Abstract
Mussels are worldwide bioindicators in pollution monitoring since they fulfil the requirements for being good sentinels. However, some methodological concerns arise in the use of particular biomarkers, particularly those displaying low enzymatic rates and/or limited responsiveness to chemicals and biological-related variability. In the present study, the suitability of oxidative stress and detoxification parameters when using mussels as sentinels of polycyclic aromatic hydrocarbon (PAH) pollution is addressed. Present results show that the S9 subcellular fraction of the digestive gland in mussels is an adequate and convenient matrix where to measure most pollution-related biomarkers. Furthermore, this work constitutes the first evidence of the potential suitability of using particular carboxylesterase (CE) activities in determining PAHs exposure in mussels. This fact could imply the replacement of more controversial cytochrome P450 components (phase I oxidation), which are only measurable in microsomal fractions, by CEs (measured in S9 fractions) as good alternatives for phase I reactions in PAH-exposed mussels. Some methodological considerations, such as the need of including commercial purified proteins in biomarker determinations for quality assurance, are evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine ecosystems are the final sink for many land-based chemicals but also from activities carried out in their waters such as transportation, spillages and aquaculture. This raises concerns regarding the toxicological consequences of those compounds on aquatic wildlife and cultured species (Nilsen et al. 2019; Tornero and Hanke 2016). Legacy persistent organic pollutants (POPs) and polycyclic aromatic hydrocarbons (PAHs) are among the chemicals that are most commonly quantified to trace anthropogenic pollution (Viñas et al. 2012; Viñas et al. 2018). Nonetheless, there is a broad range of new drugs, known as emerging contaminants or contaminants of environmental concern, which have been more recently introduced into the wastewater systems and are likely to cause long-term chronic effects (Fabbri and Franzellitti 2016; Mezzelani et al. 2018). Due to their inefficient depuration or their recalcitrant properties, some of these products can also reach marine systems (Sanchez-Avila et al. 2009).
Mussels are long-recognised sentinels in marine pollution monitoring programmes given that they have properties that enable them to integrate and reflect local chemical pollution while allowing measuring the corresponding biochemical and physiological responses (Cajaraville et al. 2000; Martinez-Gomez et al. 2017). Mussel digestive glands (Dallares et al. 2018; Gonzalez-Rey and Bebianno 2014; López-Galindo et al. 2014) or whole organisms (Freitas et al. 2017, 2019) have been used to study the effects of pollutant exposures in both laboratory and field conditions. However, methodologically speaking, there is need to include purified proteins to ensure linearity of the biochemical measures and to use them as quality control of the protocols in laboratories that perform large scale monitoring studies. The adoption of quality control protocols is especially relevant in bivalves, and mussels in particular, since some enzymatic activities are lower than those in other groups of bioindicator species such as fish (Livingstone 1998; Hansson et al. 2017). For example, even if antioxidant defences and phase II conjugation enzymes such as glutathione S-transferases (GSTs) are well-established biomarkers in mussels (Regoli and Giuliani 2014), this is not the case for cytochrome P450–related activities (CYPs). Fluorometric-based CYP assays are commonly used in fish studies, and despite the existence of CYP-like-related activities in freshwater (Aguirre-Martinez et al. 2015; Faria et al. 2009) and marine bivalves other than Mytilus galloprovincialis (Falfushynska et al. 2018; Maranho et al. 2015; Pereira et al. 2012), their activities in molluscs are relatively low and sometimes doubted to be inducible by Aryl hydrocarbon receptor (AhR) agonists (Butler et al. 2001). For this reason, some authors question the use of measuring CYP-like catalytic activity in invertebrates (Hahn, 2002) and in the marine mussel M. galloprovincialis in particular (Faria et al. 2009). Other (well-represented) metabolising enzymes in bivalves might be good alternatives to explore. We hypothesise that this is the case of phase I carboxylesterases (CEs), involved in the hydrolysis of endogenous compounds but also in the detoxification of many exogenous chemicals including pesticides and drugs in mammalian systems (Satoh and Hosokawa 1998; Satoh and Hosokawa 2006; Wheelock et al. 2008; Fukami et al. 2010; Fukami and Yokoi 2012; Imai et al. 2006). Recent studies with bivalves have demonstrated their in vivo ability to metabolise drugs such as the retroviral Tamiflu® (Dallarés et al. 2019) and their in vivo response to environmental pesticides (Dallares et al. 2018) as well as in vitro sensitivity to pesticides and drugs including plastic additives (Sole et al. 2018a; Sole et al. 2018b; Sole and Sanchez-Hernandez 2018; Nos et al. 2020).
In the context of the increasing need to determine the degree of anthropogenic impact of marine systems, for which PAHs quantification is commonly used, the present study aimed (i) to facilitate monitoring programmes by identifying the cellular fraction on which most biomarkers could be adequately measured; (ii) to determine the suitability of using phase I (e.g., CEs) as biomarkers in mussels chronically exposed to PAHs as alternatives to CYP-related catalytic activities, whose relevance in bivalves is frequently discussed and (iii) to propose some methodological improvements when using traditional biomarkers in pollution monitoring with mussels as sentinels (i.e., the inclusion of purified proteins in the measures to validate protocols and allow comparisons in large scale monitoring programmes).
Material and methods
Mussel collection
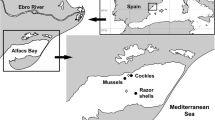
Mussels, Mytilus galloprovincialis, aimed for chemical and biochemical determinations were collected from the relatively PAH-free region of the Ebre Delta (NE Iberian Peninsula, Mediterranean Sea) at coordinates 40.622383; 0.668552 from aquaculture farms devoted to their commercialization. For the same purpose, wild specimens were additionally collected from the Barcelona harbour area (coordinates 41.377508N; 2.185741E), where natural populations are found despite the area being chronically polluted by PAHs. Additional samples were collected from this site to search for size activity relationships (n = 22). Samplings took place at the same time period (October–November 2017). Mussels from both locations were transported to the laboratory under cold conditions (~ 4 °C using ice blocks) and immediately dissected before their use in the experimental procedures described below.
Sample processing
Selection of the most suitable subcellular fraction
With the aim selecting a single subcellular fraction on which most of the potential pollution biomarkers could be analysed, a total of twenty four animals were collected at the Ebre Delta (PAH-free reference site) and six pools were made using four digestive glands in each. Pools were used in this specific case to ensure having enough microsomes to conduct the analyses. The S9 fraction was obtained by homogenising the samples in a phosphate buffer (100 mM, pH 7.4) containing 150 mM KCl, 1 mM ethylenediaminetetraacetic acid (EDTA), and 1 mM dithiothreitol (DTT) at a 1:5 (w:v) ratio and centrifuging at 10,000×g for 30 min. Resulting homogenate supernatants were further centrifuged at 100,000×g for 1 h to yield the cytosolic (supernatant) and microsomal (pellet) fractions as described in more detail by Sole and Livingstone (2005). Analyses were carried out (when possible) in each of the three fractions (cytosol, S9 and microsomes) in search of the most appropriate fraction for each assay. Antioxidant and CE activities were best measured in the S9 fraction (where results showed the lowest deviations and coefficient of variation) and thus this fraction was selected for the site comparison study (see “PAH-related site contrasts” section). Cytochrome P450–associated reductase activities were considered in S9 but only measurable in the microsomal fraction while CYP-related catalytic measures were only attempted in the microsomal fraction. Thus, these microsomal parameters were not further considered in the site comparison study.
PAH-related site contrasts
Individual digestive glands from mussels obtained at both (PAH-polluted and pristine reference) sites were dissected and immediately frozen individually in liquid nitrogen and stored at − 80 °C until analysis (n = 8 per site). Attention was paid to select animals of similar sizes to perform contrasts in bioaccumulation and enzymatic activities. For each sample, the S9 fraction was obtained following the protocol detailed before.
Biochemical determinations
Enzyme activity determinations were carried out in mussels of similar size (4.6 ± 0.21 and 4.7 ± 3.0 cm) to avoid the influence of biological traits in biomarker determinations. Antioxidant activities and CE measures were carried out spectrophotometrically on S9 fractions of digestive glands. For the first, measurements consisted on well adopted protocols for determinations for catalase (CAT) (Aebi 1984), glutathione reductase (GR) (Carlberg and Mannervik 1985), glutathione peroxidase (GPX) (Gunzler and Flohé 1985) and glutathione S-transferases (GSTs) (Habig et al. 1974). CEs were measured using four different commercial substrates, i.e., ρ-nitrophenyl acetate (ρNPA), ρ-nitrophenyl butyrate (ρNPB), α-naphthyl acetate (αNA) and α-naphthyl butyrate (αNB). The formation of ρ-nitrophenol by ρNPA and ρNPB was recorded at 405 nm as described in Hosokawa and Satoh (2005), and naphthol by αNA and αNB was measured at 235 nm according to the Mastropaolo and Yourno (1981) method. All protocols are described in more detail elsewhere (Dallares et al. 2018). Activities were all expressed nmol min−1 mg protein−1 except for CAT where results were expressed as μmol min−1 mg protein−1. Protein content was determined for each sample as described further below.
To ensure linearity of former measures following the adopted protocols, an 8-point concentration range of commercial purified proteins was considered for each enzymatic assay carried out which is the same methodological procedure as for the bivalve S9 biomarker determinations (see Table 1). This is considered a more accurate validation of the measures since there is no interference by other enzymes present in the S9 fraction of the homogenate.
Microsomal CYP-related determinations consisted in the following essays: (i) reductase activities, measured spectrophotometrically at 550 nm using NAD(P)H cytochrome c reductase and NADH-ferricyanide reductase as described by Sole and Livingstone (2005); (ii) catalytic O-deethylase activities of digestive gland CYPs, determined using fluorescent CYP-mediated substrates (ER 7-ethoxyresorufin, PR 7-pentoxyresorufin, BR 7-benzyloxyresorufin, MR methoxyresorufin; CEC 3-cyano-7-ethoxycoumarin); (iii) O-debenzyloxylase activity (BFCOD), using BFC (7-benzyloxy-4-trifluoromethylcoumarin) and (iv) O-debenzylase activity using DBF (dibenzylfluorescein). All assay conditions were adapted from fish studies and used 50 μL of mussel microsomes. To ensure that measurements could be attributable to CYP activity, an inhibition study was carried out using the broad CYP inhibitor ketoconazole. For this, microsomes were incubated for 30 min at room temperature with one of the three concentrations of ketoconazole (0.1, 1 and 10 μM) as described in detail by Koenig et al. (2013).
Total protein content of the different subcellular samples was determined by the Bradford method (Bradford 1976) adapted to microplate, using the Bradford Bio-Rad Protein Assay reagent and bovine serum albumin (BSA; 0.05–1 mg/mL) as standard. Absorbance was read at 595 nm.
All assays were carried out in a TECAN Infinite 200 microplate reader in 96-well plates in triplicate at 25 °C except for CYPs assays which were run at 30 °C. Only linear reactions were considered and these were registered using the kinetic assays mode of the Magellan V6.0 data analysis software.
Chemical analysis
A pool of about 10 g of whole soft tissue (corresponding to 5–6 mussels) was used for chemical characterisation and quantification of PAHs. The following PAHs were quantified in mussels from both pristine and polluted sites: phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo(a)anthracene (BaA), chrysene (Chr), benzo(e)pyrene (BeP), benzo(b)fluoranthene, benzo(b)fluoranthene (BkF), benzo(a)pyrene (BaP), benzo(ghi)perylene (BghiP), dibenz(ah)anthracene dB(ah)A and indeno(1,2,3-c,d)pyrene (IP). These correspond to 12 out of the 16 PAHs that the Environmental Protection Agency (US-EPA) recommends to monitor (Keith 2014). The methodology used for their quantification consisted of the Soxhlet extraction following HPLC with fluorescence detection and with the use of reference materials for quality assurance as described in detail elsewhere (Viñas et al. 2018). The limit of quantification was 0.25 ng/g wet weight (w.w.).
Statistics
Two pair comparisons were made using Student’s t test contrast after confirmation of parametric requirements (Shapiro-Wilk and Levene’s tests for normality and homoscedasticity of datasets, respectively). Correlation between biomarkers was made using Pearson’s correlation coefficient. Statistical analyses were carried out using the SPSS System Software v24 and the significance level for data analyses was set at α = 0.05. Data are presented as means ± SEM (standard error of mean).
Results and discussion
Mussel chemistry and enzymatic activities
The Ebre Delta is considered a pristine region as far as PAHs is concerned, while the Barcelona harbour is an area historically described as being heavily loaded with petrogenic PAHs (Porte et al. 2001). This was here confirmed through the chemical analysis of collected mussels, showing that the Ʃ13 PAHs reached 9 ng/g at the Ebre Delta and up to 565 ng/g at the Barcelona harbour (values in w.w.). The later value is over 5-fold higher than the 100 ng/g w.w. typically considered to correspond to heavily industrialised (and hence heavily polluted) areas (Viñas et al., 2009). Also, the calculation of diagnostic ratios between PAHs allowed us identifying their origin: petrogenic PAHs would include crude oils and refined crude oils such as gasolines, heating oils, coals or asphalt, while pyrogenic substances are the result of fires, internal combustion engines or furnaces. For instance, a Flu/(Flu+Pyr) ratio lower than 0.4 indicates petrogenic origin and it was much lower in the harbour mussel samples (0.05) than in the Ebre region mussels (0.4). Another ratio, Ant/(Ant+Pyr), sheds light on this subject, and while ratio values scoring below 0.1 is suggestive of a petrogenic origin, values over 1 indicate a pyrolytic source of PAHs. In the Barcelona harbour mussels, this value was 0.09. Altogether, data are supportive of the PAHs of the harbour being of the petrogenic source. Detailed chemical results are presented as supplementary material S1.
Since seasonality and animal size are factors described to affect biomarker activities, including some of the analysed here (Banni et al. 2009; Cravo et al. 2013; Uluturhan et al. 2019), only individuals from similar size and collected at the same time period were considered in the study. The enzyme activities of mussels S9 digestive gland fraction from these two sites showed differences in terms of antioxidant activities (Table 2). The GR, t-GPX and GSTs (antioxidant) activities were significantly increased in mussels from the harbour sampling site (p < 0.05). Antioxidant enzymes are frequently related to PAH exposure in bivalves (Regoli and Giuliani 2014). CE-related activities (Table 2) showed substrate-dependent responsiveness, while nitrophenyl substrates (ρNPA and ρNPB) did not significantly differ in mussels from the two sites; naphthyl-derived (αNA and αNB)-associated CE activities were impacted in those chronically exposed to PAHs (p < 0.05) and were highly correlated between them (r = 0.702; p < 0.05; n = 16). Since the different substrates are likely to inform on specific CE isoforms (Wheelock et al. 2008), the particular responses of naphthyl substrates suggest that they would be more adequate for PAH monitoring. To the best of our knowledge, this is the first time to relate PAH exposure to CE-dependent activities, although in mammals, CE responses are modulated by multiple xenobiotic receptors including AhR which is particular for PAHs (Zhang et al. 2012). However, it cannot be ignored that other chemicals present (e.g., metals) could be responsible for CE inhibition using these two particular substrates. Metals are regarded as B-esterase inhibitors in aquatic species, mostly from in vitro and fish studies (Frasco et al. 2005; Vieira et al. 2009; Oliva et al. 2012), although under field conditions, results are not so conclusive. Co-occurrence of higher loads of metals and petrogenic PAHs has been described in other harbours of the Iberian Peninsula (Perez-Fernandez et al. 2019).
Correlations between enzyme activities in mussels chronically exposed to PAHs
Not only there were differences in terms of total enzymatic activity in mussels collected at the two sites (Table 2), but correlations between biomarkers also differed in PAH-free and polluted sites considering a larger number of individuals from the polluted site (n = 22) since size did not affect these enzymatic activities and recent data from our group for the Ebre Delta site (Table 3). Former studies with mussels collected in the PAH-free Ebre Delta waters (with Ʃ13 PAHs levels < 10 ng/g w.w.) revealed a good correlation between biomarkers including CE-related measures using several substrates (Dallares et al. 2018). Wild mussels collected from the chronically PAH-polluted waters of the Barcelona harbour were also used to assess associations between the same biomarker activities. The lack of agreement of these formerly observed correlations (Dallares et al. 2018) with present results in chronically polluted mussels could support the particular modulation by the chemicals present in the harbour waters to the different CE isoforms. Among the antioxidant defences, CAT, GR and GSTs were positively correlated among each other (r = 0.428–0.556) but GPX was negatively correlated with GR (r = − 0.931) and GSTs (r = − 0.470). To the best of our knowledge, we are only aware of one study relating in vivo PAH exposure in fish (through water accommodated fraction (WAF)) and CEs (using ρNPB as substrate) as well as further exposure ex vivo in fish liver slices (De Anna et al. 2018). In this former study with rainbow trout, CE activity was inhibited by 42% in fish exposed in vivo to WAF for 48 h and the inhibitory action was further confirmed in ex vivo exposures. In another study with Atlantic killifish, inhabiting sites chronically polluted sites by PAHs, a low CYP1A activity expressed in this fish was associated to resistance to organophosphorus (OP) pesticides since the oxon metabolites were not formed (Clack and Di Giulio 2012), but no reference to CE activity was made. To the best of our knowledge, CE regulation by specific nuclear receptors has been studied only in rodents, with the involvement of the AhR in modulating certain CE isoforms (Zhang et al. 2012). Since mussels express low to undetectable CYP1A-related EROD activities but express other nuclear receptors involved in xenobiotic metabolism (Raingeard et al. 2013), the chronic action of PAHs and other chemicals of environmental concern on CEs activity and receptors modulation in bivalves deserves investigation. Mussel CE inhibition (using naphthyl substrates) in those collected in the harbour cannot be ascribed to PAHs or metal exposures (or both) and further independent exposures are needed to explore the mechanistic action of these environmental chemicals.
S9 determinations and CEs as alternative to CYPs components and activities
Antioxidant enzymes are mostly cytosolic but can also be measured in the post-mitochondrial S9 fraction, while the different CEs are either soluble or membrane-bound so they can be analysed in the three fractions (cytosol, S9 and microsomes). In all cases, these parameters showed good reaction rates and could be confidentially be measured. However, lower standard deviation and coefficient of variation on the S9 measures makes this easily obtained fraction more adequate (Table 4). Unspecific Cyt P450 reductases and CYP-related catalytic measures could only be reliably measured in the microsomal fraction as they are tightly associated to endoplasmic reticulum membranes. Thus, given that we aim to simplify monitoring protocols (by using, for example, a single subcellular fraction), these measurements were excluded from site comparisons. We could, however, observe that the activities (in nmol/min/mg prot) in mussels collected from the Ebre Delta followed the order NADPH cit c red. <NADH cit c red. <NADH-ferricyanide reductase as it was seen in other studies using invertebrates, including mussels (Sole and Livingstone 2005). CYP-related activities were only attempted in the microsomal fraction where these membrane-bound proteins are located. Eight fluorometric substrates, among those commonly used for measuring CYP-related activities in fish, were tested. No CYP-related activity was detected when using ER, BzRs, PR, MR and CEC as substrates (see “Materials and methods” section). Measurable fluorescence readings were only obtained when using DBF and BFC as substrates, although these measures were much lower than in fish (Sole et al. 2012). Using ketoconazole, a broad CYP inhibitor, at the concentrations of 0.1, 1 and 10 μM, BFC- or DBF-related activities were not affected (data not shown). This contrasts with previous results in fish, where a ketoconazole concentration of 10 μM causes a reduction in activity when using as substrates BFC (up to 70%) or DBF (68%) in fish (Koenig et al. 2013). Some studies report CYP-related activities in bivalves using DBF (Aguirre-Martinez et al. 2015; Almeida et al. 2015; Pereira et al. 2012). However, to our knowledge, none of these bivalve studies confirmed the CYP nature of the measures by using established CYP inhibitors or discuss the fact that maybe other enzymes could be responsible for metabolites formation. Using the CYP substrates traditionally used in fish, our observations support the lack of comparable CYP catalytic activities in M. galloprovincialis, despite the existence and purification of a CYP protein and its immunodetection in the marine mussel Mytilus edulis (Porte et al. 1995; Shaw et al. 2004) or CYP-related genes being identified in M. edulis (Zanette et al. 2013).
Because of all the above (i.e., CYPs requiring working with a unique subcellular microsomal fraction and the uncertainty of results being attributable to CYPs activities in bivalves), we suggest that biomarkers of pollution (antioxidant defences) can be confidentially measured in S9 and include CE determinations in this same fraction in future monitoring programmes using bivalves. This would have the advantage of using one single cellular fraction (S9), where all the biomarkers could be determined. CEs would be a good alternative to reductases since they are also general metabolic markers, as suggested by Satoh and Hosokawa (1998), that respond to many xenobiotics and seen in studies using the fish Solea senegalensis exposed to chemicals of environmental concern (Sole et al. 2014).
Validation of biomarker assays using commercial proteins
The use of commercial purified proteins showed good data linearity for most of the selected biomarker measures, following the particularity of the corresponding protocols and respecting the protein ranges shown in Table 1. Biomarker study results are often difficult to compare due to the use of different methodologies, nature of the extraction buffers, centrifugation steps and activities expression. Despite these limitations, the inclusion of protein standards could provide internal quality assurance on the biomarker measures in terms of good laboratory practices. At the present stage, biomarker contrasts of kinetic measures among labs are not yet possible due to the variable nature of the commercial protein standards available. However, within a same research group using the same methodology, this procedure is recommended in order to validate the quality and reproducibility of the multi-well measures in large scale comparative studies. This is a well-established practice in other fields such as chemistry, and the inclusion of purified protein in biomarker studies is, to our criteria, a good methodological practice.
Conclusions
CYP-related measurements in mussels, using the most common fish fluorometric substrates, are questionable. The present results suggest other phase I–related parameters, such as CE activities, as alternatives. This is supported by the fact that (i) they can be measured in S9 fractions (so they could be carried out along with the other biomarkers commonly considered in pollution monitoring) and that (ii) CE hydrolysis rates using any of the four proposed substrates (ρNPA, ρNPB, αNA and αNB) are high in mussel digestive glands, and they are responsive to PAHs exposure (as seen in the present study) as well to pesticides and other xenobiotics. In light of these evidences, we propose to include CE measurements as biomarkers, as well as the traditional antioxidant and biotransformation responses when using mussels as sentinels and the inclusion of purified proteins for quality assurance.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aguirre-Martinez GV, DelValls AT, Laura Martin-Diaz M (2015) Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Muller, 1774). Ecotox Environ Safe 120:142–154
Almeida A, Freitas R, Calisto V, Esteves VI, Schneider RJ, Soares AMVM, Figueira E (2015) Chronic toxicity of the antiepileptic carbamazepine on the clam Ruditapes philippinarum. Comp Biochem Physiol C 172-173:26–35
Banni M, Bouraoui Z, Ghedira J, Clearandeau C, Jebali J, Boussetta H (2009) Seasonal variation of oxidative stress biomarkers in clams Ruditapes decussatus sampled from Tunisian coastal areas. Environ Monit Assess 155:119–128
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Butler RA, Kelley ML, Powell WH, Hahn ME, Van Beneden RJ (2001) An aryl hydrocarbon receptor (AHR) homologue from the soft-shell clam, Mya arenaria: evidence that invertebrate AHR homologues lack 2,3,7,8-tetrachlorodibenzo-p-dioxin and beta-naphthoflavone binding. Gene 278:223–234
Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:295–311
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Clark BW, Di Giulio RT (2012) Fundulus heteroclitus adapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology 21:465–474
Cravo A, Lopes B, Serafim A, Company R, Barreira L, Gomes T, Bebianno MJ (2013) Spatial and seasonal biomarker responses in the clam Ruditapes decussatus. Biomarkers 18:30–43
Dallares S, Carrasco N, Alvarez-Munoz D, Rambla-Alegre M, Sole M (2018) Multibiomarker biomonitoring approach using three bivalve species in the Ebro Delta (Catalonia, Spain). Environ Sci Pollut Res 25:36745–36758
Dallarés S, Montemurro N, Pérez S, Rodríguez-Sanchez N, Solé M (2019) Preliminary results on the uptake and biochemical response to water-exposure of Tamiflu® (oseltamivir phosphate) in two marine bivalves. J. Toxicol. Environ. Health Part A 82:75–85
De Anna JS, Leggieri LR, Arias Darraz L, Carcamo JG, Venturino A, Luquet CM (2018) Effects of sequential exposure to water accommodated fraction of crude oil and chlorpyrifos on molecular and biochemical biomarkers in rainbow trout. Comp Biochem Physiol C 212:47–55
Fabbri E, Franzellitti S (2016) Human pharmaceuticals in the marine environment: focus on exposure and biological effects in animal species. Environ Toxicol Chem 35:799–812
Falfushynska H, Sokolov EP, Haider F, Oppermann C, Kragl U, Ruth W, Stock M, Glufke S, Winkel EJ, Sokolova IM (2018) Effects of a common pharmaceutical, atorvastatin, on energy metabolism and detoxification mechanisms of a marine bivalve Mytilus edulis. Aquat Toxicol 208:47–61
Faria M, Carrasco L, Diez S, Riva MC, Bayona JM, Barata C (2009) Multi-biomarker responses in the freshwater mussel Dreissena polymorpha exposed to polychlorobiphenyls and metals. Comp Biochem Physiol C 149:281–288
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2005) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10:360–375
Freitas R, Coppola F, Henriques B, Wrona F, Figueira E, Pereira E, Soares AMVM (2017) Does pre-exposure to warming conditions increase Mytilus galloprovincialis tolerance to Hg contamination? Comp Biochem Physiol C 203:1–11
Freitas R, Coppola F, Costa S, Pretti C, Intorre L, Meucci V, Soares AMVM, Solé M (2019) The influence of temperature on the effects induced by triclosan and diclofenac in mussels. Sci Total Environ 663:992–999
Fukami T, Yokoi T (2012) The emerging role of human esterases. Drug Metab Pharmacokinet 27:466–477
Fukami T, Takahashi S, Nakagawa N, Maruichi T, Nakajima M, Yokoi T (2010) In Vitro evaluation of inhibitory effects of antidiabetic and antihyperlipidemic drugs on human carboxylesterase activities. Drug Metab Dispos 38:2173–2178
Gonzalez-Rey M, Bebianno MJ (2014) Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquat Toxicol 148:221–230
Gunzler WA, Flohé L (1985) Glutathione peroxidase. In: Greenwald RA (ed) Handbook of methods for oxyradical research. CRC Press, pp 285–290
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. J Biol Chem 249:7130–7139
Hahn ME (2002) Biomarkers and bioassays for detecting dioxin-like compounds in the marine environment. Sci Total Environ 289:49–69
Hansson T, Thain J, Martinez-Gomez C, Hylland K, Gubbins M, Balk L (2017) Supporting variables for biological effects measurements in fish and blue mussel. ICES Techniques in Marine Environmental Sciences 60:1–22
Hosokawa M, Satoh T (2005) Measurement of carboxylesterase (CES) activities. In: Costa LG, Hodgson E, Lawrence DA, Ozolins TR, Reed DJ, Greenlee WF (eds) Current protocols in toxicology. John Wiley & Sons chapter 4, unit 4.7.
Imai T, Taketani M, Shii M, Hosokawa M, Chiba K (2006) Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos 34:1734–1741
Keith LH (2014) The source of U.S.EPA’ s sixteen PAH priority pollutants. Polycycl Aromat Compd:37–41
Koenig S, Guillen K, Sole M (2013) Comparative xenobiotic metabolism capacities and pesticide sensitivity in adults of Solea solea and Solea senegalensis. Comp Biochem Physiol C 157:329–336
Livingstone DR (1998) The fate of organic xenobiotics in aquatic ecosystems: quantitative and qualitative differences in biotransformation by invertebrates and fish. Comp. Biochem. Physiol. A 120:43–49
López-Galindo C, Ruiz-Jarabo I, Rubio D, Nebot E, Solé M, Mancera J (2014) Temperature enhanced effects of chlorine exposure on the health status of the sentinel organism Mytilus galloprovincialis. Environ Sci Pollut Res 21:1680–1690
Maranho LA, Andre C, DelValls TA, Gagne F, Martin-Diaz ML (2015) In situ evaluation of wastewater discharges and the bioavailability of contaminants to marine biota. Sci Total Environ 538:876–887
Martinez-Gomez C, Robinson CD, Burgeot T, Gubbins M, Halldorsson HP, Albentosa M, Bignell JP, Hylland K, Vethaak AD (2017) Biomarkers of general stress in mussels as common indicators for marine biomonitoring programmes in Europe: the ICON experience. Mar Environ Res 124:70–80
Mastropaolo W, Yourno J (1981) An ultraviolet spectrophotometric assay for α-naphthyl acetate and α-naphthyl butyrate esterases. Anal Biochem 115:188–193
Mezzelani M, Gorbi S, Regoli F (2018) Pharmaceuticals in the aquatic environments: evidence of emerged threat and future challenges for marine organisms. Mar Environ Res 140:41–60
Nilsen E, Smalling KL, Ahrens L, Gros M, Miglioranza KSB, Pico Y, Schoenfuss HL (2019) Critical review: grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ Toxicol Chem 38:46–60
Nos D, Navarro J, Saiz E, Sanchez-Hernandez JC, Solé M (2020) Tetrabromobisphenol A inhibits carboxylesterase activity in marine organisms from different trophic levels. Chemosphere 238:124592
Oliva M, Perales JA, Gravato C, Guilhermino L, Galindo-Riano MD (2012) Biomarkers responses in muscle of Senegal sole (Solea senegalensis) from a heavy metals and PAHs polluted estuary. Mar Pollut Bull 64:2097–2108
Pereira CDS, Martin-Diaz ML, Catharino MGM, Cesar A, Choueri RB, Taniguchi S, Abessa DMS, Bicego MC, Vasconcellos MBA, Bainy ACD, Sousa ECPM, DelValls TA (2012) Chronic contamination assessment integrating biomarkers’ responses in transplanted mussels-A seasonal monitoring. Environ Toxicol 27:257–267
Perez-Fernandez B, Vinas L, Besada V (2019) A new perspective on marine assessment of metals and organic pollutants: a case study from Bay of Santander. Sci Total Environ 691:156–164
Porte C, Lemaire P, Peters LD, Livingstone DR (1995) Partial-purification and properties of cytochrome-P450 from digestive gland microsomes of the common mussel, Mytilus edulis. Mar Environ Res 39:27–31
Porte C, Sole M, Borghi V, Martinez M, Chamorro J, Torreblanca A, Ortiz M, Orbea A, Soto M, Cajaraville MP (2001) Chemical, biochemical and cellular responses in the digestive gland of the mussel Mytilus galloprovincialis from the Spanish Mediterranean coast. Biomarkers 6:335–350
Raingeard D, Bilbao E, Cancio I, Cajaraville MP (2013) Retinoid X receptor (RXR), estrogen receptor (ER) and other nuclear receptors in tissues of the mussel Mytilus galloprovincialis: cloning and transcription pattern. Comp Biochem Physiol A 165:178–190
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Sanchez-Avila J, Bonet J, Velasco G, Lacorte S (2009) Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci Total Environ 407:4157–4167
Satoh T, Hosokawa M (1998) The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol 38:257–288
Satoh T, Hosokawa M (2006) Structure, function and regulation of carboxylesterases. Chem Biol Interact 162:195–211
Shaw JP, Large AT, Donkin P, Evans SV, Staff FJ, Livingstone DR, Chipman JK, Peters LD (2004) Seasonal variation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis. Aquat Toxicol 67:325–336
Sole M, Livingstone DR (2005) Components of the cytochrome P450-dependent monooxygenase system and ‘NADPH-independent benzo a pyrene hydroxylase’ activity in a wide range of marine invertebrate species. Comp Biochem Physiol C 141:20–31
Sole M, Sanchez-Hernandez JC (2018) Elucidating the importance of mussel carboxylesterase activity as exposure biomarker of environmental contaminants of current concern: an in vitro study. Ecol Indic 85:432–439
Sole M, Vega S, Varo I (2012) Characterization of type “B” esterases and hepatic CYP450 isoenzimes in Senegalese sole for their further application in monitoring studies. Ecotox Environ Safe 78:72–79
Sole M, Fortuny A, Mananos E (2014) Effects of selected xenobiotics on hepatic and plasmatic biomarkers in juveniles of Solea senegalensis. Environ Res 135:227–235
Sole M, Bonsignore M, Rivera-Ingraham G, Freitas R (2018a) Exploring alternative biomarkers of pesticide pollution in clams. Mar Pollut Bull 136:61–67
Sole M, Rivera-Ingraham G, Freitas R (2018b) The use of carboxylesterases as biomarkers of pesticide exposure in bivalves: a methodological approach. Comp Biochem Physiol C 212:18–24
Tornero V, Hanke G (2016) Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull 112:17–38
Uluturhan E, Darilmaz E, Kontas A, Bilgin M, Alyuruk H, Altay O, Sevgi S (2019) Seasonal variations of multi-biomarker responses to metals and pesticides pollution in M. galloprovincialis and T. decussatus from Homa Lagoon, Eastern Aegean Sea. Mar Pollut Bull 141:176–186
Vieira LR, Gravato C, Soares A, Morgado F, Guilhermino L (2009) Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behaviour. Chemosphere 76:1416–1427
Vinas L, Franco MA, Soriano JA, Gonzalez JJ, Ortiz L, Viñas L, Franco MA, Soriano JA, González JJ, Ortiz L, Bayona JM, Albaigés J (2009) Accumulation trends of petroleum hydrocarbons in commercial shellfish from the Galician coast (NW Spain) affected by the Prestige oil spill. Chemosphere 75: 534–541
Viñas L, Franco A, Blanco X, Bargiela J, Soriano JA, Perez-Fernandez B, Jose Gonzalez J (2012) Temporal and spatial changes of PAH concentrations in Mytilus galloprovincialis from Ria de Vigo (NW Spain). Environ Sci Pollut Res 19:529–539
Viñas L, Perez-Fernandez B, Soriano JA, Lopez M, Bargiela J, Alves I (2018) Limpet (Patella sp) as a biomonitor for organic pollutants. A proxy for mussel? Mar Pollut Bull 133:271–280
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178
Zanette J, Jenny MJ, Goldstone JV, Parente T, Woodin BR, Bainy ACD, Stegeman JJ (2013) Identification and expression of multiple CYP1-like and CYP3-like genes in the bivalve mollusk Mytilus edulis. Aquat Toxicol 128:101–112
Zhang Y, Cheng X, Aleksunes L, Klaassen CD (2012) Transcription factor-mediated regulation of carboxylesterase enzymes in livers of mice. Drug Metab Dispos 40:1191–1197
Acknowledgements
M. Sole and R. Freitas are members of CYTED network (ref. 419RT0578). Coral Hispano from the Barcelona Aquarium is thanked for providing mussel samples.
Funding
This work was financed by the Spanish Ministry of Economy, Industry and Competitivity (ref CGL2016-76332-R MINECO/FEDER, UE). Programa Operacional Competitividade e Internacionalização FEDER (POCI-01-0145-FEDER-028425) BISPECIAl - BIvalveS under Polluted Environment and ClImate chAnge.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Solé, M., Freitas, R., Viñas, L. et al. Biomarker considerations in monitoring petrogenic pollution using the mussel Mytilus galloprovincialis. Environ Sci Pollut Res 27, 31854–31862 (2020). https://doi.org/10.1007/s11356-020-09427-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09427-3