Abstract
Wide application of nanoparticles causes considerable environmental, health, and safety problems. However, their potential impact and mechanisms on plant growth are not completely clear. In the present study, the effects of different concentration of copper oxide nanoparticles (nCuO) on seed germination and seedling growth, as well as physiological parameters of Brassica pekinensis L., were investigated. The seeds were exposed to 10-, 100-, and 1000-mg L−1 nCuO suspensions and 0.8-mg L−1 Cu2+ released from 1000-mg L−1 nCuO for 7 day. The results showed that nCuO did not affect the germination rate, germination potential, and germination index of B. pekinensis but significantly affected the vitality index. The growth of roots and shoots of B. pekinensis was promoted at 10-mg L−1 nCuO, while they were inhibited under 1000-mg L−1 nCuO and Cu2+ ion treatments, and roots suffered more damage than shoots. Cu content in shoots and roots of B. pekinensis increased with increasing concentrations of nCuO, which is significantly higher in roots as compared with shoots. Roots and shoots accumulated more Cu under nCuO treatments compared with Cu2+ ion treatment. nCuO treatments led to significant lignification in roots of B. pekinensis. Furthermore, nCuO increased in the contents of soluble sugar and protein in shoots, while nCuO at 1000 mg L−1 significantly inhibited the content of soluble protein in roots. In addition, concentration-dependent augmentation of lipid peroxidation, hydrogen peroxide and superoxide generation, and antioxidant enzyme activity were noticed in shoots and roots of B. pekinensis seedlings under nCuO and Cu2+ ion treatments. Altogether, the results strongly suggested that the phytotoxicity of nCuO in B. pekinensis was caused by both the nanoparticles itself and the released Cu2+ ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper oxide nanoparticles (nCuO), one of the most important nano-products, has been successfully applied to many fields, such as electronic equipment, wood preservatives, anti-fouling paints, antimicrobials, and fungicides for agriculture (Rajput et al. 2018). Conservative and optimistic estimates of the total global market for nCuO from 2010 to 2025 were 7075 and 14,320 tons, respectively (Liu et al. 2018). However, along with the production, use, and disposal of nCuO-related products, a large number of nCuO inevitably release into the environment, which may pose risks for the ecological system including plants (Hou et al. 2017). Therefore, the impact of nCuO released into the environment is receiving increasing concern.
Plants are a potential pathway for transportation and accumulation of nanoparticles in the whole ecosystems. Many researches have reported different effects of various Cu-based nanoparticles on plants. For example, Cu nanoparticles could enhance the growth and yield of wheat (Hafeez et al. 2015). Cu-based nanoparticles did not inhibit seed germination of maize (Yang et al. 2015), common bean (Duran et al. 2017), and Arabidopsis thaliana (Wang et al. 2016). However, exposure to nCuO significantly inhibited the germination of cucumber (Wu et al. 2012), tomato (Ahmed et al. 2018), and Raphanus and Lactuca (Ko and Kong 2014). nCuO also inhibited plant growth of Brassica juncea (Nair and Chung 2015a), eggplant (Baskar et al. 2018), tomato (Ahmed et al. 2018), and Brassica rapa (Chung et al. 2019). In separate studies, the concentration of nCuO reported by Shaw and Hossain (2013) (higher than 40 mg L−1) and Costa and Sharma (2016) (higher than 100 mg L−1) inhibited the growth of rice. Wang et al. (2016) reported that Col-0 was the more sensitive ecotype to nCuO compared with Bay-0 and Ws-2, due to the different sensitivities of different species. In addition, Yang et al. (2015) found that nCuO phytotoxicity against maize and rice varied with varying concentrations of nCuO. Tiwari et al. (2019) demonstrated that lower dosages of nCuO might be beneficial for growth and development of rice seedlings, while seedling growth was decreased significantly at higher concentrations of nCuO. Moreover, Sperdouli et al. (2019) found that nCuZn has different photosynthetic efficiency and reactive oxygen species (ROS) generation in leaves of different ages in A. thaliana. The contradictory results implied that the phytotoxicity of nanoparticles is associated with plant species, nanomaterials’ concentration, plant growth age, etc.
A large number of studies have shown that nCuO phytotoxicity is related to the disturbance of metabolism in plants. Exposure to nCuO significantly reduced total chlorophyll and sugar contents and increased lipid peroxidation, resulting in a significant reduction of retardation of root growth and plant biomass of A. thaliana (Nair and Chung 2014) and B. rapa (Chung et al. 2019). nCuO inhibited plant growth by reducing the maximal quantum yield of photosystem II and transpiration rate in microalgae and barley (Che et al. 2018; Rajput et al. 2018) and by mediating root tip damage in Allium cepa (Deng et al. 2016). Hossain et al. (2015) found that nCuO damaged cells of root surface, induced the excessive accumulation of ROS-disturbed defense mechanisms, and eventually led to growth retardation of rice. Exposure to nCuO reduced the shoot and root growth due to excess lignification in B. juncea plants in a dose-dependent manner (Nair and Chung 2015a). In addition, nCuO caused cell death and callose formation and decreased the micro nutrient contents in Brassica napus seedlings (Nair and Chung 2017).
At present, there is a wide debate on the mechanisms of nCuO toxic effects, whether owing to nanoparticles themselves or the released soluble toxic metal ions (García-Gómez et al. 2017). Studies performed on A. thaliana (Tang et al. 2016) and Chlorella pyrenoidosa (Zhao et al. 2016) indicated that Cu2+ ions released from nCuO contributed partial toxicity to plant growth. Dimkpa et al. (2012) reported that the impaired growth of wheat could be explained by the dissolved Cu2+ ions from nCuO. Perreault et al. (2014) and Che et al. (2018) also found that the release of Cu2+ ions was identified as a main reason for the inhibition of growth and photosynthesis of Lemna gibba and microalgae. However, previous studied showed nCuO could be taken up by roots and translocated to shoot in plants. nCuO rather than soluble Cu2+ ions caused reduction in growth of soft-stem bulrush (Zhang et al. 2014), chickpea (Nair and Chung 2015b), buckwheat (Lee et al. 2013) and Eichhornia crassipes (Zhao et al. 2017). Therefore, whether the toxicity of nCuO is the result of the nanoparticles themselves, or an ion effect, or other factors is not clear. This contradictory makes it confusing for us to understand the nature of nCuO toxicity as well as their toxicity mechanisms.
Brassica pekinensis L. is one of the most important vegetables in Asia with high nutritional values and economic benefits, and now, its consumption demand is gradually increasing in Western countries. Studies have shown that B. pekinensis is more likely to absorb heavy metals than other vegetables (Li et al. 2019), and it is often used in seed germination toxicity tests (Xiang et al. 2015). However, to our knowledge, there is little information about the influences of nCuO on the phytotoxicity of B. pekinensis. Furthermore, it is unknown where does the toxicity of nCuO come from the nanoparticles, the ions, or a combination of both. Thus, the objective of this study was to estimate the effect of nCuO at different concentrations on seed germination and seedling growth of B. pekinensis. The Cu accumulation, contents of ROS, malondialdehyde (MDA), osmotic adjustment, lignin, and antioxidant system were also investigated in B. pekinensis, with response to nanoparticle treatment. Since it has been reported from previous studies that the toxicity mechanisms of nCuO are also associated to the released metal ions, the toxic effects of nCuO were compared with the toxicity of Cu2+ ions (0.8 mg L−1, released from the highest concentration of nCuO). This study will enhance our understanding of phytotoxicity and the mechanism of toxicity of nanoparticles and help in the development of predictive models for the safe design of nanomaterials and for the understanding nano-biological interactions.
Materials and methods
Characterization of nCuO
The nCuO was purchased from Beijing Deke DaoJin Science and Technology Co. Ltd., China. The compounds were characterized for their structure and morphology by X-ray diffractometry (XRD) and scanning electron microscopy (SEM) according to Costa and Sharma (2016). The XRD patterns of the powdered samples were recorded using a Rigaku D/MAX-2500/PC X-ray diffractometer (Rigaku Company, Tokyo, Japan) with Cu Kα radiation (λ = 1.5418 Å), and SEM images of the samples were taken using a JEOL JSM6360LA scanning electron microscope (JEOL Company, Tokyo, Japan).
Preparation of nCuO suspensions and Cu ion solution
The nCuO particles were suspended directly in deionized water and dispersed by ultrasonic vibration (100 W, 40 kHz) for 30 min according to Wang et al. (2016). Different doses of nCuO suspensions (10, 100, and 1000 mg L−1) were prepared in advance. This range includes lower dose (10 mg L−1) and higher dose (1000 mg L−1). A total of 10-mg L−1 nCuO represents environmentally relevant concentration, and higher dose (> 10 mg L−1, even up to 1000 mg L−1) was often used to evaluate the phytotoxicity of metal oxide nanoparticles (NPs) according to the US Environmental Protection Agency’s guidelines (Yang et al. 2015). These dose values are on log scale to ensure observation of relevant phytotoxic responses (Ahmed et al. 2018; Costa and Sharma 2016; Lin and Xing 2007). Cu2+ solution was prepared by dissolving CuSO4·5H2O in deionized water.
Quantification of dissolved Cu
Ten-, 100-, and 1000-mg L−1 nCuO suspensions were shaken at 150 rpm for different periods of time (0, 4, 8, 12, 24, 48, 72, 96, 120, 144, and 168 h), and then centrifuged at 10,000×g for 20 min. The supernatants were filtered by a glass membrane (0.22 μm). The Cu concentration of the filtrates was determined using flame atomic absorption spectrophotometry (FAAS, PerkinElmer AA800, USA).
Plant exposure to nCuO
Seeds of B. pekinensis were purchased from Linqu Tianhe Seed Industry Co. Ltd., China. Seeds were surface sterilized in 5.0% NaClO (v/v) for 15 min and then rinsed thoroughly with deionized water. Seeds of B. pekinensis were put into 9-cm petri dishes containing 5 mL of different concentrations (0, 10, 100, and 1000 mg L−1) of nCuO and 0.80 mg L−1 of Cu2+ ions (the amount of Cu2+ ions released from 1000-mg L−1 nCuO to the deionized water) and placed in the dark in the growth chamber (LGZ-450Y, LVBO Co. Hangzhou, China) at 25 ± 1 °C for 7 days. Each dish contained 20 seeds. There were 5 treatments, and each treatment had six replicates.
In addition, another batch of seeds were sowed for each treatment. Samples of shoots and roots were collected after 7 days of incubation and stored at − 80 °C for biochemical analysis.
Seed germination and seedling growth
In the germination experiment, when the root was up to 2 mm, seeds were considered as germinated. After 7 days of incubation, germination and seedlings growth parameters were determined. Germination rate (GR), germination potential (GP), germination index (GI), vitality index (VI), fresh weight (FW), and dry weight (DW) of shoots and roots were assayed as previously described by Ren et al. (2020). The length of shoots and roots was measured by image analysis using the Image Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The average length, FW, and DW of shoots and roots for 10 plants were a replicate.
Metal content
The Cu content in roots and shoots was determined by flame atomic absorption spectrophotometry (FAAS, PerkinElmer AA800, USA) according to He et al. (2014). At the same time, a blank digest was performed. For analytical quality assurance and quality control purposes, the certified reference material (CRM) green tea (GBW 10052, National Research Center for Certified Reference Materials, Beijing, China) was used.
Lignin, soluble sugar, and protein contents
Lignin content was extracted and determined according to Liu et al. (2019). Lignin content was expressed as OD280 g−1 DW.
Soluble sugar content in fresh root and shoot samples was estimated using the anthrone reagent method according to Rahmani et al. (2016). Glucose was used as the standard.
Protein content in fresh root and shoot samples was measured in accordance with the method of He et al. (2014). Bovine serum albumin was used as the standard.
In vivo detection of ROS
In vivo detection of superoxide (O2−·) formation in shoot and root of B. pekinensis plants was done by staining with nitroblue tetrazolium (NBT), which forms dark blue insoluble formazan when it reacts with superoxides (Nair and Chung 2014). In vivo imaging of hydrogen peroxide (H2O2) formation in shoot and root was evaluated using histochemical staining with 3′3′-diaminobenzidine (DAB) (Nair and Chung 2014). The shoots were cleared from chlorophylls after NBT and DAB staining by boiling for 20 min in acetic acid-glycerol-ethanol (1:1:3 (v/v/v)) solution in a dry bath.
MDA and H2O2 contents and O2−· generation
Contents of MDA, H2O2, and O2−· generation in fresh root and shoot samples were determined according to He et al. (2014). MDA and H2O2 contents and O2−· generation were all expressed as μmol g−1 FW.
Antioxidant enzyme activity
Fresh samples (0.1 g) were homogenized in 1-mL 50-mM potassium phosphate buffer (pH 7.8) containing 1% polyvinylpyrrolidone and 1.33-mM EDTA using a chilled pestle and mortar. The homogenate was centrifuged at 12,000×g for 20 min at 4 °C, and the supernatant was collected for the analyses of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) activities as previously described by He et al. (2014). One unit of SOD activity was defined as the amount of enzyme that would inhibit 50% of NBT photoreduction at 560 nm. One unit POD, CAT, and APX activities was defined as an absorbance change of 0.1, 0.1, and 0.1 units in 1 min at 470 nm, 240 nm, and 290 nm, respectively.
Statistical analyses
Each treatment was conducted with at least four replicates, and all the results were presented as mean ± standard deviation. The data were analyzed using analysis of variance (one-way ANOVA) and Duncan’s multiple range test at 5% level using SPSS (Version 21.0 SPSS Inc. Chicago, IL, USA) to assess statistical significance between means. The correlation analysis was also performed using the SPSS statistical software.
Results
nCuO characterization
The nanoparticle characterization studies revealed that the shape of nCuO was spherical, and the size was estimated as < 40 nm using SEM (Fig. 1a) and applying Scherer’s formula to the XRD data (Fig. 1b).
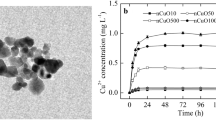
With the increase of time, Cu2+ dissolved from different concentrations of nCuO in the media increased significantly and reached equilibrium after 48 h (Fig. 2). The dissolved Cu2+ from 1000-mg L−1 nCuO was more than that from 10- and 100-mg L−1 nCuO, with a maximum of 0.8 mg L−1 (Fig. 2).
Seed germination
GR, GP, and GI were not affected under all nCuO treatments and Cu2+ ion treatment (Table 1). Compared with the control, 10-mg L−1 nCuO treatment increased VI by 11.53%, whereas 100- and 1000-mg L−1 nCuO treatments decreased VI by 5.88% and 65.39%, respectively. In addition, Cu2+ ion treatment also significantly decreased VI, but it was 2.32-fold higher than that of 1000-mg L−1 nCuO treatment.
Seedling growth
Compared with the control, 10-mg L−1 nCuO treatment increased root and shoot length by 10.86% and 7.95%, respectively, whereas 1000-mg L−1 nCuO treatment significantly decreased root and shoot length, which was 64.25% and 8.03% lower than those of the control (Table 2). The Cu2+ ion treatment also significantly inhibited the root length, which was 19.29% lower than that of the control, but it was 2.26-fold higher than that of 1000-mg L−1 nCuO treatment, indicating that exposure to the equivalent concentration of dissolved Cu2+ resulted in significantly lower toxicity. In addition, Cu2+ ion treatment did not greatly affect the shoot length of B. pekinensis as compared with the control (Table 2).
As shown in Table 2, compared with the control, 10-mg L−1 nCuO treatment increased the fresh and dry weight of roots by 22.65% and 13.58%, respectively, while higher concentrations of nCuO had adverse effects on root weight. At 1000-mg L−1 nCuO, fresh and dry weight of roots were decreased by 23.42% and 30.86%, respectively, as compared with the control. The results also showed that nCuO at all concentrations had negligible effects on fresh weight of shoots, but 10-mg L−1 nCuO treatment significantly increased dry weight of shoots compared with the control. In addition, Cu2+ ion treatment decreased fresh and dry weight of roots by 13.11% and 20.58% as compared with the control, whereas those were 13.54% and 14.88% higher than 1000-mg L−1 nCuO treatment, respectively. However, Cu2+ ion treatment had no obvious effect on fresh and dry weight of shoot compared with the control (Table 2).
Cu content
Figure 3 showed that all nCuO treatments exhibited higher Cu content in both shoots and roots compared with the control, and Cu content in roots was much higher than that in shoots. However, compared with the control, Cu2+ ion treatment had no obvious effect on Cu content in shoots and roots (Fig. 3).
Lignification of roots
nCuO significantly increased lignin content in roots in a dose-dependent manner (Fig. 4). When compared with the control, 100- and 1000-mg L−1 nCuO enhanced lignin content by 10.00% and 20.92%, respectively. However, Cu2+ ion treatment had no significant impact on lignin content in roots in comparison with the control (Fig. 4).
Soluble sugar and protein contents
As shown in Fig. 5 a and b, soluble sugar and protein contents in shoots significantly increased with the increase of nCuO concentration. Compared with the control, 1000-mg L−1 nCuO increased the soluble sugar and protein contents by 28.79% and 31.07%, respectively. In roots, only 1000-mg L−1 nCuO significantly increased the soluble sugar content. The soluble protein content in roots showed an initial increase and subsequent decrease with the increase of nCuO concentration. Compared with the control, 10-mg L−1 nCuO increased the soluble protein content by 20.37%, but 1000-mg L−1 nCuO significantly decreased it by 41.34%. Cu2+ ion treatment had no significant impact on soluble sugar and protein contents in shoots, but it increased them by 32.19% and 16.71% in roots, respectively, compared with the control (Fig. 5 a and b).
In vivo detection of ROS generation
The effect of different concentrations of nCuO on O2−· and H2O2 formations in shoots and roots of B. pekinensis plants was qualitatively analyzed using in vivo histochemical staining with NBT and DAB. A gradual increase in O2−· formation as evidenced by an increase in dark blue coloration was detected in shoots and roots of plants as a result of increasing concentrations of nCuO exposure (Fig. 6 a and b). Similarly, shoots and roots after staining with DAB were brown, indicating H2O2 generation (Fig. 6 c and d). The most serious staining was observed in shoots and roots of 1000-mg L−1 CuO-treated seedlings.
Effect of different concentrations of nCuO on superoxides generation in shoots (a) and roots (b) of B. pekinensis as revealed by NBT staining and hydrogen peroxide formation in shoots (c) and roots (d) of B. pekinensis as revealed by DAB staining (left to right: shoots and roots of B. pekinensis plants exposed to 0, 0.8 mg L−1 of Cu2+ and 10, 100, 1000 mg L−1 of nCuO)
MDA, H2O2, and O2−· contents
MDA, H2O2, and O2−· contents in roots and shoots showed a continual increase with the increase of nCuO concentrations (Fig. 7 A, B, and C). The maximum increase in MDA, H2O2, and O2−· contents at 1000-mg L−1 nCuO treatment was 2.56 folds, 1.81 folds, and 1.43 folds in roots, and 1.26 folds, 1.52 folds, and 1.65 folds in shoots of the control, respectively. Compared with the control, Cu2+ ion treatment increased the MDA, H2O2, and O2−· contents in roots by 25.14%, 22.19%, and 19.65%, whereas it had no significant impact on MDA, H2O2, and O2−· contents in shoots (Fig. 7 A, B, and C). Compared with 1000-mg L−1 nCuO, Cu2+ ion treatment led to lower MDA, H2O2, and O2−· contents in roots and shoots.
Antioxidant enzyme activity
The antioxidant enzyme activities of B. pekinensis were found to be differentially modulated by nCuO treatment (Fig. 8). In shoots, SOD, POD, CAT, and APX activities showed no significant change under 10- and 100-mg L−1 nCuO treatments, but increased by 13.58%, 18.29%, 19.68%, and 13.44% at 1000-mg L−1 nCuO treatment, respectively, compared with the control.
In roots, the activities of POD, CAT, and APX exhibited similar trends with those of shoots under different concentrations of nCuO treatment. Unlike the pattern of CAT, APX, and POD activities observed, SOD activity increased significantly at 10- and 100-mg L−1 nCuO concentrations, then decreased at 1000-mg L−1 nCuO, but it was still markedly higher than that in the control. Compared with the control, Cu2+ ions markedly promoted the activities of SOD, CAT, APX, and POD in roots, whereas these enzymes’ activities were lower than those in 1000-mg L−1 nCuO treatment. However, it had no significant impact on SOD, CAT, APX, and POD activities in shoots (Fig. 8A–D).
Discussion
Phytotoxicity of nCuO
In recent decades, the increasing use of nano-products has raised concerns about their impacts on crop plants and human health as well as ecological effects. The seed germination test has become a direct, rapid, and reliable biological test method to assess phytotoxicity (Zhang et al. 2015). Nanoparticles have solubility and could release metal ions, which leads to their more complex environmental behavior or toxic effects.
Previous studies have shown that Cu-based nanoparticles had no impact on seed germination, such as maize (Yang et al. 2015), common bean (Duran et al. 2017), and A. thaliana (Wang et al. 2016). Lin and Xing (2007) found that nZnO did not affect the germination of radish, rape, ryegrass, lettuce, and cucumber seeds. López-Luna et al. (2019) also reported that wheat seed germination was not affected by Ag@CoFe2O4 nanoparticles. Similar to these observations, our results showed that 10–1000-mg L−1 nCuO had no significant impact on GR, GP, and GI of B. pekinensis (Table 1). Wang et al. (2016) suggested that seed coats have selective permeability, which can protect the seed from harmful external factors. Nevertheless, Wu et al. (2012) found the treatment of nCuO significantly inhibited the germination of cucumber. Ko and Kong (2014) also found that 1-mg L−1 nCuO treatment almost completely inhibited seed germination of Raphanus and Lactuca. In addition, seed germination of rice was promoted at lower concentration of nCuO and inhibited significantly at higher concentration of nCuO (Costa and Sharma 2016; Tiwari et al. 2019). Furthermore, Andersen et al. (2016) found that eight of the 10 species tested responded to nTiO2, and 5 species responded to nCeO2. So, it is obvious that no general patterns were observed in seed germination under nCuO, and restriction of seed germination varied with the applied nCuO concentration as well as with the plant species. In addition, this study showed that VI of B. pekinensis was increased by 10-mg L−1 nCuO while decreased by 100- and 1000-mg L−1 nCuO (Table 1). Similar results were also found in eggplant (Baskar et al. 2018) and tomato (Ahmed et al. 2018) under nCuO treatment. Additionally, no marked negative effect of Cu2+ ion treatment on GR, GP, and GI of B. pekinensis was observed compared with the control (Table 1). Although Cu2+ ion treatment significantly inhibited the VI of B. pekinensis, it showed much less inhibition than 100- and 1000-mg L−1 nCuO treatment. These results implied that the toxicity of higher concentrations of nCuO to VI was caused by not only dissolved Cu2+ released but also by nCuO particle itself.
The present study showed that 10-mg L−1 nCuO markedly increased the length of roots and shoots (Table 2), as well as fresh and dry weight of B. pekinensis (Table 2). This indicated that nCuO at lower concentrations can promote B. pekinensis growth. However, 1000-mg L−1 nCuO significantly reduced root and shoot growth of B. pekinensis, and the inhibition of roots was more obvious than that of shoots (Table 2). The reason might be that nCuO was in direct contact with plant roots (Rahmani et al. 2016). This result was consistent with earlier studies in eggplant (Baskar et al. 2018), tomato (Ahmed et al. 2018), A. thaliana (Nair and Chung 2014), and B. napus (Rahmani et al. 2016). López-Luna et al. (2019) reported that Ag@CoFe2O4 nanoparticles inhibited root and shoot lengths of wheat seedlings. Mahajan et al. (2011) found that nZnO retarded the seedling growth of V. radiate and Ccicer arietinum. Similar result was also reported with the application of nano-iron oxide on soybean. In addition, Cu2+ ion treatment decreased root and shoot length of B. pekinensis, whereas the inhibition by Cu2+ ion treatment was less than 1000-mg L−1 nCuO treatment, indicating that Cu2+ ions released from nCuO produced some toxicity to plant growth.
Mechanism of phytotoxicity by nCuO
Copper, one of the important nutrient elements, can promote plant growth at an appropriate concentration. Excessive copper has adverse impacts on plant growth and development (Vatansever et al. 2017). The result in this study indicated that Cu content in roots and shoots of B. pekinensis seedlings was dose-dependent under different concentrations of nCuO treatment, and Cu content was much higher in roots than in shoots (Fig. 3), which may be one of the reasons that roots exhibit greater inhibition than shoots (Table 2). Similar results were also found in B. rapa (Chung et al. 2019). The Cu contents in both roots and shoots under all nCuO treatments were significantly higher than that under Cu2+ ion treatment. Yanik and Vardar (2015) suggested that NPs can penetrate to the cortex layer and endodermis and accumulate in the cells of the central cylinder of roots. This may be explained that roots could absorb directly nCuO and transport it to shoots (Wang et al. 2012). Correlation analysis showed that root and shoot length of B. pekinensis were markedly negatively correlated with Cu content in the roots (R = − 0.794, P < 0.05) and shoots (R = − 0.674, P < 0.05), respectively. Therefore, excess Cu accumulation by B. pekinensis may be responsible for the growth inhibition from nCuO at higher concentrations. Ogunkunle et al. (2019) also found nCu became phytotoxic to the root and shoot of cowpea in a concentration-dependent manner due to the accumulation in roots and shoots. Increased lignification of roots resulting in roots growth retardation under excessive nanoparticle treatment has been reported from many plants (Nair and Chung 2014). Similarly, it was observed in this study that 100- and 1000-mg L−1 nCuO significantly increased lignin content in roots (Fig. 4). Correlation analysis also showed that root length of B. pekinensis was negatively correlated with the lignin content (R = − 0.857, P < 0.05). Lignin deposition has been found in roots of tomato and cauliflower under nCuO treatment (Singh et al. 2017) and A. thaliana (Nair and Chung 2014). Moreover, the inhibition of nAl2O3 (Yang et al. 2015) and nZnO (Prakash and Chung 2016) stress on root growth of Triticum aestivum was related to lignin accumulation. However, the lignin content in the roots of B. pekinensis did not change significantly under Cu2+ ion treatment (Fig. 4). Therefore, it could be assumed that the enhanced lignification was one of the reasons for the decrease in root growth of B. pekinensis exposure to nCuO. In addition, the inhibition in root growth under nCuO stress might be due to hormonal imbalances (Nair and Chung 2014) and DNA damage (Rajeshwari et al. 2015). Many previous studies indicate that heavy metal stress causes changes in osmotic balance in plants (Singh et al. 2016). The accumulation of solutes under stress, such as soluble sugars and soluble protein, can stabilize the cell structure and function by stabilizing osmotic balance of cytoplasm and interacting with cellular macromolecules (Ahmed et al. 2018). The present result indicated that soluble sugar content in roots and shoots increased under Cu2+ ions and higher concentrations of nCuO treatments (Fig. 5a). Our finding is consistent with previous study on B. napus seedlings under nCuO treatment (Rahmani et al. 2016). Sugar accumulation was also found in B. napus (Rahmani et al. 2016) under nZnO treatment. Moreover, soluble protein content in roots increased under Cu2+ ions and 10- and 100-mg L−1 nCuO treatments and decreased under 1000-mg L−1 nCuO, while it constantly increased in shoots (Fig. 5b). The increase in protein content was probably owing to the de novo synthesis of new proteins or the prevention of protein degradation by the accumulation of sugar (Amist et al. 2017). Similar to our results, soluble protein contents in tomato roots and shoots showed different trends under nCuO treatment (Ahmed et al. 2018).
Many studies have reported that nanoparticles could enter through cell membrane, and their accumulation in the cytoplasm may trigger ROS overproduction and lead to oxidative stress in various plants (Singh et al. 2017). In this study, we found that nCuO and Cu2+ ions induced the generation of H2O2 and O2−· in vivo by quantitative and qualitative assessments (Figs. 6 and 7). Through the Haber-Weiss reaction, H2O2 and O2−· can be transformed to the highly reactive oxidant OH·, which causes lipid peroxidation in plant cell (Costa and Sharma 2016). Nanoparticle toxicity is reported to induce the increase of MDA in A. thaliana (Tang et al. 2016). Similar results were also detected in this study. The increase of ROS and MDA contents demonstrate that nCuO induced oxidative stress in B. pekinensis. In addition, a high and negative correlation coefficient (R = − 0.895, P < 0.05) was found between MDA content and root length of B. pekinensis, indicating that the oxidative damage caused by nCuO was another reason for reduced root growth of B. pekinensis. In accordance with our results, exposure to nAg and other nanoparticles resulted in oxidative stress in different plant species (Andersen et al. 2016). The increased ROS generation and lipid peroxidation as observed in this study might be due to the uptake and direct interaction of nCuO with different subcellular compartments of the cell (Nair and Chung 2015a). Moreover, MDA and H2O2 contents treated with 1000-mg L−1 nCuO were significantly higher than those under Cu2+ ion treatments, suggesting that the nCuO itself was the main factor that caused membrane damage on B. pekinensis by nCuO.
Plants have evolved a complex antioxidant mechanism (including SOD, CAT, POD, and APX) to protect cells from oxidative damage (Costa and Sharma 2016). The current results showed an increased SOD activity in shoots and roots of B. pekinensis under Cu2+ ions and low concentrations of nCuO treatments; however, the SOD activity in roots was decreased at 1000-mg L−1 nCuO treatment (Fig. 8A). Similar observations were also found by Yasur and Rani (2013) and Costa and Sharma (2016). According to Singh et al. (2016), the upregulated SOD activity can be owing to de novo synthesis of enzyme protein, and the decreased SOD activity at highest concentration of nCuO treatment might be attributed to enzyme damage by the excessive ROS. Our data revealed that CAT, POD, and APX activities in roots increased significantly under higher concentrations of nCuO and Cu2+ ion treatments (Fig. 8B, C, and D). Similar result was also found in Lemna minor (Song et al. 2016) and wheat (Dimkpa et al. 2012). The high activities of POD, CAT, and APX enable the plants to scavenge excessive H2O2 (Faizan et al. 2018). Even so, the observed increase in CAT, POD, and APX activities in roots does not seem to give full protection to the nCuO-treated seedlings in scavenging excessive ROS (Figs. 6 and 7). In contrast, some other studies differ from our results, which reported a reduction in CAT activity under nCuO stress (Hong et al. 2015; Singh et al. 2017). Tamez et al. (2019) found that APX and CAT activities decreased in zucchini roots treated with nCu. Chen et al. (2016) also found that the activities of CAT and APX in pumpkin were significantly reduced under nNd2O3 treatment. Thus, it is difficult to confirm positive or negative impacts of nanoparticles on various enzymes, which are related to the type, nature, and concentration of nanoparticles.
Conclusions
In summary, nCuO (10–1000 mg L−1) had no significant effect on GR, GP, and GI of B. pekinensis. However, the effects of nCuO on VI and seedling growth exhibited a concentration-dependent manner. The Cu content in shoots and roots of B. pekinensis seedlings significantly increased under nCuO exposure and was substantially greater than that under Cu2+ ion treatment. Also, higher concentration of nCuO treatments led to excessive levels of lignin, soluble sugar, and soluble protein; ROS and lipid peroxidation; and antioxidant enzyme activities. Overall, our results suggested that the inhibitory impact on seed germination and seedling growth upon exposure to nCuO was associated with high Cu accumulation, oxidative damage, and lignification. The phytotoxicity was caused by nCuO derived from nCuO itself and released Cu2+ ions. The present study confirmed the potential adverse effects of nCuO at higher concentration on B. pekinensis. The results of the present research can contribute in better understanding of the behavior and toxic mechanism of nCuO in plants. Therefore, the concentrations of nCuO should be taken into account for environmental safety when using it.
References
Ahmed B, Khan MS, Musarrat J (2018) Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): a study on growth dynamics and plant cell death. Environ Pollut 240:802–816
Amist N, Singh NB, Yadav K, Singh SC, Pandey JK (2017) Comparative studies of Al3+ ions and Al2O3 nanoparticles on growth and metabolism of cabbage seedlings. J Biotechnol 254:1–8
Baskar V, Nayeem S, Kuppuraj SP, Muthu T, Ramalingam S (2018) Assessment of the effects of metal oxide nanoparticles on the growth physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech 8(8):362–373
Che X, Ding R, Li Y, Zhang Z, Gao H, Wang W (2018) Mechanism of long-term toxicity of CuO NPs to microalgae. Nanotoxicology 12(8):923–939
Chen X, O’Halloran J, Jansen MA (2016) The toxicity of zinc oxide nanoparticles to Lemna minor (L.) is predominantly caused by dissolved Zn. Aquat Toxicol 174:46–53
Chung IM, Rekha K, Venkidasamy B, Thiruvengadam M (2019) Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut 230:48
Costa MVJD, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54(1):110–119
Deng F, Wang S, Xin H (2016) Toxicity of CuO nanoparticles to structure and metabolic activity of Allium cepa root tips. Bull Environ Contam Toxicol 97(5):702–708
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ (2012) CuO and ZnO nanoparticles: phytotoxicity metal speciation and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14(9):1125–1139
Duran NM, Savassa SM, Lima RGD, de Almeida E, Linhares FS, van Gestel CA, Pereira de Carvalho HW (2017) X-ray spectroscopy uncovering the effects of Cu based nanoparticle concentration and structure on Phaseolus vulgaris germination and seedling development. J Agr Food Chem 65(36):7874–7884
Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56(2):678–686
García-Gómez C, Obrador A, González D, Babín M, Fernández MD (2017) Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci Total Environ 589:11–24
Hafeez A, Razzaq A, Mahmood T, Jhanzab HM (2015) Potential of copper nanoparticles to increase growth and yield of wheat. J Nanosci Adv Technol 1(1):6–11
He J, Ren Y, Chen X, Chen H (2014) Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L) under cadmium stress. Ecotoxicol Environ Saf 108:114–119
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ Sci Process Impacts 17(1):177–185
Hossain Z, Mustafa G, Komatsu S (2015) Plant responses to nanoparticle stress. Int J Mol Sci 16(11):26644–26653
Hou J, Wang X, Hayat T, Wang X (2017) Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ Pollut 221:209–217
Ko KS, Kong IC (2014) Toxic effects of nanoparticles on bioluminescence activity seed germination and gene mutation. Appl Microbiol Biotechnol 98(7):3295–3303
Lee S, Chung H, Kim S, Lee I (2013) The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut 224(9):1668–1678
Li FR, Dian WEN, Wang FH, Sun FF, Wang X, Du YQ, Liu XX, Wan K (2019) Derivation of soil Pb/Cd/As thresholds for safety of vegetable planting: a case study for pakchoi in Guangdong Province, China. J Integr Agr 18(1):179–189
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150(2):243–250
Liu J, Dhungana B, Cobb GP (2018) Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ Toxicol Chem 37(1):11–20
Liu Y, Lv H, Yang N, Li Y, Liu B, Rensing C, Dai J, Fekin IB, Wang L, Mazhar S, Kehinde SB, Xu J, Su J, Zhang R, Wang R, Fan Z, Feng R (2019) Roles of root cell wall components and root plaques in regulating elemental uptake in rice subjected to selenite and different speciation of antimony. Environ Exp Bot 163:36–44
López-Luna J, Cruz-Fernández S, Mills DS, Martínez-Enríquez AI, Solís-Domínguez FA, del Carmen G-CM, Carrillo-González R, Martinez-Vargas S, Mijangos-Ricardez OF, del Carmen C-DM (2019) Phytotoxicity and upper localization of Ag@CoFe2O4 nanoparticles in wheat plants. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06668-9
Mahajan P, Dhoke SK, Khanna AS (2011) Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Ccicer arietinum) seedling using plant agar method. J Nanotechnol 1(1):1–7
Nair PMG, Chung IM (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ Sci Pollut Res 21:12709–12722
Nair PMG, Chung IM (2015a) Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L). Ecotoxicol Environ Saf 113:302–313
Nair PMG, Chung IM (2015b) Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant 37:1–11
Nair PMG, Chung IM (2017) Evaluation of stress effects of copper oxide nanoparticles in Brassica napus L. seedlings. 3. Biotech 7:293
Ogunkunle CO, Bornmann B, Wagner R, Fatoba PO, Frahm R, Lützenkirchen-Hecht D (2019) Copper uptake, tissue partitioning and biotransformation evidence by XANES in cowpea (Vigna unguiculata L) grown in soil amended with nanosized copper particles. Environ Nanotechnol Monit Manag 12:100231
Perreault F, Samadani M, Dewez D (2014) Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology 8:374–382
Prakash MG, Chung IM (2016) Determination of zinc oxide nanoparticles toxicity in root growth in wheat (Triticum aestivum L.) seedlings. Acta Biol Hung 67:286–296
Rahmani F, Peymani A, Daneshvand E, Biparva P (2016) Impact of zinc oxide and copper oxide nano-particles on physiological and molecular processes in Brassica napus L. Indian J Plant Physiol 21(2):122–128
Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A (2015) Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip effects of oxidative stress generation and biouptake. Environ Sci Pollut Res 22(14):11057–11066
Rajput V, Minkina T, Fedorenko A, Sushkova S, Mandzhieva S, Lysenko V, Duplii N, Fedorenko G, Dvadnenko K, Ghazaryan K (2018) Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci Total Environ 645:1103–1113
Ren Y, Wang W, He J, Zhang L, Wei Y, Yang M (2020) Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxcol Environ Saf 187:109785
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93(6):906–915
Singh A, Singh NB, Hussain I, Singh H, Yadav V, Singh SC (2016) Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J Biotechnol 233:84–94
Singh A, Singh NB, Hussain I, Singh H (2017) Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var botrytis. J Biotechnol 262:11–27
Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H (2016) Effects of CuO nanoparticles on Lemna minor. Bot Stud 57(1):3–10
Sperdouli I, Moustaka J, Antonoglou OS, Adamakis ID, Dendrinou-Samara C, Moustakas M (2019) Leaf age-dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 12(15):2498
Tamez C, Hernandez-Molina M, Hernandez-Viezcas JA, Gardea-Torresdey JL (2019) Uptake transport and effects of nano-copper exposure in zucchini (Cucurbita pepo). Sci Total Environ 665:100–106
Tang Y, He R, Zhao J, Nie G, Xu L, Xing B (2016) Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ Pollut 212:605–614
Tiwari PK, Shweta SAK, Singh VP, Prasad SM, Ramawat N, Tripathi DK, Chauhan DK, Rai AK (2019) Liquid assisted pulsed laser ablation synthesized copper oxide nanoparticles (CuO-NPs) and their differential impact on rice seedlings. Ecotoxicol Environ Saf 176:321–329
Vatansever R, Ozyigit II, Filiz E (2017) Essential and beneficial trace elements in plants and their transport in roots: a review. Appl Biochem Biotechnol 181(1):464–482
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L). Environ Sci Technol 46(8):4434–4441
Wang Z, Xu L, Zhao J, Wang X, White JC, Xing B (2016) CuO nanoparticle interaction with Arabidopsis thaliana: toxicity parent-progeny transfer and gene expression. Environ Sci Technol 50(11):6008–6016
Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ (2012) Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J Pet Environ Biotechnol 3(4):126–130
Xiang L, Zhao HM, Li YW, Huang XP, Wu XL, Zhai T, Yuan Y, Cai QY, Mo CH (2015) Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ Sci Pollut Res 22(14):10452–10462
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants maize (Zea mays L) and rice (Oryza sativa L). Int J Environ Res Public Health 12(12):15100–15109
Yanik F, Vardar F (2015) Toxic effects of aluminum oxide (Al2O3) nanoparticles on root growth and development in Triticum aestivum. Water Air Soil Pollut 226(9):296
Yasur J, Rani PU (2013) Environmental effects of nano silver: impact on castor seed germination, seedling growth, and plant physiology. Environ Sci Pollut Res 20(12):8636–8648
Zhang D, Hua T, Xiao F, Chen C, Gersberg RM, Liu Y, Ng WJ, Tan SK (2014) Uptake and accumulation of CuO nanoparticles and CdS/ZnS quantum dot nanoparticles by Schoenoplectus tabernaemontani in hydroponic mesocosms. Ecol Eng 70:114–123
Zhang R, Zhang H, Tu C, Hu X, Li L, Luo Y, Christie P (2015) Phytotoxicity of ZnO nanoparticles and the released Zn (II) ion to corn (Zea mays L) and cucumber (Cucumis sativus L) during germination. Environ Sci Pollut Res 22(14):11109–11117
Zhao J, Cao X, Liu X, Wang Z, Zhang C, White JC, Xing B (2016) Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: adhesion uptake and toxicity. Nanotoxicology 10(9):1297–1305
Zhao J, Ren W, Dai Y, Liu L, Wang Z, Yu X, Zhang J, Wang X, Xing B (2017) Uptake, distribution, and transformation of CuO NPs in a floating plant Eichhornia crassipes and related stomatal responses. Environ Sci Technol 51:7686–7695
Funding
This study was funded by the National Natural Science Foundation of China (31460100, 31660477, and 31360413), Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX19-0635 and SJCX19-0650), and Scientific Research Foundation for Talents of Changzhou University (201709 and 201710).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Ren, Y., He, J. et al. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L.. Environ Sci Pollut Res 27, 31505–31515 (2020). https://doi.org/10.1007/s11356-020-09338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09338-3