosion or loss of polish is observed on the corresponding copper coupon; while judged temporarily usable if slight overall corrosion or a few very localized spots are observed on the lead coupon, or slight discoloration is observed on the copper coupon compared with the control; and the final overall judgment is based on the worst single judgment result for the three coupons. In comparison with the lead coupons for other wood samples, the lead coupon for the wood sample aged for 100 years produced larger corrosion spots with darker color, which was especially conspicuous in the affected coupons. Therefore, the wood sample aged for 100 years was judged to be unusable, while the other two wood samples were regarded as temporarily usable.
Species and amount of the volatile compounds
Major volatile compounds identified by GC-MS are listed in Table 1. The TVOC values were 600, 670, and 780 ppb for the wood samples aged for 30, 50, and 100 years, respectively. According to Table 1, terpenes and their derivatives occupied a predominant part of the volatile species emitted by the samples. These matters are typical volatile organic compounds (VOCs) which produce aroma and play the role of repelling insects and inhibiting microbes. Specifically, D-(+)-Camphor and Eucalyptol were detected as major compounds in all the three cases. During the whole aging period of the camphor wood material, both the relative contents of D-(+)-Camphor and Eucalyptol increased to the top at about the fiftieth year, and after that, they decreased to lower values. D-(+)-Camphor did not hold the first place in data for the wood sample aged for 100 years; instead, α-Copaene occupied the position. Such differences may have direct or indirect effects on corrosion and other chemical reactions on metallic coupons for different wood samples. On the other hand, as the most important lower fatty acid, acetic acid is also the commonest volatile component emitted by many wooden materials (Tétreault et al. 1998). Since acetic acid emitted only occupied 0.08%, 0.01%, and 0.05% of the total peak area for the wood samples aged for 30, 50, and 100 years, respectively, acetic acid was not significant in both relative and absolute concentrations in the camphor wood samples. Other typical lower fatty acids such as formic acid and propionic acid were more hardly detected, indicating that lower fatty acids did not occupy an important position in the volatile compounds. Furthermore, less common acidic volatile compounds including inorganic acids and carboxylic acids with more complex structures were also hardly detected.
Since VOCs were more significantly detected than acids, it is even more debatable to apply the camphor wood material to the conservation of ancient organic objects like documents or fabrics than metallic objects. The reference (Jia et al. 2017) shows that paper deteriorates when camphor wood is used as the storage material, which is noteworthy for conservators from both eastern and western museums. Therefore, although the application of camphor wood may be helpful for repelling harmful insects and inhibiting microbes (Wang et al. 2019; Wu et al. 2019), further and deeper studies are required if that influence on the condition of ancient organic objects is to be understood more clearly.
Corrosion products
Coupon phases identified by XRD are shown in Fig. 3. Pb10(CO3)6(OH)6O (JCPDS 19-0680) was identified as the corrosion product of lead coupons affected by the camphor wood samples, but no new significant diffraction peaks were found on the patterns for copper and silver coupons. Differing from the fact that silver is resistant to certain severe environmental conditions, the failure of detecting the corrosion products of copper coupons was more likely to be attributed to their low yields. The recognition of them has to be achieved by other methods.
The EDS results provided semi-quantitative information about the relative content of elements involved in the corrosion. As shown in Table 2, C and O were commonly detected on the coupon surface, which may be originated from the VOCs emitted by the camphor wood samples, the corrosion products, or the chamber contaminants. It can be seen that for the original control coupons, both C% and O% values were around 5%. Compared with that, an increasing was observed in both values of C% and O% for the copper control coupon after the Oddy test, indicating the normal oxidation of copper during the Oddy test (Thickett and Lee 1996); while for the affected copper coupons, the increasing was more significant, which indicates the corrosion caused by the VOCs. Considering that the increasing of O% was obvious and the corroded position featured a black appearance, it is reasonable to deduce that tenorite (CuO) was the most likely corrosion product of the copper coupons. In the case of Ag, the increasing of both C% and O% values was also observed, but much less significant than that in the case of Cu, especially for C%, implying that carbonates or organics were unlikely the corrosion products of the silver coupons, while the increasing of C% for the affected copper coupons compared with the copper control may include both the adsorbed VOCs and the carbonates or organics (as corrosion products). On the other hand, element S was not significantly detected in the case of Ag, indicating that sulfides were not the noteworthy corrosion product of the silver coupons. In the case of Pb, the increasing of C% and O% values was similar to that in the case of Cu. Compared with the initial condition, the lead control showed a higher C% after the Oddy test, which means the normal conversion of lead during the Oddy test, probably producing carbonates (Peng 2003). This phenomenon was more significantly observed on the affected lead coupons, implying further carbonation of lead.
The atomic % values of the metallic elements are useful for determining the corrosion products. The atomic % values of Cu or Pb in several pure corrosion products are listed in Table 3. When copper is corroded through the route: Cu → Cu2O → CuO (see “Corrosion mechanisms” below), it can be found that the Cu% value for the corrosion products presents a monotonic decline. The theoretical values (66.7% and 50.0%) can be used as critical values for differentiation of the corrosion stages of copper. For instance, the copper control coupon after the Oddy test was regarded as corroded between the stages of Cu and Cu2O, while the affected copper coupons were corroded between the stages of Cu2O and CuO. This deduction is based on the fact that all the other corrosion products of copper are less stable and are converted into Cu2O and CuO under the experimental conditions. In other words, CuO can be regarded as the final corrosion product of copper, and simultaneously the simplest chemical formula having the highest Cu% value among the Cu(II) corrosion products, thus if the Cu% is measured as a value < 50.0%, active corrosion products (such as malachite, copper hydroxide, and copper acetate) must be included. In the case of Pb, the Pb% in the corrosion products is usually a low value if lead is corroded completely according to the theoretical equations. This case is totally different from the case of Cu, as the lead oxides (PbO, Pb3O4, Pb2O3, and PbO2) are unlikely to be permanently stable under the experimental conditions (Tétreault et al. 1998), and thus the Pb% cannot be a higher value. Based on this, when lead is corroded through the route: Pb → Pb(OH)2 → Pb10(CO3)6(OH)6O (see “Corrosion mechanisms” below), the Pb% value is around 20.0% after lead is completely converted into Pb(OH)2 if no side reactions happen. The measured Pb% values for the affected lead coupons were higher than the theoretical value, which was related to the feature of EDS, as element H composing certain corrosion products of lead cannot be detected by this characterization; also, it may be caused by the detection of the lead substrate (incomplete conversion). For the lead coupon affected by the camphor wood sample aged for 100 years, the larger size of the corrosion product particle may lower the possibility of detecting the substrate by chance. It should be noticed that the halogens are ruled out of consideration in both the cases of Cu and Pb, otherwise the above calculation will be inapplicable. This rule is reasonable in the Oddy test because both volatile inorganic and organic halogens are uncommon in the volatile compounds emitted by most potential materials. Similarly, the possibility of other non-metallic elements except O and S is also ignored because most non-metallic elements are either inactive or uncommon in air or in the volatile compounds, while oxygen is the primary oxidant in air and plays the key role in many corrosion cases in the Oddy test, and sulfur should only be considered when absolutely necessary.
Last but not least, the EDS results should only be regarded as a reference rather than an absolute standard, because the measurement accuracy is commonly influenced by some factors such as working voltage and chamber cleanliness which make EDS a semi-quantitative characterization in most cases. Typically, lower working voltage may lead to the failure of detecting heavy elements, while excessive working voltage may cause the detection of additional elements located at the substrate; chamber contaminants and other substances adsorbed on the sample surface may also lead to the detection of additional elements such as C and O. It can be seen from Table 2 that the Cu/O ratios for the affected copper coupons were close to 2:1 rather than 1:1, which may cause a misconception to assume that Cu2O was the predominant surface oxide, but actually it was probably caused by the combined effect of surface adsorption of VOCs (thus C% relatively increased while O% relatively decreased) and detection of additional pure metals from the coupons (thus metal % relatively increased). In a word, a general judgment of the corrosion stage using EDS is based on reasonable interpretation of the measured data.
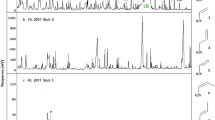
The secondary ion mass spectra (negative, m/z = 5–100) for copper, silver, and lead control coupons and those affected by the camphor wood aged for 100 years after the Oddy test are shown in Figs. 4, 5, and 6, respectively. It should be noticed that ToF-SIMS only reflects the information about the species adhered to the coupon surface while GC-MS characterizes all the volatile compounds emitted. It can be seen that for each kind of coupon, the two spectra were similar in general, but for each ion species, the intensity values differed; meanwhile, different kinds of coupon showed quite different intensity values at some critical m/z positions. Subtle clues hidden in complex phenomena can be found by comparing the corresponding peaks carefully. For example, the intensity value for CO3− (m/z = 60) detected on the affected lead coupon was much higher compared with that on the control, while the corresponding values for copper and silver were generally constant and the absolute values were much lower than in the case of lead. This result indicates that carbonates tended to emerge from the lead coupon surface in air, and this process was significantly promoted during the Oddy test when the camphor wood was coexisted, which was in accord with the detection of Pb10(CO3)6(OH)6O in XRD result. The other information obtained was the content of surface oxygen on different coupons. It can be found that for the three kinds of coupon, the order of the intensity values at m/z = 16 (O−) was Pb > Cu > Ag, which was in accord with the order of metal activity, reflecting the difference of affinity for oxygen. Last but not least, it is noteworthy that for the affected lead coupon, the intensity values at m/z = 45 (CHO2−), 59 (C2H3O2−), and 73 (C3H5O2−) (both the difference values were 14, indicating a –CH2 group) were significantly higher than the corresponding values for the lead control, while in the cases of copper and silver, the values for the affected coupons were even lower than those for the corresponding control coupons, which may be related to the intrinsic corrosion mechanisms. According to the reference (Tétreault et al. 1998), the peaks imply the existence of formate, acetate, and propionate groups, which may be originated from the intermediate corrosion products. Meanwhile, there still remained some peaks which cannot be definitely recognized due to the complexity of the secondary ion mass spectra. On the other hand, compared with the lower mass range spectra, the spectra of mass range 100–800 were much more complicated while the intensity counts for each secondary ion species were much lower, thus deeper studies as well as more experiences on recognizing critical details are required for extracting meaningful information from the higher mass range spectra.
Corrosion mechanisms
Although silver is usually regarded as a noble metal, silver surface is easy to react with some non-metallic species in air (Costa 2001). Tarnishing is the commonest form of silver corrosion, which typically appears as a brown or even black film under severe conditions. The innate character of tarnishing is the formation of silver sulfide (t’Hart et al. 2016). Typical reactions occurring in the tarnishing are listed as follows (Costa 2001):
According to the literature (Costa 2001), the whole process is promoted by high humidity, which coincides with the condition of the Oddy test. In fact, tarnishing is also an important evidence for judging the corresponding material unusable or temporarily usable in the Oddy test (Thickett and Lee 1996). Since tarnishing was not observed on the silver coupons, it is confirmed that H2S was not a significant factor in this study. Mineralization, localized corrosion, selective corrosion, and stress-induced corrosion are regarded as less common types of silver corrosion (Costa 2001).
As a weak-metallic element, lead tends to be corroded into Pb(OH)2 in air through the oxygen absorption process (Peng 2003). Then, carbon dioxide participates in the reactions, producing Pb10(CO3)6(OH)6O [which is also written as 3Pb(OH)2·6PbCO3·PbO]. The whole process is promoted by the combination of high temperature and high relative humidity which coincides with the experimental conditions of the accelerated corrosion test. The relevant reactions can be generalized as follows:
Another route to producing Pb10(CO3)6(OH)6O was given in the reference (Tétreault et al. 1998):
The role of acetic acid in Eq. 6 can be replaced by other lower fatty acids such as formic acid and propionic acid. In fact, it can be found that the generation of Pb10(CO3)6(OH)6O is not related to acids or other special substances but the common elements in air when combining Eq. 6 with Eq. 7:
Both Eq. 8 and Eq. 4 indicate that acids are unnecessary in the corrosion of the lead coupons, but they can promote the process.
The corrosion route of copper is even more complex compared with the case of lead. Acids, air, and water play requisite roles in the conversion. According to the reference (Shen et al. 2018), the key reactions can be generalized as follows:
Lower fatty acids are traditionally regarded as the main body of acids participating in these reactions. In fact, when Eq. 9 is combined with Eq. 10, it can be found that acids only play a catalytic role in the overall reaction:
Thus, acids are unnecessary in the corrosion processes of lead and copper expressed by Eqs. 4, 8, and 11, which is in accord with the above result that acetic acid, the most typical lower fatty acid, was not significant in both relative and absolute contents. Nevertheless, the existence of acids promotes the corrosion processes.
Acids participating in these reactions may also have other sources. It is well known that ozone is efficient in oxidizing terpenes in the troposphere and one major product is carboxylic acids (Atkinson and Arey 2003; Calogirou et al. 1999). The typical reactions can be written as follows:
The indoor environment is totally different from the troposphere, but some studies show that unsaturated terpenes have a high potential to react with ozone indoors and carboxylic acids are produced as a result as well (Weschler and Shields 1997; Wolkoff et al. 1997; Wolkoff et al. 2000). Since the Oddy test was performed in a thermo-hygrostatic chamber without lighting, ozone was unlikely to be originated directly from the photolysis of NO2; rather, it was more likely to be generated mainly from electrical discharging according to the reference (Weschler 2000). The factors influencing the indoor ozone concentration include the indoor ozone source, the outdoor ozone concentration, the indoor-outdoor air exchange rate, the indoor ozone remove rate, and the ozone-related reactions in air. As an essential element for producing ozone, electrical equipment was available in this study. However, the corresponding oxidized products, the carboxylic acids, or the corresponding carboxylates, were not reflected significantly by GC-MS or ToF-SIMS, which may be due to rearrangement, decomposition, or other complex organic reactions.
The role of terpenes and their derivatives in the corrosion of collections is crucial to museums but it has not been adequately recognized and studied. One reference (Oikawa et al. 2005) shows that artifact materials made of iron, copper, or some inorganic pigments are strongly affected by the wooden interior materials used in museum storage rooms. Hinokitiol, a species of terpene derivative, together with acetic acid, is regarded as the causal component emitted by the wooden materials influencing the artifacts. In the following study (Oikawa et al. 2006), the role of Hinokitiol and acetic acid is further confirmed, and the importance of studying the role of specific VOCs other than acetic acid, the most concerned corrosive substance by museums (Ghiara et al. 2014), is emphasized, while there still remains some interesting points such as the conversion mode of Hinokitiol, the corrosion process of the artifact materials and the investigation of other unknown causal components. In a word, it is interesting to further study the corrosion mechanisms in the wooden storage material–cultural object systems (typically, the VOCs-metal system).
Conclusions
Although camphor wood is useful for repelling insects, it is unsuitable to be permanently used as a museum storage material, especially for the conservation of objects composed of copper, lead, or any active metals. The corrosion is probably promoted by traces of acids emitted by the camphor wood samples, which is a big challenge to the researchers trying to modify camphor wood to a more reliable material and may advance the tendency of abandoning wooden storage materials in museums. The catalytic role of acids in the corrosion requires more attention from museums on a global level. The predominant VOCs emitted by the camphor wood (Cinnamomum camphora) samples are terpenes and their derivatives, typically D-(+)-Camphor (in the samples aged for 30 and 50 years), Eucalyptol (in the samples aged for 50 and 100 years), and α-Copaene (in the sample aged for 100 years). Each of the three components occupies at least 10% of TVOC emitted by the corresponding camphor wood samples. With traces of ozone coexisted, terpenes have a high potential to be oxidized to carboxylic acids which may also promote the corrosion, especially on lead and copper. The main corrosion products of lead and copper coupons are Pb10(CO3)6(OH)6O and CuO, respectively. As advanced instrumental methods, ToF-SIMS is helpful for deeper understanding of the corrosion process, while EDS provides useful information for generally judging the corrosion stage of the metallic coupons. The relations among volatile compounds, metallic coupons, and the environmental factors in the system need further investigations.
References
Atkinson R, Arey J (2003) Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos Environ 37:S197–S219
Borrego S, Molina A (2019) Fungal assessment on storerooms indoor environment in the National Museum of Fine Arts, Cuba. Air Qual Atmos Health 12:1373–1385
Calogirou A, Larsen BR, Kotzias D (1999) Gas-phase terpene oxidation products: a review. Atmos Environ 33:1423–1439
Chen W, Vermaak I, Viljoen A (2013) Camphor – a fumigant during the Black Death and a coveted fragrant wood in Ancient Egypt and Babylon – a review. Molecules 18:5434–5454
Costa V (2001) The deterioration of silver alloys and some aspects of their conservation. Stud Conserv 46:18–34
Ding N (2018) A survey report of collections pests on museums in Lingnan area. Museum 2:94–103 (in Chinese)
Fabbri K (2018) Indoor microclimate. In: Pretelli M, Fabbri K (eds) Historic indoor microclimate of the heritage buildings. Springer, Cham, pp 23–71
Fu Z (1955) Comments on the cultural relic conservation work in museums. Cult Relics 6:15–20 (in Chinese)
Ghiara G, Campodonico S, Piccardo P, Martini C, Storme P, Carnasciali MM (2014) Micro Raman investigation on corrosion of Pb-based alloy replicas of letters from the museum Plantin-Moretus, Antwerp. J Raman Spectrosc 45:1093–1102
Jia Z, Li Y, Bao T, Zhou T (2017) The effect of camphorwood volatiles on the durability of paper archives. China Pulp Paper 36:43–48 (in Chinese)
Lee C, Kim Y, Nagajyothi PC, Thammalangsy S, Goung S (2011) Cultural heritage: a potential pollution source in museum. Environ Sci Pollut Res 18:743–755
Li S (2012) Imperial archival vault: the archival repository of Ming and Qing dynasties. Beijing Arch 29:8–11 (in Chinese)
Oddy WA (1973) An unsuspected danger in display. Museums J 73:27–28
Oikawa T, Matsui T, Matsuda Y, Takayama T, Niinuma H, Nishida Y, Hoshi K, Yatagai M (2005) Volatile organic compounds from wood and their influences on museum artifact materials I. Differences in wood species and analyses of causal substances of deterioration. J Wood Sci 51:363–369
Oikawa T, Matsui T, Matsuda Y, Takayama T, Niinuma H, Nishida Y, Hoshi K, Yatagai M (2006) Volatile organic compounds from wood and their influences on museum artifact materials II: inference of causal substances of deterioration based on intercomparison of laser Raman spectra of deteriorated products. J Wood Sci 52:140–146
Peng K (2003) Corrosion mechanism and corrosive-inhibitive of lead ingot. Dissertation, Central South University (in Chinese)
Robinet L, Thickett D (2003) A new methodology for accelerated corrosion testing. Stud Conserv 48:263–268
Shen J, Shen Y, Xu F, Zhou X, Wu L (2018) Evaluating the suitability of museum storage or display materials for the conservation of metal objects: a study on the conformance between the deposited metal film method and the Oddy test. Environ Sci Pollut Res 25:35109–35129
t’Hart L, Storme P, Anaf W, Nuyts G, Vanmeert F, Dorriné W, Janssens K, de Wael K, Schalm O (2016) Monitoring the impact of the indoor air quality on silver cultural heritage objects using passive and continuous corrosion rate assessments. Appl Phys A Mater Sci Process 122:923
Tétreault J, Sirois J, Stamatopoulou E (1998) Studies of lead corrosion in acetic acid environments. Stud Conserv 43:17–32
Thickett D, Lee LR (1996) Selection of materials for the storage or display of museum objects, 1st edn. The British Museum Press, London
Wang W, Li D, Huang X, Yang H, Qiu Z, Zou L, Liang Q, Shi Y, Wu Y, Wu S, Yang C, Li Y (2019) Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 24:3792
Weschler CJ (2000) Ozone in indoor environments: concentration and chemistry. Indoor Air 10:269–288
Weschler CJ, Shields HC (1997) Measurements of the hydroxyl radical in a manipulated but realistic indoor environment. Environ Sci Technol 31:3719–3722
Wolkoff P, Clausen PA, Jensen B, Nielsen GD, Wilkins CK (1997) Are we measuring the relevant indoor pollutants? Indoor Air 7:92–106
Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD (2000) Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air 10:82–91
Wu K, Lin Y, Chai X, Duan X, Zhao X, Chun C (2019) Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci Nutr 7:2546–2555
Acknowledgments
The author sincerely acknowledges Shanghai Museum Research Project for providing financial support for this work.
Funding
This work was funded by Shanghai Museum Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Responsible Editor: Michel Sablier
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, J. Camphor wood, a potentially harmful museum storage material: an analytical study using instrumental methods. Environ Sci Pollut Res 28, 46458–46468 (2021). https://doi.org/10.1007/s11356-020-09446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09446-0