Abstract
In this work, some trace elements (As, Cd, Cr, Cu, and Pb) were determined for the first time in the crustacean Sergio mirim (Decapoda: Thalassinidea: Callianassidae) from Southern Coast (Rio Grande, RS) of Brazil. The trace element determination was carried out by graphite furnace atomic absorption spectrometry (GF AAS) and inductively coupled plasma mass spectrometry (ICP-MS). Different microwave radiation-based sample digestion methods were evaluated. The analyte concentration ranged from 1.45 to 3.70 μg g−1 for As, 0.615 to 0.942 μg g−1 for Cd, 0.884 to 7.20 μg g−1 for Cr, 122 to 275 μg g−1 for Cu, and 0.390 to 0.916 μg g−1 for Pb. The limits of quantification for As, Cd, Cr, Cu, and Pb were 0.12, 0.01, 0.16, 0.92, and 0.06 μg g−1, respectively. The accuracy was evaluated by results comparison between GF AAS and ICP-MS techniques, as well as by analysis of certified reference materials of fish muscle and oyster tissue, with agreement from 92 to 108%. The feasibility of using Sergio mirim as a promising environmental bioindicator candidate was evaluated, since that it is an abundant organism in the studied area (South cost of Brazil) as well as in other places around the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Daily, considerable amounts of pollutants reach the marine/estuarine environment through transport and atmospheric deposition, natural erosion, geochemical cycles, and products from anthropogenic activities (Peirrce et al. 1998). Contamination by metals is currently one of the major environmental problems nowadays. This type of pollution also significantly affects the aquatic systems (estuaries, coastal lagoons, etc.), which are very dynamic systems, making it essential to study the effects on the organisms that inhabit them (Núñez-Nogueira et al. 2012). Additionally, it is well known that crustaceans are very sensitive to contaminants and environmental disturbances and therefore are particularly useful for detecting degradation and for site remediation (Roast et al. 2002; Madeira et al. 2018; Plenty et al. 2018). The industrial zone of Rio Grande city is characterized by the presence of an important complex of industries, including fertilizers, food, and naval, as well as populated areas fishing village and small groups of unstructured dwellings, all of them comprising the impact zone of the industries emissions. Until the implementation of a drinking water supply system in 2002, this population (more than 200,000 people) have used groundwater for consumption. However, for socio-economic reasons, these characteristics are still common in some dwellings, increasing the health risks (Mirlean et al. 2005).
In the marine environment, the benthic crustaceans are considered potential indicators of site contamination and are often used as the most suitable for environmental biomonitoring (Rosenberg and Resh 1993). The toxic effects of metals depend mainly on their species and bioavailability, but also on the absorption, accumulation, and excretion rate of the organisms (Götze et al. 2014). Biomonitoring can be defined as the use of organisms/materials to obtain quantitative information of certain characteristics of the environment in which they are found (Wolterbeek 2002). These contaminants (e.g., metals or metalloids) can reach the biota and thus extend up to the sediments which represent an important source of contamination for many benthic organisms. The concentration of key elements in the tissues of some organisms provides relevant information for biomonitoring purposes (Smodiš and Bleise 2002).
It has been observed a growing interest in the study of aquatic organisms as bioindicators of environmental contamination. Some organisms that live in close contact with water or sediment have been used as metal bioindicators in marine environment, as fish (Naccari et al. 2015), mussel (Baraj et al. 2011), oysters (Rodriguez-Iruretagoiena et al. 2016), shrimp (Garcia and Niencheski 2012), barnacles (Reis et al. 2011), and others. It is important to mention that for this kind of study, the use native species is mandatory and important because they are less tolerant to contaminants than invasive species (Bielen et al. 2016).
Taking into account the local aspect, but not restricted to it, the biota of the beaches of Rio Grande do Sul State presents a considerable variety of marine invertebrate species. Sergio mirim (Decapoda: Thalassinidea: Callianassidae) is a digger decapod crustacean, which belongs to the family Callianassidae and is abundant in this region (Pezzuto 1998; Coelho and Rodrigues 2001; Rosa et al. 2018). These organisms meet the main prerequisites to be considered as bioindicators such as pollution resistant, abundant in the studied region, ensure a lifetime according to the period of the experiments, size enough to provide material for the analysis, and resistant to brackish water (Haug et al. 1974). Surprisingly, there are no studies on the concentration of trace elements for Sergio mirim. These organisms living in a transitional environment named subterranean estuary (ES), but up to now it is a poorly understood area due to the complexity of geochemical processes (Beck et al. 2010; Gonneea et al. 2014). Worldwide, the organisms from the family Callianassidae can be found in several places, as Callichirus laurae in the Gulf of Aqaba, Jordan (Red Sea), as well as Glypturus acanthochirus are abundant in the Caribbean region. Although there are a few studies about these crustaceans, they are potential to be used as model organisms in several studies (Manning 1987; Abu-Hilal et al. 1988).
Anthropogenic factors such the contamination of coastal waters and groundwater attributed to the population growing and intense occupation of coastal areas can modify the dynamics of trace elements in the ES or induce the increasing of harmful elements to the biota (Niencheski et al. 2006). Currently, there is a great interest on monitoring some elements (in the past known as “heavy metals,” but now known as “toxic elements”) in organisms, some of them with high toxicity (e.g., As, Cd, Hg, and Pb) and others with metabolic activity (e.g., Cu, Fe, and Zn) (Schmidt et al. 2013). Additionally, there is an increasing interest in the development of simple, rapid, and accurate methods, and preferably with multielemental capability for the determination of contaminants in marine organisms (Soares et al. 2012; Muller et al. 2014).
Among the main analytical techniques used for the determination of trace elements in biological samples, graphite furnace atomic absorption spectrometry (GF AAS) (Sardans et al. 2010), inductively coupled plasma optical emission spectrometry (ICP-OES) (Pereira et al. 2012), and inductively coupled plasma mass spectrometry (ICP-MS) (Ashoka et al. 2009; Schmidt et al. 2015) can be highlighted. However, these techniques usually require a sample digestion prior to the detection, such as wet or dry digestion, in open or closed vessels and under conventional heating or microwave radiation.
In general, methods assisted by microwave radiation in closed systems are preferred, taking into account its efficiency, negligible analyte loss, low risk of contamination, quickness, among others (Hartwig et al. 2016). Microwave-assisted digestion (MAD) in closed vessels has been usually applied for the digestion of biological samples (Pereira et al. 2012). However, in the last years, some studies have highlighted the use of MAD combined with ultraviolet radiation (MAD-UV) (Mesko et al. 2015), as well as MAD with pressurized oxygen (MAD-O2) (Bizzi et al. 2011). These two relatively new approaches (MAD-UV and MAD-O2) have been investigated to minimize some drawbacks of conventional MAD, mainly by using diluted acids.
In the MAD-UV system, an electrodeless discharge lamp (Cd with main emission line at 228.8 nm) is used for in situ UV generation by immersion into the digestion vessel used for MAD (Florian and Knapp 2001). The MAD-O2 system is based on the sample digestion using diluted acids (generally HNO3) which is regenerated during the heating. The regeneration process occurs due to the gradient of temperature inside the reaction vessel during the initial step of digestion. The oxygen pressurization inside the vessel and the poor absorption of microwave radiation by the gas phase result in reaction products that can be oxidized also at the upper atmosphere of the reaction vessel and further reabsorbed in the liquid phase. This process allows the regeneration of HNO3, increasing digestion efficiency (Bizzi et al. 2010).
Therefore, this study aimed to evaluate microwave-assisted sample preparation methods for subsequent As, Cd, Cr, Cu, and Pb determination by spectrometric techniques in Sergio mirim samples. Additionally, a database will be created that will provide a baseline for future works taking into account the characteristics of Sergio mirim as a promising indicator of metal contamination.
Materials and methods
Instrumentation
The determination of As, Cd, Cr, Cu, and Pb was carried out in an atomic absorption spectrometer (model AA800, Perkin Elmer, USA), equipped with graphite furnace atomization mode, a background correction system based on Zeeman effect, an autosampler (model AS92, Perkin Elmer), a transversal heating tube, and a hollow cathode lamp as radiation source. Measurements were carried out at 199.7 nm for As, 228.8 nm for Cd, 357.9 nm for Cr, 324.8 nm for Cu, and 283.3 nm for Pb. For all the analytes, the spectral slit width was 0.7 nm. The current applied to the hollow cathode lamps was 18, 4, 25, 15, and 10 mA for As, Cd, Cr, Cu, and Pb, respectively. The conditions for analyte determination by GF AAS are shown in Table 1.
The analytes were also determined using an inductively coupled plasma mass spectrometer (model ELAN DRC II, Perkin Elmer, Canada), equipped with a concentric nebulizer (Meinhard® type A, USA), a cyclonic spray chamber (Glass Expansion, Inc., Australia), and a quartz torch with quartz injector tube. An inductively coupled plasma optical emission spectrometer (model Optima 4300 DV, Perkin Elmer, USA) with axial view configuration and equipped with a quartz torch (Glass Expansion, Inc.) with quartz injector tube, a cyclonic spray chamber (Expansion Glass, Inc.), and a concentric nebulizer (Meinhard®) was used for the determination of residual carbon content (RCC) in the digests. The operating conditions of plasma-based instruments are shown in Table 2.
For the proposed sample preparation methods (MAD, MAD-O2, and MAD-UV), a microwave sample preparation system (Multiwave 3000, Anton Paar, Austria), equipped with a rotor with capacity for up to eight quartz vessels with internal volume of 80 mL and monitoring of temperature and pressure (maximum of 280 bar and 80 °C, respectively), was used. For the MAD-UV method, UV quartz lamps (activated by microwave radiation) filled with Ar and Cd vapor at low pressure were used.
The samples were lyophilized using a freeze dryer (model L101 LIOTOP, Liobras, Brazil) and milled in a cryogenic mill (model 6750, Spex CertiPrep, USA). The ultrapure water (18.2 MΩ cm resistivity) was obtained from a Milli-Q® purification system (model UV3 Direct-Q, Millipore, USA).
Statistical treatments were carried out using InStat software (version 3.0, Copyright 1992–1998 GraphPad Software Inc., Microsoft Corporation). For comparison of means, the t Student test was used and for the comparison of two or more means, the analysis of variance (ANOVA) was used, applying the Tukey-Kramer multiple comparisons test with a confidence level of 95%.
Reagents and samples
All solutions were prepared using ultrapure water. For sample digestion, concentrated or diluted HNO3 (Merck, Germany) and H2O2 30% (Merck) were used. Nitric acid was purified in a duoPUR sub-boiling distillation system (Milestone). All reagents used in this work were of analytical grade or better.
The aqueous reference solutions for the determination by GF AAS were prepared by suitable dilution of stock solutions of 1000 mg L−1 of As, Cd, Cu, Cr, and Pb (Merck) in 5% HNO3. A Pd solution (1000 mg L−1) was used as chemical modifier (GF AAS determination), which was prepared by dilution from a 20 g L−1 Pd (Sigma-Aldrich, St. Louis, USA) stock solution. The calibration of ICP-MS instrument was performed by using reference solutions prepared from a multielement stock solution (SCP33MS, SPC Science, Canada) containing 10 mg L−1 of each element. The carbon reference solutions used for RCC determination by ICP-OES were prepared by dissolving citric acid (Merck) in ultrapure water. An yttrium stock solution (1000 mg L−1, Spex CertiPrep) was used as internal standard for carbon determination by ICP-OES, which was added to reference solutions and digests at final concentration of 1 mg L−1.
For the determination by ICP-MS and ICP-OES, argon (99.996%, White Martins, Brazil) was used for plasma generation, nebulization, and as auxiliary gas. The pressurization of quartz vessels for MAD-O2 method was performed with oxygen (99.999%, White Martins).
The accuracy and precision were evaluated by analysis of CRMs of dogfish muscle (DORM-2) and oyster tissue (NIST 1566b), which were purchased from the National Research Council of Canada (NRCC, Canada) and National Institute of Standards and Technology (NIST, USA), respectively.
Sample collection
The organisms were collected in the intertidal region and the collection was taken at low tide. The sample collections were performed in two points of Cassino beach (Rio Grande city, Rio Grande do Sul State, Brazil), in November 2012 (“Altair ship” at 32° 17′ 31″ S 52° 15′ 38″ W) and in May 2013 (“Sarita lighthouse” at 32° 37′ 48″ S and 52° 25′ 26″ W). For sample collection, a PVC suction pump with 10 cm of diameter and 50 cm of length was used, where were collected at each sampling point between 7 and 10 organisms of each sex and size (about 30 organisms). After collection, the samples were transported to the laboratory (refrigerated), washed with ultrapure water, and stored at − 20 °C. Then, the organisms were classified by sex and size. After classification and separation, the samples were lyophilized (for 24 h) and milled in a cryogenic mill (2 cycles for freezing for 2 min and 2 cycles for grinding for 2 min). The development and optimization of digestion methods (MAD, MAD-O2, and MAD-UV) were performed using a polled sample (lyophilized and milled) without classification of sex and size.
Microwave-assisted digestion
About 500 mg of sample was weighted and transferred to the quartz vessels, which were previously filled with 6 mL of concentrated HNO3. After closing, the vessels were placed inside the microwave oven and the irradiation program was applied as follows: (i) 1000 W for 30 min (ramp of 5 W min−1) and (ii) 0 W for 20 min (cooling step). After cooling, the digests were diluted up to 25 mL with ultrapure water for further analyte determination. The vessel cleaning was carried out with 6 mL of concentrated HNO3 using the following irradiation program: (i) 1400 W for 10 min and (ii) 0 W for 20 min (cooling step).
Microwave-assisted digestion with oxygen pressurization
Based on previously described procedures using MAD-O2, about 500 mg of sample were weighed and transferred to the quartz vessels, which were previously filled with 6 mL of 3 mol L−1 HNO3. Vessels were closed and pressurized with oxygen (10 bar) through the valve originally used for pressure release (Bizzi et al. 2010, 2011). The irradiation program was the same used for MAD method. After the digestion, the pressure in the vessels was carefully released and the digests were diluted up to 25 mL with ultrapure water for subsequent analyte determination.
Microwave-assisted digestion with ultraviolet radiation
About 500 mg of sample was weighed and transferred to the quartz vessels of conventional MAD system. The vessels were previously filled with 15 mL HNO3 (1, 2, 4, or 7 mol L−1) or 15 mL of a mixture containing 1 mol L−1 HNO3 and 1, 2, or 3 mL H2O2 (30%). Then, the UV lamps were inserted vertically into the quartz vessels. The volume of digestion solution (15 mL) was selected according to the recommendation of manufacturer, since the bulb UV lamps must be fully submerged into the solution. The irradiation program used for MAD-UV method was (i) 400 W for 10 min (ramp of 10 W min−1); (ii) 900 W for 10 min (ramp of 10 W min−1); and (iii) 0 W for 20 min (cooling step). After the digestion, digests were diluted up to 25 mL with ultrapure water for subsequent analyte determination. The vessel cleaning was carried out with 6 mL of concentrated HNO3 using the following irradiation program: (i) 1000 W for 10 min; (ii) 0 W for 20 min (cooling step).
Residual carbon content determination
Previously to the RCC determination, an yttrium solution was added as internal standard in the blanks, reference solutions, and samples with final concentration of 1 mg L−1. The solutions were purged with argon for 1 min to remove the dissolved CO2 in the digests and to avoid overestimated results. After purging, the solutions were promptly analyzed by ICP-OES to prevent the CO2 reabsorption (Flores et al. 2008).
Results and discussion
Initially, a careful optimization of sample preparation methods and element determination was performed. These studies were mandatory to ensure the achievement of suitable conditions to provide accurate results about the trace element concentration in Sergio mirim.
Evaluation of sample preparation methods
All the sample preparation methods were evaluated taking into account that this is the first time that such analytes were determined in this kind of sample. For the evaluation of sample preparation methods, MAD was used as reference method for results comparison with MAD-O2 and MAD-UV (Meche et al. 2010; Pereira et al. 2012). For MAD-UV, different concentrations of HNO3 (1, 2, 4, and 7 mol L−1) and a mixture of 1 mol L−1 HNO3 with 1, 2, and 3 mL of H2O2 were evaluated. The digests from MAD and MAD-UV (except 1 mol L−1 HNO3) presented a clear solution with evidence of complete digestion. MAD-O2 digests showed evidence of incomplete digestion of organic matter. The results for RCC and the comparison among the sample preparation methods are shown in Table 3.
The RCC values after sample digestion were about 5.0% for MAD, 13.1% for MAD-O2, and from 7.1 to 8.3% for MAD-UV. During the digestion by MAD-O2, the maximum temperature and pressure were 190 °C and 58 bar, respectively. It is important to emphasize that these values of temperature and pressure are below the maximum conditions allowed by the manufacturer of microwave oven (280 °C and 80 bar, respectively), which can influence negatively the digestion efficiency.
Regarding to the accuracy, in general the results by MAD-O2 and MAD-UV (for all the evaluated conditions) were in agreement with MAD method for all elements (As, Cd, Cr, Cu, and Pb), ranging from 87 to 106% for MAD-O2 and from 83 to 116% for MAD-UV. The only exception was using 1 mol L−1 HNO3 for MAD-UV method, where the agreement with MAD for Cr was not better than 53%. It can be attributed to the availability of analytes in the solution due to the conditions such as pH and oxidizing environment. However, the addition of only 1 mL of H2O2 to 1 mol L−1 HNO3 solution was enough to increase significantly the digestion efficiency and agreements for Cr, Cu, and Pb. Although most of alternative methods evaluated showed suitable results comparing to MAD, this method was chosen for the element determination due to its relative simplicity and lower operation cost.
Optimization of operational conditions for analyte determination by GF AAS
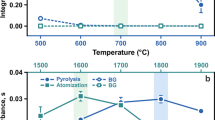
In order to compare the results obtained by ICP-MS after digestion by MAD (as shown in Table 3) and to provide a cheaper alternative for analyte determination, the GF AAS technique was also evaluated and carefully optimized. One of the main requirements for an accurate determination by GF AAS is to achieve a compromise condition of the heating program for sample and reference solutions. Thus, the pyrolysis and atomization temperatures were evaluated for sample and reference solutions with and without using of Pd as chemical modifier (Supplementary material - Fig. S1). The thermal stability of the analytes during the pyrolysis using Pd was increased, allowing the use of higher pyrolysis temperatures and with lower background signals. However, the thermal stability for Cd, Cr, and Pb in the sample was not significantly increased by using Pd. On the other hand, the signal intensity was increased for As and Pb in the samples using Pd as chemical modifier.
In the pyrolysis temperature for As for sample and reference solution (both using Pd), the signal intensity begins to decrease at 1300 °C. Thus, 1200 °C was chosen as pyrolysis temperature. Taking into account the atomization temperature for As, sample and reference solution showed the highest signal intensity at 2000 °C and this temperature was selected.
The pyrolysis temperature for Cd showed the same profile for sample and reference solution (both with Pd) and 500 °C was chosen. When 1300 and 1400 °C were used for Cd atomization, the signals without Pd were overestimated for sample and reference solution. Thus, 1500 °C was selected as atomization temperature for Cd using Pd as chemical modifier.
For Cr, from 1000 to 1500 °C, no significant difference (p > 0.05) was observed for pyrolysis for sample and reference solution (both with Pd) and 1400 °C was chosen. Although the maximum intensity for atomization was achieved (using Pd) at 1800 °C, 2000 °C was selected as atomization temperature for Cr taking into account the lower standard deviation.
The thermal stabilization for Cu during the pyrolysis was achieved up to 1200 °C for sample using Pd. However, the pyrolysis temperature for reference solution remained constant only up to 1100 °C, when the analyte starts to be volatilized. Thus, considering the pyrolysis temperature with highest signal intensity (for both sample and reference solution), 800 °C was chosen for the next experiments. The atomization temperature of 1900 °C was chosen taking into account the highest signal for sample and reference solution.
For Pb, the use of Pd as chemical modifier did not improve its thermal stability, but an increasing (about 4 times) on integrated absorbance for the sample, as well as a reduction of the standard deviation for reference solution was observed. Using Pd, sample and reference solution were thermally stable up to 750 °C, but volatile elements such as Cd and Pb can be lost at this temperature, even using Pd as chemical modifier. Thus, as the highest signal intensity for sample and reference were achieved at 650 °C, this temperature was selected as pyrolysis for Pb. The atomization for Pb was feasible at 1600 °C, also using Pd as chemical modifier.
Analytical performance
After the optimization of operational conditions for the measurements by GF AAS, the instrument calibration was carried out using aqueous reference solution (in 0.1 mol L−1 HNO3 solution) for each element. The calibration curves for GF AAS showed good linearity, with R2 better than 0.99, whereas the R2 for ICP-MS measurements was better than 0.9998 for all analytes.
The limit of detection (LOD) and limit of quantification (LOQ) were defined as 3 and 10-fold, respectively, the standard deviation of ten measurements of the blank divided by the slope of the calibration curve (Magnusson and Ornemark 2014). The LOD and LOQ values, as well as the results for analyte determination by both detection techniques (GF AAS and ICP-MS) after MAD are shown in Table 4. In general, the LOQ values for GF AAS were similar to those obtained by ICP-MS. Regarding to the discrepancy of the results for As (Table 4) by both detection techniques, it can be attributed to the high salt content (mainly Cl−) in the Sergio mirim, since that its habitat is in contact with seawater (Beck et al. 2010; Gonneea et al. 2014). Several studies have been reported about interferences by Cl− in the determination of 75As+ by ICP-MS due to the formation of polyatomic ions in the plasma by combining 40Ar and 35Cl, increasing significantly the signal for 75As+ (Todolí and Mermet 2006; Antes et al. 2010). On the other hand, the high carbon content may also contribute to the increasing of As signal due to the interferences by charge transfer, which also increases the signal for 75As+ (Jarvis et al. 1992; Thomas 2013). It is important to mention that both types of interferences (salt and carbon content) can be reduced using some strategies described in the literature (Jarvis et al. 1992; Thomas 2013), but they were not used in this work. This overestimated result makes the ICP-MS technique infeasible for As determination without the use of ways to minimize such interference as the use of mathematical corrections in this kind of sample (Thomas 2013) and the authors also recommend the use of GF AAS for this purpose in this work.
The accuracy for the determination of As, Cd, Cr, Cu, and Pb in Sergio mirim was evaluated by results comparison from both detection techniques (Table 4), as well as by analysis of certified reference materials (CRMs) of dogfish muscle (DORM-2) and oyster tissue (NIST 1566b). Results are shown in Table 5 and no significant difference (p > 0.05) was observed. The values found in the CRMs were in agreement (from 94 to 108%) with the certified values for all the analytes. The relative standard deviation values for all the measurements were below 8%.
Sergio mirim as a promising bioindicator for trace elements
These organisms (Sergio mirim) live in a complex region of mixing of seawater with high salinity (when the tide is high) and with the fresh groundwater that reaches the beach regions when the tide is low. Coastal aquifers and permeable sediments produce a reaction zone where the mixture of fresh water with salt water becomes similar to happens in the water column of the estuarine mixing zone, characterizing this environment like a subterranean estuary (Moore 1999). In subterranean estuary, the water flow and its reaction with the solid phases and mix the continental freshwater-marine water migrate in response to the seasonal and tidal level variations. In this region, important processes can influence the presence and contribution of several substances to the coastal environment (Robinson et al. 2018; Alorda-Kleinglass et al. 2019), as well as the organisms that living there (Encarnação et al. 2013).
Considering the complexity of the region under study and the need for understanding the dynamics of some trace elements (As, Cd, Cu, Cr, and Pb), Sergio mirim samples were classified by sex, size (age), and sampling site in order to collect different information from the level of absorption of the studied elements. The results of trace element concentration after sample classification are shown in Table 6.
Some organisms used as bioindicators throughout the region under study such as mussels (Baraj et al. 2003, 2011) and shrimps (Garcia and Niencheski 2012), combined to the comparison between the elements accumulated in these organisms will provide important information about the quality of the water resources. Although there is a distinction in feeding habits and places of occurrence among the organisms, some characteristics stand out because they present a low mobility (similar to the condition of fixed organisms such as mussels and barnacle) and, on the other hand, regarding to the mode of feeding resemble the shrimp. The element concentration in the males in the two sampling points ranged from 2.02 to 3.70 μg g−1 for As, from 0.615 to 1.13 μg g−1 for Cd, from 2.46 to 7.20 μg g−1 for Cr, from 122 to 275 μg g−1 for Cu, and from 0.390 to 0.916 μg g−1 for Pb. In the females, the element concentration was found in the same range (no statistical difference, p > 0.05) as those found in males in both sampling points. Although the organisms catch has been performed at two locations and at different times, no trend in the concentration related to size, sex, or sampling site was observed. Increasing the number of individuals analyzed, regular sampling in locations with different uses can provide more details about the availability of trace metals in the environment.
In general, the concentration of essential elements (e.g., Zn and Cu) tends to be several orders of magnitude higher than those of non-essential elements. In this case, Cu is not an exception, since it performs several biological functions and is found in high levels in crustaceans. Until now, the element concentration in the studied region was evaluated only in organisms as shrimp (Garcia and Niencheski 2012) and mussels (Baraj et al. 2003, 2011), but no information about the concentration trace elements in Sergio mirim has been reported in the literature.
Comparing with the results from a study that determined the concentration of trace elements in mussels Perna perna along the Brazilian coast region (including the studied area), the levels of Cd, Cr, and Pb were similar to those found in Sergio mirim. The levels of Cu found in this study were about 10 times higher than in mussel Perna perna (Baraj et al. 2003, 2011). The data of present work represent the first study involving the determination of trace elements in Sergio mirim as a potential bioindicator for trace elements. This study can be considered the starting point for the establishment of a baseline of toxic element content in this region related to this organism, as well as the possibility of this data to support future studies for understanding the dynamics of trace elements in the subterranean estuary that is the habitat of Sergio mirim (Baraj et al. 2003; Niencheski et al. 2006; Beck et al. 2010).
Conclusion
The concentration of trace elements determined in Sergio mirim showed that the values are within the regional background found for the other aquatic organisms. The sample preparation methods using microwave radiation in closed systems were considered suitable for Sergio mirim digestion and subsequent determination of As, Cd, Cr, Cu, and Pb by GF AAS. The crustacean Sergio mirim can be considered as a good candidate for trace element bioindicator in coastal regions and can be used for environmental monitoring purposes, especially for studies about dynamic of underground estuaries in sandy beaches. It is important to highlight the necessity of increasing the number of individuals analyzed and the application of regular sampling in locations with different uses to provide more detailed information about the availability of trace elements in the environment.
References
Abu-Hilal A, Badran M, de Vaugelas J (1988) Distribution of trace elements in Callichirus laurae burrows and nearby sediments in the Gulf of Aqaba, Jordan (Red Sea). Mar Environ Res 25:233–248. https://doi.org/10.1016/0141-1136(88)90014-1
Alorda-Kleinglass A, Garcia-Orellana J, Rodellas V, Cerdà-Domènech M, Tovar-Sánchez A, Diego-Feliu M, Trezzi G, Sánchez-Quilez D, Sanchez-Vidal A, Canals M (2019) Remobilization of dissolved metals from a coastal mine tailing deposit driven by groundwater discharge and porewater exchange. Sci Total Environ 688:1359–1372. https://doi.org/10.1016/j.scitotenv.2019.06.224
Antes FG, Duarte FA, Mesko MF, Nunes MA, Pereira VA, Müller EI, Dressler VL, Flores EM (2010) Determination of toxic elements in coal by ICP-MS after digestion using microwave-induced combustion. Talanta 83:364–369. https://doi.org/10.1016/j.talanta.2010.09.030
Ashoka S, Peake BM, Bremner G, Hageman KJ, Reid MR (2009) Comparison of digestion methods for ICP-MS determination of trace elements in fish tissues. Anal Chim Acta 653:191–199. https://doi.org/10.1016/j.aca.2009.09.025
Baraj B, Niencheski LF, Corradi C (2003) Trace metal content trend of mussel Perna perna (Linnaeus, 1758) from the Atlantic Coast of Southern Brazil. Water Air Soil Pollut 145:205–214. https://doi.org/10.1023/A:1023614822121
Baraj B, Niencheski F, Fillmann G, de Martinez GMC (2011) Assessing the effects of Cu, Cd, and exposure period on metallothionein production in gills of the Brazilian brown mussel Perna perna by using factorial design. Environ Monit Assess 179:155–162. https://doi.org/10.1007/s10661-010-1725-8
Beck AJ, Cochran JK, Sañudo-Wilhelmy SA (2010) The distribution and speciation of dissolved trace metals in a shallow subterranean estuary. Mar Chem 121:145–156. https://doi.org/10.1016/j.marchem.2010.04.003
Bielen A, Bošnjak I, Sepčić K, Jaklič M, Cvitanić M, Lušić J, Lajtner J, Simčič T, Hudina S (2016) Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci Total Environ 543:449–459. https://doi.org/10.1016/j.scitotenv.2015.11.049
Bizzi CA, Flores EMM, Picoloto RS et al (2010) Microwave-assisted digestion in closed vessels: effect of pressurization with oxygen on digestion process with diluted nitric acid. Anal Methods 2:734–738. https://doi.org/10.1039/c0ay00059k
Bizzi CA, Barin JS, Müller EI, Schmidt L, Nóbrega JA, Flores EM (2011) Evaluation of oxygen pressurized microwave-assisted digestion of botanical materials using diluted nitric acid. Talanta 83:1324–1328. https://doi.org/10.1016/j.talanta.2010.10.015
Coelho VR, Rodrigues SDA (2001) Setal diversity, trophic modes and functional morphology of feeding appendages of two callianassid shrimps, Callichirus major and Sergio mirim (Decapoda: Thalassinidea: Callianassidae). J Nat Hist 35:1447–1483. https://doi.org/10.1080/002229301317067638
Encarnação J, Leitão F, Range P et al (2013) The influence of submarine groundwater discharges on subtidal meiofauna assemblages in south Portugal (Algarve). Estuar Coast Shelf Sci 130:202–208. https://doi.org/10.1016/j.ecss.2013.04.013
Flores EMM, Mesko MF, Moraes DP, Pereira JS, Mello PA, Barin JS, Knapp G (2008) Determination of halogens in coal after digestion using the microwave-induced combustion technique. Anal Chem 80:1865–1870. https://doi.org/10.1021/ac8000836
Florian D, Knapp G (2001) High-temperature, microwave-assisted UV digestion: a promising sample preparation technique for trace element analysis. Anal Chem 73:1515–1520. https://doi.org/10.1021/ac001180y
Garcia JG, Niencheski LFH (2012) Avaliação temporal da acumulação de elementos traço no camarão-rosa Farfantepenaeus paulensis no estuário da Lagoa dos Patos, RS, Brasil. Trop Oceanogr 40:327–339. https://doi.org/10.5914/tropocean.v40i2.5410
Gonneea ME, Charette MA, Liu Q et al (2014) Trace element geochemistry of groundwater in a karst subterranean estuary (Yucatan Peninsula, Mexico). Geochim Cosmochim Acta 132:31–49. https://doi.org/10.1016/j.gca.2014.01.037
Götze S, Bose A, Sokolova IM et al (2014) The proteasomes of two marine decapod crustaceans, European lobster (Homarus gammarus) and edible crab (Cancer pagurus), are differently impaired by heavy metals. Comp Biochem Physiol C Toxicol Pharmacol 162:62–69. https://doi.org/10.1016/j.cbpc.2014.03.012
Hartwig CA, Pereira RM, Rondan FS et al (2016) The synergic effect of microwave and ultraviolet radiation for chocolate digestion and further determination of As, Cd, Ni and Pb by ICP-MS. J Anal At Spectrom 31:523–530. https://doi.org/10.1039/c5ja00388a
Haug A, Melsom S, Omang S (1974) Estimation of heavy metal pollution in two Norwegian fjord areas by analysis of the brown alga Ascophyllum nodosum. Environ Pollut 7:179–192. https://doi.org/10.1016/0013-9327(74)90065-2
Jarvis KE, Gray AL, Houk RS (1992) Handbook of inductively coupled plasma mass spectrometry, 1st edn. Chapman and Hall, New York
Madeira C, Leal MC, Diniz MS, Cabral HN, Vinagre C (2018) Thermal stress and energy metabolism in two circumtropical decapod crustaceans: responses to acute temperature events. Mar Environ Res 141:148–158. https://doi.org/10.1016/j.marenvres.2018.08.015
Magnusson B, Ornemark U (2014) Eurachem guide: the fitness for purpose of analytical methods—a laboratory guide to method validation and related topics, 2nd edn. Eurachem. http://www.eurachem.org. Accessed 24 June 2015
Manning RB (1987) Notes on Western Atlantic Callianassidae (Crustacea: Decapoda: Thalassinidea). Proc Biol Soc Wash 100:386–401
Meche A, Martins MC, Lofrano BESN et al (2010) Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Microchem J 94:171–174. https://doi.org/10.1016/j.microc.2009.10.018
Mesko MF, Picoloto RS, Ferreira LR et al (2015) Ultraviolet radiation combined with microwave-assisted wet digestion of Antarctic seaweeds for further determination of toxic elements by ICP-MS. J Anal At Spectrom 30:260–266. https://doi.org/10.1039/c4ja00264d
Mirlean N, Machado MI, Osinaldi GM et al (2005) O impacto industrial na composição química das águas subterrâneas com enfoque de consumo humano (Rio Grande, RS). Quim Nova 28:788–791. https://doi.org/10.1590/s0100-40422005000500010
Moore WS (1999) The subterranean estuary: a reaction zone of ground water and sea water. Mar Chem 65:111–125. https://doi.org/10.1016/S0304-4203(99)00014-6
Muller CC, Muller ALH, Pirola C et al (2014) Feasibility of nut digestion using single reaction chamber for further trace element determination by ICP-OES. Microchem J 116:255–260. https://doi.org/10.1016/j.microc.2014.04.013
Naccari C, Cicero N, Ferrantelli V, Giangrosso G, Vella A, Macaluso A, Naccari F, Dugo G (2015) Toxic metals in pelagic, benthic and demersal fish species from Mediterranean FAO Zone 37. Bull Environ Contam Toxicol 95:567–573. https://doi.org/10.1007/s00128-015-1585-6
Niencheski LFH, Baraj B, Windom HL, França RG (2006) Natural background assessment and its anthropogenic contamination of Cd, Pb, Cu, Cr, Zn, Al and Fe in the sediments of the southern area of Patos Lagoon. J Coast Res 2004:1040–1043
Núñez-Nogueira G, Fernández-Bringas L, Ordiano-Flores A, Gómez-Ponce A, de León-Hill CP, González-Farías F (2012) Accumulation and regulation effects from the metal mixture of Zn, Pb, and Cd in the tropical shrimp Penaeus vannamei. Biol Trace Elem Res 150:208–213. https://doi.org/10.1007/s12011-012-9500-z
Peirrce JJ, Weiner RF, Vesilind PA (1998) Environmental pollution and control, 4th edn. Butterworth-Heinemann, Oxford
Pereira ER, Soares BM, Vieira AP et al (2012) Assessment of inorganic contaminants in golden mussel (Limnoperna fortunei) in Southern Brazil. J Braz Chem Soc 23:846–853. https://doi.org/10.1590/S0103-50532012000500009
Pezzuto PR (1998) Population dynamics of Sergio mirim RODRIGUES 1971 Decapoda: Thalassinidea: Callianassidae in Cassino Beach, Southern Brazil. Mar Ecol 19:89–109. https://doi.org/10.1111/j.1439-0485.1998.tb00456.x
Plenty SJ, Tweedley JR, Bird DJ et al (2018) Long-term annual and monthly changes in mysids and caridean decapods in a macrotidal estuarine environment in relation to climate change and pollution. J Sea Res 137:35–46. https://doi.org/10.1016/j.seares.2018.03.007
Reis PA, Salgado MA, Vasconcelos V (2011) Barnacles as biomonitors of metal contamination in coastal waters. Estuar Coast Shelf Sci 93:269–278. https://doi.org/10.1016/j.ecss.2010.12.022
Roast SD, Widdows J, Jones MB (2002) Behavioural responses of estuarine mysids to hypoxia and disruption by cadmium. Mar Environ Res 54:319–323. https://doi.org/10.1016/S0141-1136(02)00134-4
Robinson CE, Xin P, Santos IR et al (2018) Groundwater dynamics in subterranean estuaries of coastal unconfined aquifers: controls on submarine groundwater discharge and chemical inputs to the ocean. Adv Water Resour 115:315–331. https://doi.org/10.1016/j.advwatres.2017.10.041
Rodriguez-Iruretagoiena A, Rementeria A, Zaldibar B, de Vallejuelo SF, Gredilla A, Arana G, de Diego A (2016) Is there a direct relationship between stress biomarkers in oysters and the amount of metals in the sediments where they inhabit? Mar Pollut Bull 111:95–105. https://doi.org/10.1016/j.marpolbul.2016.07.025
Rosa LC, Freire KMF, Souza MJM (2018) Spatial distribution and population dynamics of Callichirus major (Crustacea, Callianassidae) in a tropical sandy beach, northeastern Brazil. Invertebr Biol 137:308–318. https://doi.org/10.1111/ivb.12228
Rosenberg DM, Resh VH (1993) Freshwater biomonitoring and benthic macroinvertebrates. Springer, Berlin
Sardans J, Montes F, Peñuelas J (2010) Determination of As, Cd, Cu, Hg and Pb in biological samples by modern electrothermal atomic absorption spectrometry. Spectrochim Acta - Part B At Spectrosc 65:97–112. https://doi.org/10.1016/j.sab.2009.11.009
Schmidt L, Bizzi CA, Duarte FA et al (2013) Evaluation of drying conditions of fish tissues for inorganic mercury and methylmercury speciation analysis. Microchem J 108:53–59. https://doi.org/10.1016/j.microc.2012.12.010
Schmidt L, Bizzi CA, Duarte FA et al (2015) Evaluation of Hg species after culinary treatments of fish. Food Control 47:413–419. https://doi.org/10.1016/j.foodcont.2014.07.040
Smodiš B, Bleise A (2002) Internationally harmonized approach to biomonitoring trace element atmospheric deposition. Environ Pollut 120:3–10. https://doi.org/10.1016/S0269-7491(02)00123-9
Soares BM, Vieira AA, Lemões JS, Santos CM, Mesko MF, Primel EG, Montes D'Oca MG, Duarte FA (2012) Investigation of major and trace element distribution in the extraction-transesterification process of fatty acid methyl esters from microalgae Chlorella sp. Bioresour Technol 110:730–734. https://doi.org/10.1016/j.biortech.2012.01.148
Thomas R (2013) A practical guide to ICP-MS—a tutorial for beginners, 3rd edn. CRC Press, Boca Raton
Todolí JL, Mermet JM (2006) Sample introduction systems for the analysis of liquid microsamples by ICP-AES and ICP-MS. Spectrochim Acta B At Spectrosc 61:239–283. https://doi.org/10.1016/j.sab.2005.12.010
Wolterbeek B (2002) Biomonitoring of trace element air pollution: principles, possibilities and perspectives. Environ Pollut 120:11–21. https://doi.org/10.1016/S0269-7491(02)00124-0
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) – Finance Code 001. The authors also thank to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/Brazil, process number 12/1853-8) for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 119 kb).
Rights and permissions
About this article
Cite this article
Mandlate, J.S., Soares, B.M., Andrade, C.F.F. et al. Determination of trace elements in Sergio mirim: an evaluation of sample preparation methods and detection techniques. Environ Sci Pollut Res 27, 21914–21923 (2020). https://doi.org/10.1007/s11356-020-08766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08766-5