Abstract
A protocol for an ultra-rapid screening toxicity test is described using the rotifer Philodina acuticornis/roseola. The test can be executed in 30 min starting from the rehydration of desiccated life stages called tuns. Philodina tuns remain viable for years when maintained dry and at low temperature. They are very useful for conducting toxicity tests because the test animals do not require cultivation and are available to initiate tests anytime and anywhere. The swimming/crawling activity of rehydrated Philodina tuns is used as an endpoint to compare activity in control dilution water with inhibition of activity in an environmental sample. The Rotifer Activity Inhibition Test (RAIT) estimates toxicity semi-quantitatively using four toxicity categories: non-toxic, slightly toxic, very toxic, and 100% toxic. As proof of principle, RAIT has been tested on environmental samples from a variety of habitats and RAIT results have been compared with those obtained from traditional toxicity tests with bacteria, algae, Daphnia, and fish. Broad congruence between the effect signals of the rapid RAIT screening test and traditional assays has been found for river surface waters, industrial wastewaters, and sludge leachates from waste water treatment plants. Rotifers are an important group of animals in aquatic and soil food webs, and RAIT is a welcome new method for simple, ultra-rapid, and low-cost toxicity screening with a representative of this ecologically important group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental toxicity testing
In the history of ecotoxicology, toxicity testing has been conducted from two different viewpoints which can be classified as fundamental versus applied, respectively.
In the fundamental approach, the objective of toxicity testing is to investigate the toxicity of individual chemicals, i.e., to determine the magnitude of their effects on biota exposed to increasing concentrations of the chemical during a defined exposure period.
During the last century, many thousands of tests have been performed in this regard with the following variables: (a) the test species, (b) the chemical, (c) the test conditions, (d) the time of exposure, and (e) the toxicity endpoint (Newman 2020). The combination of these 5 variables has generated thousands of scientific publications, the data of which can be found in ecotoxicological journals and textbooks. Although each of the studies has its own merits, the findings have limitations with regard to extrapolation of the effects of environmental pollution to real world situations.
In the practical approach, scientists are trying to address the magnitude of the effects of real world aquatic and terrestrial pollution caused by the discharge of chemicals, as assessed by toxicity tests on selected species at either organismal or suborganismal level.
During the last decades, a variety of toxicity tests have been developed and are implemented in many countries in which the toxicity of waters and soils is monitored geographically and temporally to follow the changes in toxic hazards.
Contrary to the fundamental approach of toxicity testing in which the aspects of the speed, convenience, and costs of the tests are often not considered, these factors can be a major financial obstacle for the practical performance of toxicity tests. In practical applications, exposure time is another factor determining how widely a toxicity test is implemented in the real world. For certain applications, a quick answer is needed so that mitigation can be initiated to limit toxic impacts.
Rapid toxicity tests
During the last half century, a substantial number of rapid toxicity tests have been developed with different test species, including bacteria, algae, protozoa, rotifers, crustaceans, and fish. The impact of toxicants is typically studied on various biological activities (physiological, metabolic, or behavioral) with short exposure times of minutes to a few hours.
Several of these rapid toxicity tests are available commercially, and in 2003 and 2006, the US EPA contracted the company Battelle to perform a study on rapid bioassays in the framework of their Environmental Technology Verification program (ETV). The following commercially available rapid assays were included in these 2 studies: Deltatox, Microtox, ToxTrak, Polytox, ToxScreen, Daphnia IQ, Biotox, Abratox, ToxiChromo, LuminoTox, and Rapidtoxkit.
All these tests were applied on various types of contaminants like industrial chemicals, pesticides, rodenticides, pharmaceuticals, nerve agents, and biological toxins which were added in different concentrations to drinking water. The objective of the ETV study was to determine the sensitivity of the rapid tests for these chemicals and to see which of them could detect the lethal toxicity of the analyzed chemicals following consumption of 250 ml of the contaminated water by a person of 75-kg weight. Reports for each of the rapid tests applied in these 2 studies—which also address the aspects of their practicality and costs—have been published by the EPA and can be found in the US EPA ETV Archives.
It is beyond the scope of this article to address the many papers dealing with the development and specific applications of rapid toxicity tests. Information on a number of these rapid assays can be found in 2 reviews (Persoone et al. 2004; Persoone 2006), which detail tests based on bioluminescent, enzymatic, behavioral, and physiological endpoints.
Regardless of the merits of each of these rapid tests, even taking into account that some of them have a sensitivity similar to that of conventional toxicity tests of much longer duration, the reality is that to date only a few rapid toxicity tests are used worldwide for repeated daily toxicity analyses and monitoring.
Despite the speed of the answers in detecting and quantifying toxicity, rapid tests indeed have their own technical or financial obstacles which limit their large-scale applicability.
A major disadvantage of many rapid tests is that they are dependent on the continuous culturing/maintenance of live stocks of the test species (with the intrinsic infrastructure and work load required). In some of these tests, this drawback is eliminated by the use of dormant or immobilized stages of the test species, which can be hatched or reactivated. However, this does not eliminate the often intensive workload for the preparatory steps of the assay, nor the equipment needed their performance.
The most widely utilized rapid test that is employed worldwide is the bacterial luminescence inhibition assay, mostly known under the name “Microtox.” This 30-min test has been in use for more than 40 years and is based on the marine bacterium Aliivibrio fischeri and is commercialized by different companies under different names.
The rapid luminescence inhibition test has been published by ISO in 2009 as a standard test (ISO 11348-3), but despite its present international use, it unfortunately also has its own drawbacks. For example, the lyophilized test species is a marine bacterium that has to be shipped and kept frozen until the performance of the assay. The analyzed samples must first be adjusted to “seawater salinity,” which can change their properties and alter their toxicity. The method makes use of a quite expensive luminometer provided with a cooling block in order to perform the assay at a specific temperature, and it has limitations when testing colored or turbid materials that interfere with the bioluminescence emitted.
An alternative kinetic flash assay was developed by Lappalainen et al. (1999, 2001) which does not suffer from color nor turbidity interferences (ISO 2010) and in which the luminescence is measured even more rapidly than in the 30-min luminescence inhibition test.
Ultra-rapid toxicity tests
During the last few years, a team of scientists of RECETOX at the Faculty of Science of Masaryk University in Brno, Czech Republic, worked out a further improvement of the kinetic flash assay procedure with the aid of a small portable battery-operated luminometer. The results of this “ultra-rapid” test procedure are available after 30 s of exposure (Masner et al. 2017). This publication reports on the use of an alternative bioluminescent bacterium, namely the freshwater Photorhabdus luminescens, in comparison to the marine Aliivibrio fischeri, and shows that the sensitivity of both test species is comparable. The use of the freshwater bacterial test species is definitely an advantage since it bypasses the salinization requirement of the A. fischeri test and also allows performance of the P. luminescens assay at room temperature, eliminating the need to keep the stocks frozen.
Yet, these authors indicate that “there are limitations in the interpretation of bacterial bioluminescence assays for real world applications,” such as whole effluent toxicity testing (WET). Inhibition of luminescence is indeed the endpoint evaluated in all bioluminescence tests, but the data of their own study show that WET testing based on luminescence often shows inhibitions as well as stimulations. One of the conclusions of this publication is therefore that “further research and debate on the interpretation of stimulatory responses in bacterial bioluminescence is needed” and this is hence an important question mark for this new promising ultra-rapid toxicity test
Another ultra-rapid toxicity testing approach is the Bioluminescent Enzyme System Technology (BEST). This assay uses bacterial coupled enzyme systems (NADH:FMN oxidoreductase and luciferase) as biosensors in replacement of intact bacterial cells. This assay which is unique in its kind since it is “test species independent” is more than 30 years old. It has been the subject of intense research at the Laboratory of Bioluminescence Technologies of the Siberian Federal University in Krasnoyarsk, Russia (Kratasyuk 1990). More than 50 publications have been written on this enzymatic test, the references of which can be found in a review article by Kratasyuk and Esimbekova (2015).
The ultra-rapid BEST test is a very simple and practical assay which gives results in a few minutes. A multitude of BEST tests have been performed over the years in the Laboratory for Bioluminescence Technologies for a large array of applications including environmental studies, medical diagnostics, safety monitoring and control of food quality, biotechnology, and education.
In 2018, a collaboration on the BEST test was established between the Laboratory of Bioluminescence Technologies in Russia and the Laboratory for Environmental Toxicology and Aquatic Ecology of the Ghent University in Belgium with the goal to further optimize the test procedure and determine its sensitivity and precision for assays on wastewater samples.
During 2018, hundreds of BEST tests were performed in both laboratories, whereby various technological and experimental changes have been tried.
The outcome of these investigations revealed that satisfactory results can be obtained with regard to sensitivity, but there is in turn substantial variability between replicates and repeated tests which require further investigation.
Need for simple, practical, low-cost rapid toxicity tests for routine applications
All the considerations given above on rapid toxicity tests indicate that none of them actually meets the criteria which are prerequisite for a toxicity test that can be routinely applied at large scale. Such a test must indeed fulfill the aspects of simplicity, practicality, sensitivity, precision, and costs.
Since unfortunately this is not yet the case with the rapid toxicity tests that are presently available, we decided to investigate a new rapid test that would fulfill these prerequisites to the largest extent possible.
Development of a new rapid toxicity test with a bdelloid rotifer species
In aquatic environments, rotifers are quantitatively one of the most abundant groups of animals. They play a major role in ecological processes like nutrient cycling and are a significant food source for larval and adult fish. Rotifers also are abundant in soils and in water films and water-filled pores where they play a key role in nutrient cycling.
A variety of toxicity tests with rotifers have been developed over the last half century. Several recent review papers summarize the broad range of approaches for toxicity tests with rotifer species, which range from molecular and physiological to behavioral and population endpoints (Snell and Janssen 1995; Dahms et al. 2011; Rico-Martinez et al. 2013; Rico-Martinez et al. 2016; Won et al. 2017; Snell and Marcial 2017).
Rapid toxicity tests with rotifers have been developed, but they are virtually all based on the use of resting eggs (cysts) of brachionid rotifers (in particular Brachionus calyciflorus for freshwater tests and Brachionus plicatilis for marine tests). These rapid cyst-based rotifer tests bypass the need for culturing/maintenance of live stocks of test animals, but the hatching of the cysts takes about 1 day to obtain the live animals to start the assays. This hatching step is an inherent handicap from the point of view of a rapid testing since it requires a 24-h preliminary step.
Whereas sexual reproduction in monogonont rotifers leads to the production of cysts, bdelloid rotifers do not reproduce sexually and do not produce diapausing cysts. Instead, many species survive unfavorable conditions (starvation, low temperature, desiccation) by anhydrobiosis. These bdelloid species can indeed desiccate in a few hours whereby they alter their morphology and physiology and enter a state of anhydrobiosis as a result of water loss. The dry rotifers have a compact shape called a xerosome or tun and fully recover their crawling and swimming activity within a few minutes after rehydration.
The anhydrobiosis ability of bdelloid rotifers (which also exists in some tardigrade and nematode species) has been the subject of a large number of fundamental studies, and a first review was published more than a century ago (Jacobs 1909).
The findings that bdelloid rotifers survive desiccation, remain viable in the dry state for years, and are reactivated in minutes upon rehydration have recently attracted the interest of ecotoxicologists. A first study in this regard (Robles-Vargas and Snell 2010) compared the effects of anhydrobiosis of the bdelloid rotifer Philodina acuticornis/roseola with the diapause of the monogonont Brachionus calyciflorus on their toxicant sensitivity after reactivation from desiccation as tuns or cysts, respectively. As explained in Snell et al. (2017), the taxonomy of Philodina acuticornis/roseola is still uncertain, thus its compound species name.
Snell et al. (2017) introduced an interesting new concept for the desiccation and storage of the dry rotifers in view of subsequent toxicity testing after rehydration. This procedure consists of pipetting a 0.5 ml of a high-density P. acuticornis/roseola culture into 0.6-ml microcentrifuge tubes, then allowing the tubes to air dry at room temperature until they are fully desiccated. During the drying, the rotifers attach themselves to small paper discs placed in the tubes. The tubes are then stored in a refrigerator prior to rehydration of the rotifers for the experiments. In the Snell et al. (2017) study, rehydrated free swimming P. acuticornis/roseola were pipetted from the tubes for performance of toxicity tests at 3 exposure times and with measurement of 3 different endpoints: a 1-h (ingestion) test, a 24-h (survival) test, and a 5-day (reproduction) test exposed to inorganic and organic toxicants. The work reported here extends this study, developing a new endpoint: reactivation of desiccated tuns in the presence of toxicants. This test has the advantage of being ultra-rapid because it can be evaluated in only 30 min.
Methods
Details of the methods for performing toxicity tests with Philodina are described in Snell et al. (2017). We briefly describe below the rationale for the analysis and how methods have been modified for using Philodina rehydration as an endpoint in toxicity testing.
The reactivation of the desiccated rotifers is very rapid, taking as little as 5 min. The long-term preservation of viable desiccated Philodina means that in contrast to the 24 h required to hatch Brachionus cysts to produce test animals, toxicity tests with desiccated P. acuticornis/roseola can be started virtually immediately at any location.
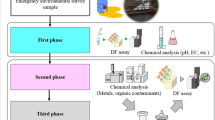
These interesting facts triggered our research to develop a new rapid toxicity test based on the use of desiccated P. acuticornis/roseola (Fig. 1).
The first part of this research project addressed the selection of appropriate conditions for the mass production and storage of desiccated P. acuticornis/roseola on small paper discs, followed by analysis of the reactivation potential of the tuns as a function of the time of storage (shelf life) and the storage conditions.
The second part of the research then focused on the development of a toxicity test exploring a variety of test conditions, the materials and the equipment needed, and the best endpoint of the new assay. Each of these selection aspects was addressed from the point of view of simplicity, practicality, sensitivity, precision, and costs for a rapid toxicity test for routine screening purposes.
Philodina acuticornis/roseola culture
P. acuticornis/roseola was maintained in serial dilution culture in 250-mL bottles incubated at 25 °C in low light. This species was originally collected from an artificial goldfish pond in Provincetown, MA, by David Mark Welch in May 2000 (Robles-Vargas and Snell 2010). Rotifers were cultured in EPA medium (US EPA 1985), consisting of 96 mg NaHCO3, 60 mg CaSO4·2H2O, 60 mg MgSO4, and 4 mg KCl in 1 L of deionized water with pH 8.0. Rotifer stock cultures were fed a mixture of live green alga Chlorella kessleri grown in BBM medium (Nichols 1973) and S.parkle commercial rotifer feed from INVE Aquaculture (http://www.inveaquaculture.com). S.parkle stock solution was prepared by mixing 10 mg/mL in EPA medium and vortexing for 5 min.
Once a Philodina culture reached high density (≥ 50 rotifers/mL), bottles were vortexed for about a minute to release attached rotifers. One-half-milliliter aliquots were added to 0.6-ml centrifuge tubes, each tube containing a small circle of 2.5-μm filter paper about 6 mm in diameter as modified from Ricci et al. (2003). Subsamples from the stock culture were counted to estimate Philodina density in each tube. Tubes were left vertically on a bench-top to air-dry at room temperature (20–22 °C) until all were fully desiccated (about 4 days). During desiccation, food is present, so the Philodina dry with food in their stomachs. As desiccation proceeds, Philodina aggregate around the filter paper and eventually adhere to it as they dry. Desiccated tubes were stored at 4 °C for up to 1 year until ready to rehydrate for experiments.
Operational procedure for a rapid toxicity test starting from dried P. acuticornis/roseola
Our first approach was to determine the percentage reactivation of desiccated P. acuticornis/roseola in control dilution water versus in a test sample, for different exposure times and temperatures. The procedure consisted in adding 0.5-ml control dilution water (a natural water or an artificial water such as the ISO medium (ISO 1996)) to a microcentrifuge tube containing a paper disc with attached tuns, and 0.5-ml test sample to a second tube with a similar disc. Both tubes were then vortexed for 30 s, during which most attached tuns separated from the discs. Two hundred microliters was then pipetted from each tube (in 4 replicates) and put on a 75 × 25-mm glass slide that was then covered with a 22 × 22-mm coverslip. The number of Philodina tuns under the 4 coverslips with control water and the test sample were then counted under a dissection microscope at 10× magnification. Then, the glass slides were incubated for increasing periods of time (from a few minutes up to 30 min) at room temperature. After each incubation, the slides were placed again under the microscope and the number of reactivated Philodina was counted under each coverslip. The percentage reactivation in the control and the test sample was then compared for different incubation times. The effect percentage (i.e., the toxicity) was calculated with the formula (A − B)/A × 100, where A = % reactivation in the control and B = % reactivation in the sample. The same tests were repeated at two higher incubation temperatures (25 °C and 30 °C) to evaluate the influence of temperature on reactivation of the Philodina tuns.
A simplification of this procedure was subsequently implemented, which consisted of putting 2 discs with tuns on the left and on the right side of a glass slide. The discs on the left were hydrated with 200-μl control water, and the discs on the right with the same volume of test sample. Both discs (and the large water drops on top of them) were covered with a cover slip. The glass slide was subsequently also incubated for different periods of time at room temperature, with counting of the number of swimming rotifers under the 2 coverslips. The assays were then repeated with incubation at 25 °C and 30 °C.
On the basis of these results, although some tuns reactivated within a few minutes, 30 min was judged the best exposure time to obtain the maximum percentage reactivation.
With regard to the temperature, it was found that the assays can be performed at room temperature, which should not be lower than 20 °C for a good reactivation of the tuns. However, it was also found that assays performed in an incubator at a temperature of 30 °C gave the most uniform results.
With regard to the simplicity, practicality, and the precision of these 2 test procedures, all findings eventually revealed that the precise counting of all the reactivated (and rapidly swimming) rotifers was difficult and time consuming and yielded different results in repeated tests as well as in scorings by different investigators.
These difficulties in the counting of the reactivated Philodina eventually led us to the conclusion that a quantitative test with determination of an exact percentage of effect was unnecessary for a rapid screening toxicity test. We therefore developed a semi-quantitative test based on analysis of the degree of reactivation of the rehydrated rotifers instead of exact quantitative counting.
Rotifer Activity Inhibition Test (RAIT)
A test procedure has been worked out for this semi-quantitative approach which evaluates two criteria. The assay compares the decrease (= inhibition) of the reactivation of the hydrated tuns in the analyzed sample versus the control water.
This test has been named “Rotifer Activity Inhibition Test (RAIT)” and distinguishes four activity classes on the basis of reactivation and activity criteria, which are then assigned to a toxicity class.
Table 1 shows the four activity classes of the tuns after rehydration and exposure to test samples, with the corresponding magnitude of toxicity and the toxicity class.
The experimental procedure of RAIT is in fact identical to that described above for the second test, but instead of counting the total number of swimming rotifers, a semi-quantitative evaluation is made of the overall degree of reactivation of the tuns and the activity (crawling, swimming) of the reactivated rotifers.
Based on the microscopic observations during the development of the RAIT method, the following general activity criteria have been selected for the final test procedure and for the assignment of the analyzed sample to one of the four toxicity classes:
-
I.
No activity inhibition
-
Reactivation of the majority of the tuns
-
Substantial number of swimming rotifers
-
High activity of all reactivated rotifers
-
II.
Slight activity inhibition
-
Lower proportion of swimming rotifers
-
Full body stretching and crawling dispersion of the reactivated rotifers over the entire surface of the coverslip
-
Ciliary activity of the corona in some organisms
-
III.
Strong activity inhibition
-
Low proportion of reactivated tuns
-
No or only a few swimming rotifers
-
Slow body stretching and crawling movement
-
Little ciliary activity of the corona
-
IV.
Total activity inhibition
-
Very low or no reactivation of tuns
-
No swimming rotifers
-
No full body stretching of reactivated rotifers
-
No ciliary activity of the corona
-
Most reactivated rotifers are immobile
The technical performance of RAIT is as follows:
-
1.
Place 2 discs with desiccated P. acuticornis/roseola on the left and right sides of a glass slide.
-
2.
Pipet 200-μl natural water onto the discs on the left side (= control) and 200 μl of test sample on to the discs on the right side.
-
3.
Cover both wet discs with a coverslip and incubate for 30 min at room temperature (at least 20 °C, or preferably even 30 °C)
-
4.
Analyze the reactivation pattern and score the activity of rotifers from both discs under a dissection microscope.
The degree of toxicity of the test sample is then assigned to one of the four toxicity classes on the basis of the reactivation and activity characteristics of the rotifers.
An important consideration and the first requirement for the acceptability of the RAIT assay is that the control be classified as not toxic, with the majority of the tuns reactivated and all of the rotifers actively swimming or crawling.
In order to evaluate the reproducibility of the final RAIT procedure, repeated tests have been performed on a variety of natural samples, as well as on individual chemicals in increasing test concentrations. These assays consistently showed that the results of repeated tests were in most cases all of the same toxicity class as that of the first test.
Yet, and similarly to results of conventional toxicity tests in which the effect percentage of repeated tests can vary by up to 20%, we have observed similar variability in repeated RAIT assays. To increase the precision of RAIT toxicity evaluations, it is therefore advised to perform RAIT with two replicates per assay.
Results
Precision of the RAIT test
Repeated RAIT tests were performed concurrently and at different times, with discs from the same batch and different batches. The assays were made on several types of samples, comprising wastewaters as well as natural waters spiked with chemicals.
The test samples belonged to different toxicity classes according to the RAIT classification method. The effect signal obtained on each of the analyzed samples in the assays performed in duplicate consistently gave a similar response in terms of either “not toxic, slightly toxic, very toxic or 100% toxic.” The precision of RAIT is hence quite satisfactory from the point of view of repeatability and reproducibility.
There is a quite simple way to verify the credibility of the outcome of a RAIT test in terms of its assignment to a toxicity class. One can simply perform the assay with one duplicate (thus prepare 2 slides with discs instead of only one). Like in the findings reported above for the precision testing, the outcome of the duplicates should also be the same.
Toxicity detection and quantification thresholds of RAIT
A major question about the usefulness of the RAIT rapid screening test for routine application in ecotoxicology is to find out its potential and limits with regard to its sensitivity in comparison to other toxicity tests, and in particular for monitoring studies of natural waters and wastewaters.
A series of investigations was made in collaboration with three organizations in Belgium that regularly perform toxicity tests on environmental samples. These organizations are ISSEP (Scientific Institute of Public Service) in Wallonia, the company ECCA (Environmental Consulting and Chemical Analysis), and the University College Gent (Hogeschool Gent) in Flanders. These organizations provided samples on which they performed standard toxicity tests and on which we performed the RAIT in parallel.
The standard toxicity tests performed by these organizations included:
-
1.
The bacterial luminescence inhibition test using Aliivibrio fischeri (Microtox) (ISO 11348-3)
-
2.
The algae growth inhibition test on Pseudokirchneriella subcapitata (ISO 8692)
-
3.
The acute Daphnia magna test (ISO 6341)
-
4.
The Daphnia magna reproduction test (ISO 10706)
-
5.
The acute fish test with Brachydanio rerio (ISO 7346-1)
-
6.
The chronic rotifer growth test on Brachionus calyciflorus (ISO 20666)
Not all these standard tests were performed by all three organizations on each sample. Each organization decided on its own, depending on the demand of their customers, which tests they performed on a particular sample.
Application of the RAIT to river surface waters
During 2019, 18 samples of river surface waters were collected by ISSEP from 12 rivers in Wallonia, for analysis of the toxicity of surface waters as part of the implementation of the Water Framework Directive 2000/60/CE (Table 2).
The following tests have—in parallel to the RAIT—been performed on all the samples: the bacterial luminescence inhibition test, the algae growth inhibition test, the Daphnia magna reproduction test, and the chronic rotifer growth inhibition test.
NT not toxic
The results of this study revealed that, with the exception of a slight effect (26%) with the bacterial test in one sample, the 18 river waters were not toxic to the test species of the four standard tests nor for the RAIT. These results show that this RAIT test gives an identical effect signals as the standard toxicity tests with neither false negatives nor positives.
The advantage of the RAIT test is clearly that an estimate of animal toxicity is obtained after half an hour of exposure, whereas tests with other animal species require at least 2–3 days exposure and even up to 2 weeks for the Daphnia magna reproduction test.
Toxicity monitoring of the influent and effluent of an industrial wastewater
In the last months of 2018, a toxicity monitoring study was performed by the University College Gent on the wastewaters of an industry in Flanders, Belgium, containing inorganic and organic chemicals, as well as domestic biodegradable compounds. This industry has a specific wastewater treatment system, and the influents and effluents have been collected at four weekly intervals in November and December 2018. Acute Daphnia magna tests were performed by the Ecotox laboratory of the University College Gent, and the RAIT was performed in parallel. The results of this study are shown in Table 3 and indicate that the effluent was not toxic in any of the samples, indicating the effectiveness of the wastewater treatment.
In turn, in both the acute Daphnia magna and RAIT tests, the toxicity of the influent was quite different between the sampling periods. The influent was not toxic to Daphnia magna nor to the RAIT on Nov 13, but highly toxic on Nov 20, slightly toxic on Dec 12, and again highly toxic on Dec 11.
These data on wastewater influent toxicity demonstrate that the 30-min RAIT screening assay gave for each of the eight analyzed samples the same order of magnitude toxicity signal as the 48-h acute Daphnia magna test, in a fraction of the time and for a fraction of the costs.
Toxicity analysis of wastewaters
The company ECCA routinely performs chemical and ecotoxicological analyses on various types of wastewaters originating from industries, research centers, and laboratories. A comparative study of the toxicity tests performed by ECCA and the RAIT screening assays has been performed on a series of nine wastewater samples collected by ECCA and originating from four research centers, one chemical industry, one domestic wastewater treatment plant, and three pharmaceutical industries.
Table 4 shows the results of the toxicity tests performed by ECCA on these wastewaters, in comparison to the RAIT assays. As can be seen in this table, the toxicity evaluation of the wastewater samples by ECCA was in most cases limited to the Microtox test, with application of the acute Daphnia magna test to only four samples and the acute fish test to only two samples.
With regard to the conventional tests performed by ECCA, 2 of the 9 wastewaters were highly toxic for Daphnia but not to the bacteria, whereas 2 other wastewaters were highly toxic to both test organisms. One wastewater was not toxic to the fish but highly toxic to the bacteria, whereas another wastewater was virtually not toxic to the fish and the bacteria.
Comparison of the RAIT results with those of the acute Daphnia magna tests showed that two wastewaters were very toxic to both Daphnia and Philodina, but two other wastewaters were highly toxic for Daphnia but not toxic for the rotifer.
Interestingly, the results found with the two rapid toxicity tests (the bacterial luminescence test and RAIT) showed a very similar responsiveness to the toxic effects. The three wastewaters which were highly toxic to the bacteria were indeed also highly toxic for the rotifer, and the six waste waters which were not toxic to the bacteria were not toxic to the rotifers.
Toxicity analysis of leachates of sludges of wastewater treatment plants
ISSEP regularly performs toxicity analyses on sludges of domestic wastewater treatment plants to determine the efficiency of wastewater treatment (which can differ from one plant to another).
To evaluate the residual toxicity of the sludges, toxicity tests were performed on leachates of the sludges that were prepared according to the standard procedure (NBN EN 12457-2 2002). ISSEP performed four toxicity tests on the sludge leachates: the bacterial luminescence inhibition test (Microtox), the algae growth inhibition test, the acute Daphnia magna test, and the chronic rotifer test. Leachates of sludges from seven wastewater treatment plants (WWTP) have been kindly provided by ISSEP for parallel performance of RAIT assays.
Table 5 shows the results of the toxicity tests performed on the sludge leachates, in comparison to the results of the RAIT assays. Except for the sludge leachate of WWTP II, in which the percentage toxicity found with the acute Daphnia magna test was low (25%), all six other sludge leachates showed quite high toxicity, and in many cases even 100% toxic effects with all the traditional toxicity tests.
A quite high toxicity of all the sludge leachates was also found with the RAIT (toxicity class III and IV), except for WWTP II which was found “not toxic” in the RAIT. Interestingly, this sludge leachate was not very toxic for Daphnia magna either.
Discussion and conclusions
It is generally recognized in ecotoxicology that toxicity is species and chemical dependent. This is once again demonstrated for the sublethal endpoint of Philodina tun reactivation as utilized in the rapid RAIT screening test described in this paper. Indeed, it would be an exception to this rule if an identical effect would always have been observed between RAIT and the other toxicity tests applied on the different samples. RAIT was found for some samples as sensitive and for others less sensitive than the longer and more complicated traditional toxicity tests. Consequently, the results of the comparative studies detailed above showed that this very rapid new animal toxicity test basically gives a comparable toxicity signal as the battery of traditional toxicity tests. RAIT is therefore definitely worth further exploration for its potential as a rapid, practical, and low-cost assay for toxicity screening and routine toxicity monitoring.
RAIT takes advantage of the biology of bdelloid rotifers to improve the speed and convenience of toxicity testing. Philodina is a well-characterized representative of this class of animals which play an important role in aquatic as well as in terrestrial ecosystems and is known for its remarkable ability to survive desiccation and revive after a few minutes of rehydration. Reactivation and activity of rehydrated Philodina tuns is inhibited by a variety of toxicants in a dose-dependent manner, making this criterion quite useful for toxicity testing.
The new rapid screening toxicity test RAIT has several attractive features for routine, practical, and low-cost application in ecotoxicology. RAIT can be performed anytime without preparation of test animals and without culturing of live stocks. It can be applied anywhere and only requires a dissection microscope and an incubator as equipment, and it provides results after only 30 min.
For simplicity, the RAIT was designed as a semi-quantitative test, with classification of the results into four toxicity categories based on the degree of inhibition of the reactivation and swimming activity of the animals after a 30-min exposure.
Since RAIT is a “screening” test, it is performed on non-diluted samples (limit test) to evaluate the magnitude of the toxic effect of the sample on the rotifer test species. Yet, RAIT can also provide additional interesting information by performing the assay in a 1:1 dilution series of the sample. Such a test with dilutions of a sample will indicate how much the original sample must be diluted to reach the lowest ineffective dilution (LID), i.e., the dilution corresponding to the NOEC (No-observed-Effect-Concentration). This information is important for industry with regard to, e.g., the efficiency of their wastewater treatment procedure and discharge permits.
Lastly, RAIT can also be an interesting rapid, simple, and practical new tool in testing and comparing the inherent order of magnitude of the toxicity of particular chemicals or classes of chemicals (e.g., metals, organics, pesticides, petroleum) to Philodina, as a representative test species for this important group of animals in aquatic and terrestrial environments.
A website has been constructed for RAIT (www.rotiferactivityinhibitiontest.com) which contains information on the development of the RAIT screening test and the test procedure, and shows results from its first applications. The RAIT website also indicates that tubes containing discs with desiccated Philodina can be obtained from the company ROTOX in the USA.
References
Dahms HU, Hagiwara A, Lee JS (2011) Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol 101:1–12
ISO 21338 (2010) Water quality – Kinetic determination of the inhibitory effects of sediment, other solids and coloured samples on the light emission of Vibrio fischeri (kinetic luminescent bacteria test). 21 pages
ISO 6341 (1996) Water quality – Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) – Acute toxicity test. 9 pages
Jacobs H (1909) The effects of desiccation on the rotifer Philodina roseola. J Exp Zool 6(2):207–265
Kratasyuk VA (1990) Principle of luciferase biotesting. In: Jezowska-Trzebiatowska (eds) Proceedings of the first international school “Biological luminescence”, Wroclaw, Poland, 20-23 June 1989. World Scientific Publishing Co, Singapore, pp 550–558
Kratasyuk VA, Esimbekova EN (2015) Applications of luminous bacteria enzymes in toxicology. Comb Chem High Throughput Screen 18:952–959
Lappalainen J, Juvonen R, Vaajassari K, Karp M (1999) A new flash method for measuring the toxicity of solid and colored samples. Chemosphere 38(5):1069–1083
Lappalainen J, Juvonen R, Nurmi J, Karp M (2001) Automated color correction method for Vibrio fischeri toxicity tests. Comparison of standard and kinetic assays. Chemosphere 45(4-5):635–641
Masner P, Javurkova B, Blaha L (2017) Rapid in situ toxicity testing with luminescent bacteria Photorhabdus luminescens and Vibrio fischeri adapted to a small portable luminometer. Environ Sci Pollut Res 24:3748–3758
NBN EN 12457-2 (2002) Characterization of waste – leaching – compliance test for leaching of granular waste materials and sludges – part 2: one stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 4 mm (without or with size reduction)
Newman MC (2020) Fundamentals of ecotoxicology, fifth edn. Taylor and Francis Group, CRC Press, Boca Raton, FL
Persoone G (2006) Recent advances in rapid ecotoxicity testing. In: Gray J (ed) Thompson KC. RSC Publications, Water Contamination emergencies – Enhancing our response, pp 192–202
Persoone G, Wadhia K, Thompson KC (2004) Rapid toxkit microbiotests for water contamination emergencies. In: Thompson KG (ed) Gray J. RSC Publications, Water contamination emergencies. Can we cope, pp 128–130
Ricci C, Melone G, Santo N, Caprioli M (2003) Morphological response of a bdelloid rotifer to desiccation. J. Morphol. 257:246–253
Rico-Martinez R, Perez-Legaspi IA, Arias-Almeida JC, Santos-Medrano GE (2013) Rotifers in ecotoxicology. In: Ferard JF, Blaise C (eds) Encyclopedia of Aquatic Toxicology. Springer-Verlag, Berlin, pp 973–996
Rico-Martinez R, Arzate-Cardenas MA, Robles-Vargas D, Perez-Legaspi IA, Alvarado-Flores J, Santos-Medrano GE (2016) Rotifers as models in toxicity screening of chemicals and environmental samples. In: Larramendy ML, Soloneski S (eds) Invertebrates – experimental models in toxicity screening. Rijeka, Croatia Chapter 4, 44 pages
Robles-Vargas D, Snell TW (2010) Effects of desiccation on the toxicant sensitivity of rotifers. Hydrobiologia. 652:185–193
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313/314:231–247
Snell TW, Marcial HS (2017) Using rotifers to diagnose the ecological impacts of toxicants. In: Hagiwara A, Yoshinaga T (eds) Rotifers: aquaculture, ecology, gerontology, and ecotoxicology. Springer, Tokyo, pp 129–147
Snell TW, Johnston RK, Matthews AB (2017) Freshwater toxicity testing using rehydrated Philodina sp. (Rotifera) as test animals. Environ Toxicol 32:2267–2276
US Environmental Protection Agency (1985) Methods for measuring acute toxicity of effluents to freshwater and marine organisms. EPA600/4-85-013
Won EJ, Han J, Kim DH, Dahms HU, Lee JS (2017) Rotifers in ecotoxicology. In: Hagiwara A, Yoshinaga T (eds) Rotifers: aquaculture, ecology, gerontology, and ecotoxicology. Springer, Tokyo, pp 149–176
Acknowledgments
The authors hereby acknowledge the cooperation of the 3 organizations in Belgium which kindly provided them samples for the RAIT tests and the results of the various toxicity tests which they themselves performed on these samples.
We are in this regard very grateful to the following people: Dr Y.Marneffe and Mrs C.Carole (ISSEP), Mr S. De Vriese and Mr F.Noyelle (ECCA), Mrs C. Van der Heyden (University College, Ghent), and Ms Jillian Brashear (Georgia Tech, Atlanta).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Snell, T.W., Persoone, G. A rapid, simple screening toxicity test using desiccated bdelloid rotifers: Rotifer Activity Inhibition Test (RAIT). Environ Sci Pollut Res 28, 3810–3819 (2021). https://doi.org/10.1007/s11356-020-09255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09255-5