Abstract
Phosphorus (P) is responsible for algal growth and the structural changes in algal communities. Therefore, it is essential to know whether the different phosphorus availability to different algae can change the community structure. In this study, the interspecific competition was investigated at two bloom-forming cyanobacterium, Cylindrospermopsis raciborskii and Microcystis aeruginosa, when both were treated with five different phosphate compounds, including K2HPO4, β-glycerol phosphate, (2-aminoethyl)-phosphinic acid, glyphosate, and P-free. The results of mono-culture experiments showed that the two species could utilize the dissolved organic phosphorus (DOP) and K2HPO4 (DIP) as the sole P resource. Moreover, the specific growth rates and the endogenous alkaline phosphatase activity in M. aeruginosa cells were much lower than those in C. raciborskii under DOP and DIP treatments. In the co-cultured experiments, however, a significant biomass increase in C. raciborskii was observed in all experimental P treatments, except for glyphosate, regardless of its initial cell density proportion. A 31.8–63.4% increase in cell number of C. raciborskii was found after incubated into K2HPO4, while the highest biomass of mixed samples, 17.72 × 106 cell mL−1, was observed in the (2-aminoethyl)-phosphinic acid treatment (50C50M). Additionally, higher specific growth rate was also found in C. raciborskii when compared with M. aeruginosa under P-free; the increasing proportion of C. raciborskii were 29.1% (50C50M), 16.4% (75C25M), and 36.7% (25C75M), respectively. When the mixed samples were co-cultivated under glyphosate, C. raciborskii cells appeared to be depressed, whereas the cell density of M. aeruginosa increased rapidly. The findings indicated that an excellent P competition might give some advantages for C. raciborskii dominance in natural waters with DIP limitation or DOP abundance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the aggravation of eutrophication and global climatic change, cyanobacteria harmful algae bloom (cyanoHAB) has considered to be one of the most serious problems in aquatic ecosystems (Paerl and Huisman 2009). As the two of major cyanoHAB species in the field waters, filamentous Cylindrospermopsis and colonial Microcystis have gained increasing attention from both the scientific community and managers of water facilities (Padisák and Reynolds 1998; Nixdorf et al. 2003; Tomioka et al. 2011).
Previous reports have shown Cylindrospermopsis raciborskii is well regarded as an invasive cyanobacterial species since it is now becoming prevalent in more temperate regions (Padisák 1997; Saker and Griffiths 2001; Hamilton et al. 2005; Stüken et al. 2006; Moreira et al. 2015). Furthermore, C. raciborskii can produce toxic substances, such as cylindrospermopsin (CYN) and paralytic shellfish-poisoning toxin (PSP) that may cause serious health risks to both humans and wildlife (Byth 1980; Bourke et al. 1983; Saker and Griffiths 2001; Lagos et al. 1999). Therefore, C. raciborskii has become one of the most notorious and conspicuous harmful algae. Numerous factors might have facilitated the fast expansion and invasion of C. raciborskii, such as its high phenotypic plasticity and high level of flexibility to critical environmental factors (light and nutrients) (Wu et al. 2009; Isvánovics et al. 2000; Bonilla et al. 2011; Bai et al. 2014; Burford et al. 2016; Braind et al. 2004; Coles and Jones 2000).
C. raciborskii is replacing other bloom formers, such as Microcystis aeruginosa and Anabaena spp., or is becoming co-dominant in some tropical reservoirs (Marinho and Huszar 2000; Molica et al. 2005; Soares et al. 2009; Moisander et al. 2012; Yatigammana and Perera 2017). Long-term monitoring of Lake Julius in Australia has also shown a gradual shift within the cyanobacterial composition, from dominance by M. aeruginosa (Finlayson et al. 1985; Boland 1993) to C. raciborskii dominance after 1991 (Saker and Griffiths, 2001). Shifts in phytoplankton community are often thought to be a result of changes in nutrient factors (Fujimoto and Sudo 1997; Piehler et al. 2004; Hlaili et al. 2006) and physical factors (Gomes and Miranda, 2001; Moisan et al. 2002). For instance, Karadžić et al. (2013) found that on account of the heterocyst and the low optimal light requirements for growth, Cylindrospermopsis raciborskii occupied the dominance for collapse of Aphanizomenon flos-aquae in the Ponjavica River in Serbia, a eutrophic river with the higher biochemical oxygen demand and turbidity and lower NO3–N concentrations.
Simultaneously, nutrient composition and concentration of water indeed influence the formation of different algae blooms. As the most essential of nutrients in most ecosystems, the quantity and forms of phosphorus in the environment mediate phytoplankton biomass and influence phytoplankton species composition (Dyhrman et al. 2007). In natural waters, dissolved phosphorus comprises orthophosphate (DIP) (Diaz et al. 2008) and dissolved organic phosphorus (DOP) derived from the degradation of glycolipids, glycoproteins, and antibiotics (Clark et al. 1998; Kolowith et al. 2001; Dyhrman et al. 2006; White and Metcalf 2007). Although DIP is considered the most important P source for microalgae, the DIP is often low near the detection limit using standard analytical methods in many aquatic environments (Anagnostou and Sherrell 2008). However, organic phosphorus species have accounted for a substantial amount of the dissolved P found in many aquatic environments, such as nucleic acids (DNA and RNA), nucleotides (e.g., ATP, GTP, AND, AMP), inositol phosphates, pyrophosphate, phospholipids, and phosphonates (Baldwin 2013). Numerous studies showed that DOP is available for the production of both phytoplankton and bacteria in some freshwater systems, when the DIP concentration is insufficient (Rinker and Powell 2006; Wang et al. 2011; Bai et al. 2014; Li et al. 2015).
Our previous study has revealed that C. raciborskii could utilize organic phosphorus effectively (Bai et al. 2014). Otherwise, high storage capacity and high affinity for phosphorus might play essential roles in the dominance of C. raciborskii (Isvánovics et al. 2000; Wu et al. 2009). Furthermore, Wu et al. (2009) and Posselt et al. (2009) have shown that effective uptake and utilization to phosphate might play important roles in its occurrence and invasive behavior. Padisák (1997) revealed that the replacement of several cyanobacteria species by C. raciborskii in tropical aquatic ecosystems might be related to competition for resources. Therefore, we can suppose that the flexibility of the utilization for phosphorus might be a competitive strategy in C. raciborskii. Nevertheless, whether this factor of C. raciborskii might contribute to its success in interspecific competition is not yet well determined.
As the prevalent bloom-forming species of cyanoHAB, the growing properties of M. aeruginosa have been studied extensively under different environmental conditions. Ren et al. (2017) revealed that M. aeruginosa could utilize DOP under low P conditions and also exhibited a strong potential to scavenge DOP, which led to better compete with other species in the co-cultures. Moreover, M. aeruginosa could accumulate and store cellular P to ensure that its growth rates did not immediately decline (Li et al. 2015).
Base on the similarity of P utilization of the two species, we questioned (1) whether the phosphorus availability of the two strains was different or not and (2) which strain will be a winner in interspecific competition when the two strains co-existed under varying P sources. Therefore, to clarify the questions, the growth and competition between two bloom-forming cyanobacteria, C. raciborskii and M. aeruginosa, were investigated after the two species were mono-cultured and co-cultured at various phosphate treatments.
Materials and methods
Culture conditions
The Cylindrospermopsis raciborskii (Woloszýnska) strain FACHB 1096 we used in our experiments was obtained from the Culture Collections of the freshwater algae section of the Institute of Hydrobiology, Chinese Academy of Sciences (FACHB-Collection; Wuhan, China). A previous study had demonstrated the strain, C. raciborskii, was non-CYN (Bai et al. 2014). The unicellular strain Microcystis aeruginosa (Kützing) strain 205 was separated from the Three Gorges Reservoir and was non-toxic determined by toxin detection using HPLC method. The axenic strains were cultivated in MA medium (Ichimura 1979) at 25 ± 1 °C, and the light illumination was controlled at 25 μmol photons m−2 s−1, with a light and dark cycle of 12 h:12 h. The culture condition of the two strains was kept consistent. To guarantee the bacterial contamination remained less than 1% of the culture biomass, regular fluorescence microscopic inspection after acridine orange and 4′, 6-diamidino-2-phenylindole staining was analyzed in our whole experiments.
Experimental design
Before treatment with different phosphorus source, exponentially growing cells at MA medium were collected by centrifugation at 4000 rpm for 8 min, washed three times with a P-free medium, and then inoculated in a P-free medium for 3 days to remove excess phosphate of the cell.
After the P starvation, five phosphorus treatments, including β-glycerol phosphate, (2-aminoethyl)-phosphinic acid, glyphosate, dipotassium phosphate (K2HPO4, there for name after DIP), and P-free, were carried out in this study. The P element concentration in all media was about 10.13 mg L−1. The different organophosphorus was used to test in this study, because it has been reported to be important and representative for P loading in eutrophic freshwater lakes in China (Bai et al. 2009). An adequate concentration of nitrogen, 15.84 mg L−1, was supplied in all media in order to avoid the influence of nitrogen on both C. raciborskii and M. aeruginosa. For the mono-cultures, the algae were separately inoculated in 250-mL standard medium with different forms of phosphorus. To the co-culture experiments, the two species cells exposed to the P starvation were mixed and co-cultured in 500-mL culture flasks with different phosphorus treatments. The initial cell density proportions are as follows: (1) 25C75M: 25% C. raciborskii–75% M. aeruginosa, (2) 50C50M: 50% C. raciborskii–50% M. aeruginosa, and (3) 75C25M: 75% C. raciborskii–25% M. aeruginosa, respectively. The initial cell density of all the samples was 1 × 106 cells mL−1. All experiments were performed in triplicate.
Mono-culture and co-cultures experiments were carried out for 16 days under the same culture conditions as described above. Each flask was shaken twice a day manually. The algal cells were sampled at 0, 2, 4, 6, 8, 10, 13, and 16 days of inoculation.
Growth analyses
The cell density was determined using a hemocytometer chamber under a Nikon CE-I microscope (Nikon, Tokyo, Japan). The cell number of M. aeruginosa was obtained by direct counting, whereas the cell number of C. raciborskii was obtained according to methods provided by Bai et al. (2014). The specific growth rate (μ) in the experiment was calculated based on the cell density according to the following equation: μ = (lnCt2 - lnCt1)/(t2 - t1), in which Ct2 and Ct1 reflect the cell density at times day 4 and day 13, respectively.
Orthophosphate detection and extracellular alkaline phosphatase activity
Algae were filtered out, and the phosphorus molybdenum blue method was used for the measurement of orthophosphate concentration (Martin and Doty 1949). The extracellular alkaline phosphatase activity (APA) was assayed according to the protocol by Shen and Song (2007).
Statistical analyses
One-way ANOVA was performed using the Microcal Origin Version 8.5 (OriginLab Corporation, Northampton, MA, USA), to determine differences in growth rates between treatments, species, and culture methods. In all the analyses, the p value < 0.05 was considered as statistically significant. The statistical analyses were performed using the SPSS 17.0 Version (IBM, Armonk, New York City, USA).
Results
Mono-culture experiments
Growth
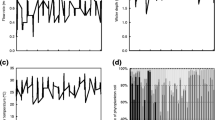
The relationship between the growth curve and various phosphorus resources for M. aeruginosa and C. raciborskii was displayed in Fig. 1. In the P-free cultivation, the growth rate was kept a slightly increase in M. aeruginosa and C. raciborskii, and the cell number increased to 2.94 ± 0.21 × 106 cells mL−1 and 4.42 ± 0.14 × 106 cells mL−1 after 16-d culture, respectively. Both cells could keep the growth when the two strains were exposed to treatments of DIP, (2-aminoethyl)-phosphinic acid, and β-glycerol phosphate, respectively. After 16-d culture at treatments of DIP, (2-aminoethyl)-phosphinic acid, and β-glycerol phosphate, the final cell densities of C. raciborskii were 11.13 ± 0.25 × 106, 17.00 ± 0.23 × 106, and 20.78 ± 0.14 × 106 cells mL−1, which was 2.60, 2.81, and 3.17 times higher than those of M. aeruginosa, respectively (ANOVA, P < 0.05). The growth rates (μ) of the two strains cultivated with (2-aminoethyl)-phosphinic acid and β-glycerol phosphate were significantly higher than those under P-free condition (Table 1, ANOVA, P < 0.05). However, growth inhibition was found in C. raciborskii cultured at glyphosate treatment, while M. aeruginosa showed an increasing growth; the specific growth rate was 0.11 ± 0.01 d −1 (ANOVA, P < 0.05).
Alkaline phosphatase activity (APA)
The extracellular alkaline phosphatase activity was displayed a similar tendency in both strains (Fig. 2); APA was rapidly increased and then decreased to a relatively constant level. Nevertheless, APA in M. aeruginosa was much lower than that in C. raciborskii. At treatments of P-free, DIP, (2-aminoethyl)-phosphinic acid, β-glycerol phosphate, and glyphosate, APA in C. raciborskii was 6.51, 1.94, 2.97, 3.78, and 4.02 times higher in contrast with M. aeruginosa at the 2 days (ANOVA, P < 0.05).
Phosphorus fraction
The change of phosphorus concentration in culture medium accompanying the growth of M. aeruginosa and C. raciborskii was showed in Fig. 3. The DIP concentration showed a similar trend between M. aeruginosa and C. raciborskii, representing a markedly increase at the early phase of their growth (day 0–6) under the treatments of β-glycerol phosphate and (2-aminoethyl)-phosphinic acid and then began to decline at after 6 days. In treatments of P-free and glyphosate, DIP concentration kept a relatively low level in both of the two species during the whole phase.
Co-culture experiments
Co-culture under P-free conditions
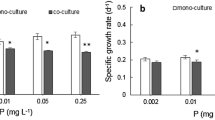
As the results showed in Fig. 4, the proportions of C. raciborskii revealed a mean 27.3% increase in the culture regardless of its initial cell density under P-free conditions. The lower the initial proportion of C. raciborskii, the higher an increase was observed in its proportion in the co-culture. The greatest change (36.7%) was found in 25C75M. Additionally, the specific growth rate (μ) of C. raciborskii was markedly higher than that of M. aeruginosa under three of the initial ratios (Table 1, ANOVA, P < 0.05). Moreover, compared with the μ values in mono-culture, the μ values of M. aeruginosa decreased by a mean of 34.3%, while the values of C. raciborskii increased by a mean of 63.5% (Table 1, ANOVA, P < 0.05).
During the entire experiment and in all investigated samples, a relatively high and rapid increase of extracellular APA was observed at the beginning of the experiment. The most significant up-regulation was observed in the 75C25M sample, in which APA was increased to a constant value of 0.65 ± 0.09 μmol (108 cells)−1 h−1 (Fig. 5).
Co-culture experiment under dissolved inorganic phosphorus
A proportion of C. raciborskii was increased under the treatment of K2HPO4, with a mean increasing rate of 31.8% for the three initial proportions (Fig. 6). Here, the highest increasing rate of C. raciborskii also was found (42.4%) among all of the co-culture experiments when the initial proportion was 75C25M. When the co-culture included K2HPO4 treatment, the growth curve of C. raciborskii showed a rapid increase, and the ratio of C. raciborskii increased to 63.4% when the initial proportion was 25C75M. Also, the growth rates of M. aeruginosa showed a lower value when compared with the mono cultivation (Table 1, ANOVA, P < 0.05).
Co-culture experiment using dissolved organic P sources
The results of the co-culture experiment using dissolved organic P treatments were shown in Fig. 7. After 16 days in culture, the cell density of C. raciborskii increased rapidly, when the co-culture cells were cultured in treatments of β-glycerol phosphate and (2-aminoethyl)-phosphinic acid and an increasing proportion of C. raciborskii was revealed in these two treatments regardless of the initial ratio. The mean increasing rate was 22.8% and 20.87%, respectively (Fig. 7a and b). The highest biomass of co-culture cells was observed in the 50C50M samples when the co-culture was incubated with (2-aminoethyl)-phosphinic acid, in which the cell density was increased to 17.72 ± 0.52 × 106 cell mL−1. In the meantime, a decreasing proportion was found in M. aeruginosa co-cultured at β-glycerol phosphate and (2-aminoethyl)-phosphinic acid (Fig. 7a and b). However, the specific growth rates in the two strains did not show a difference between the mono-culture and the co-culture (Table 1, ANOVA, P˃0.05).
By contrast, under glyphosate treatment, the ratio of C. raciborskii showed a distinctly decrease compared with the initial rate (Fig. 7c), with a depressed rate of 25.3% (25C75M), 40.8% (50C50M), and 43.2% (75C25M), respectively. However, the growth rate for C. raciborskii displayed an increase with the value of 0.02 ± 0.01, 0.04 ± 0.02, and 0.03 ± 0.02 d−1 for each initial proportion, which was much higher than those in the mono-culture. There was no difference of the growth rate between the mono-culture and the co-culture for M. aeruginosa under the glyphosate condition.
Discussion
Numerous researches have shown that phosphorus availability in aquatic ecosystems is crucial to control harmful algae blooms (Laws et al. 2011; Wang et al. 2011; Abell and Hamiltion 2013). Some algae that pose a capacity to use various phosphorus forms as sole phosphorus source have been widely investigated (Stihl et al. 2001; Dyhrman et al. 2002; Dyhrman et al. 2006; Shi et al. 2011; Li et al. 2015). In this study, the specific growth rate in C. raciborskii and M. aeruginosa was 0.09–0.20 d−1 and 0.06–0.11 d−1, respectively (Fig. 1 and Table 1) in the mono-cultures, which supported the results of Wu et al. (2009). A similar result was also proposed in the study of Xiao et al. (2017a, b) that the specific growth rate in C. raciborskii and M. aeruginosa was 0.15–0.70 d−1 and 0.16–0.55 d−1 when cultured at different light and temperature, suggesting that the utilization ability for phosphorus in C. raciborskii might be much stronger than that in M. aeruginosa and the two species showed apparent physiological plasticity (ecotype) (Willis et al. 2015; Burford et al. 2016; Robarts and Zohary 1992; Briand et al. 2004).
Mono-culture in C. raciborskii and M. aeruginosa
Reynolds (1997) indicated that aquatic microbial organisms could only directly assimilate DIP. Ren et al. (2017) also showed that DIP was the preferred phosphorus substrate in both culture types for M. aeruginosa, Chlorella pyrenoidosa, and Pseudokirchneriella subcapitata. However, in this study, higher growth rates were found in the two strains exposed to β-glycerol phosphate and (2-aminoethyl)-phosphinic acid in contrast to DIP treatment (Table 1), indicating that both Cylindrospermopsis and Microcystis could be efficient to use DOP. It was consistent with previous findings that the two strains could utilized the DOP as a solo P source in the mono-culture (Shi et al. 2011; Bai et al. 2014; Li et al. 2015; Ren et al. 2017). A similar result was also reported by Wang et al. (2011), who revealed that Prorocentrum micans and Chattonella marina used phosphomonoesters (β-glycerol phosphate and glyphosate) more efficiently than inorganic phosphorus. Dyhrman et al. (2007) showed that β-glycerol phosphate and (2-aminoethyl)-phosphinic acid were efficient to support algal growth when DIP was insufficient in natural waters. These suggested that the utilization of DOP by cyanobacteria might play essential roles in the frequent occurrence of cyanoHABs.
Wong (2000) and Forlani et al. (2008) showed that different appropriate concentration glyphosate might stimulate the growth of different bloom algae, such as Scenedesmus quadricauda at 0.0037 mg L−1, Leptolyngbya boryana at 6.2 mg L−1, and Nostoc punctiforme at 3.1 mg L−1. Qiu et al. (2013) had shown that M. aeruginosa could utilize glyphosate (a typical exogenous DOP caused by anthropogenic activity) at a low concentration. In the present study, an increasing growth was found in M. aeruginosa exposed to glyphosate, whereas C. raciborskii showed an inhibiting growth, even more cell death at the treatment of glyphosate (Fig. 1 and Table 1), supporting our previous result that C. raciborskii could not utilize the glyphosate (Bai et al. 2014). The result suggested that glyphosate might be toxic to C. raciborskii (Ren et al. 2017). Tsui and Chu (2008) stated that glyphosate could influence the microbial communities in freshwater lakes.
Alkaline phosphatase (AP) activity is often recommended as an indicator of P status, since it was induced to hydrolyze organic phosphorus to compensate for the deficiency in ambient DIP (Gillor et al. 2002; Dyhrman and Ruttenberg 2006), indicating that C. raciborskii could produce AP as a spontaneous response to P limitations (Gillor et al. 2002; Li and Brett 2013; Prentice et al. 2019). Reynolds (1997) demonstrated that cyanobacteria could first hydrolyze DOP through AP to assimilate the DOP. In the present study, alkaline phosphatase activity in both C. raciborskii and M. aeruginosa was significantly increased at mono-cultures of P-free, β-glycerol phosphate, (2-aminoethyl)-phosphinic acid, and glyphosate (Fig. 2). Tan et al. (2007) found that a lower capacity to excrete AP of M. aeruginosa was determined than other cyanobacteria, A. flos-aquae and O. planctonica, under P-stress. A similar result was also observed in the present study, in that APA in M. aeruginosa was much lower than that in C. raciborskii (Fig. 2), reflecting that high bioavailability of DOP in C. raciborskii at treatments of DOP and P-free.
Studies also declared that DOP might be associated with surface enzymes, which was then hydrolyzed to DIP by algae for direct utilization or transient accumulation in culture medium (Jacobson and Halmann 1982; Van Moorleghem et al. 2013). Generally, AP played a vital role in the utilization of phosphomonoesters and phosphonate (Reynolds 1997; Wan et al. 2019). In this study, DIP release showed a significant increase at treatments of β-glycerol phosphate and (2-aminoethyl)-phosphinic acid (Fig. 3), indicating that cyanobacteria could assimilate P from complex organic P sources by AP to recalcitrant and non-bioavailable (Ilikchyan et al. 2009). However, DIP release was not found in treatments of P-free and glyphosate (Fig. 3), implying that high bulk APA did not necessarily lead to DIP release in glyphosate as solo P source (Ren et al. 2017).
Co-culture between C. raciborskii and M. aeruginosa
Studies have indicated that M. aeruginosa and C. raciborskii could co-occur in some places (Soares et al. 2009; Dantas et al. 2011). It has already been shown that C. raciborskii is capable of dominating to M. aeruginosa in a lake or lowland river (Soares et al. 2009; Karadžić et al. 2013). Marinho et al. (2013) found that C. raciborskii can either dominate or be displaced by M. aeruginosa when exposed to different conditions of light or phosphate limitation. In the present study, when the co-culture was implemented in P-free medium, the proportion of C. raciborskii showed an increase under different treatments of the initial cell density (Fig. 4), suggesting that competition for resources and the regulatory mechanisms may have developed between these two species. In this study, a relatively high and rapid increase of extracellular APA was observed at the beginning of the experiment (Fig. 5). Moreover, the higher the initial proportion of C. raciborskii, the more APase was synthesized (Fig. 5), which is consistent with the result of mono-culture experiment. Tan et al. (2007) indicated that the tolerance to P-stress in M. aeruginosa was weaker than that observed in two other bloom-forming cyanobacteria species, Aphanizomenon flos-aquae and Oscillatoria planctonica. Additionally, the capacity to excrete AP was determined for three cyanobacteria under P-stress: A. flos-aquae > O. planctonica > M. aeruginosa (Tan et al. 2007). Shen and Song (2007) determined that the unicellular M. aeruginosa consumed more P to maintain the growth, resulting in a weaker tolerance to P-free conditions. These suggested that C. raciborskii could synthesize more APase to acclimatize and tolerance to P-starvation (Wu et al. 2012; Bai et al. 2014; Rzymski et al. 2014).
Studies have shown that the stronger competitive capacity to P might lead to the dominance of algae (De Nobel et al., 1997). When co-cultured under the treatment of K2HPO4, increasing proportions of C. raciborskii were the highest (Fig. 6). The growth rates of C. raciborskii were significantly higher than of M. aeruginosa in three initial proportions (Table 1). Wu et al. (2009) found that in comparison with M. aeruginosa and A. flos-aquae, C. raciborskii exhibited a more effective uptake and utilization of phosphate. The result further supported that high uptake to DIP of C. raciborskii provides an advantage for its dominance (Isvánovics et al. 2000). Interestingly, the growth curve of C. raciborskii occurred at a great fluctuation in the 25C75M group. One explanation could be that chemical substances were secreted by a large number of M. aeruginosa cells and then induced a suppression of C. raciborskii (Mello et al. 2012).
In this study, the co-culture in media treated with β-glycerol phosphate revealed a mean 22.8% increase of C. raciborskii proportion at the expense of M. aeruginosa growth, independent of its initial cell density. Similarly, an 20.87% increase of C. raciborskii proportion in media treated with (2-aminoethyl)-phosphinic acid was observed in all samples (Table 1, Fig. 7a and b). Shi et al. (2011) showed that the growth rates and cell abundances decreased when M. aeruginosa was inoculated into DOP, such as glucose-1-phosphate, adenosine triphosphate, and cyclic-adenosine monophosphate, in contrast with DIP group. Smith and Kalff (1982) found that the interspecific variation of phosphate uptake kinetics is mainly dependent on phytoplankton cell size, nutrition quota in the cell, and environmental factors. In this study, the size of C. raciborskii filament was 20 μm; however, the cell diameter of M. aeruginosa is mainly less than 3.5 μm, suggesting that large cell size might be responsible for the competition of C. raciborskii by phosphate uptake, storage, and capacity.
Previous results have shown that the cell density of M. aeruginosa increased rapidly when the strain was cultured at different appropriate concentration glyphosate (Wong, 2000; Forlani et al. 2008; Qiu et al. 2013). Bai et al. (2014) found that C. raciborskii did not survive at the treatment of glyphosate. Similar result was also found at co-culture between C. raciborskii and M. aeruginosa explored to glyphosate. When the co-culture was incubated into the media with glyphosate, the decreased contribution of C. raciborskii was found in the present study (Table 1, Fig. 7c), reflecting that a difference in the competition and the regulatory for glyphosate between C. raciborskii and M. aeruginosa was developed.
In conclusion, the study has shown that both C. raciborskii and M. aeruginosa could utilize the DOP as a useful P source in the mono-culture. However, a significant increase of C. raciborskii proportion was observed independent of its initial contribution in the co-culture, except for glyphosate treatment, revealing that C. raciborskii was a strategist with high bioavailable P and a good competitor (Shen and Song 2007; Willis et al. 2015, 2017). Moreover, Cylindrospermopsis is known to dominate in water bodies with very low DIP (< 0.06 μm) (Padisák 1997; Burford and O’Donohue 2006; Marinho et al. 2013). Therefore, studies on Pi deficiency and DOP utilization capability by Cylindrospermopsis are important to control Cylindrospermopsis bloom. However, Xiao et al. (2017b) indicated cyanobacterial competition was highly variable due to be depended on strains present and environmental conditions, such as light and temperature, etc., suggesting that there was no absolute “winner” under all conditions as there were always strains predicted to co-exist with the dominant strains. Therefore, in order to clarify P storage and utilization mechanisms of phytoplankton further, studies about the different strains and different cultivation environment are needed in the future.
Abbreviations
- APA:
-
Alkaline phosphatase activity
- C–P:
-
Carbon–phosphorus bond
- C–O–P:
-
Carbon–oxygen–phosphorus bond
- DIP:
-
Dissolved inorganic phosphorus
- DOP:
-
Dissolved organic phosphorus
- DRP:
-
Dissolved reactive phosphorus
References
Abell JM, Hamiltion DP (2013) Bioavailability of phosphorus transported during storm flow to a eutrophic, polymictic lake. N Z J Mar Freshw Res 4:481–489
Anagnostou E, Sherrell RM (2008) A MAGIC method for sub-nanomolar orthophosphate determination in freshwater. Limnol Oceanogr Methods 6:64–74
Bai F, Liu R, Yang Y, Ran X, Shi J, Wu Z (2014) Dissolved organic phosphorus use by the invasive freshwater diazotroph cyanobacterium, Cylindrospermopsis raciborskii. Harmful Algae 39:112–120
Bai XL, Ding SM, Liu T, Shi D, Zhang L (2009) Organic phosphorus species in surface sediments of a large, shallow, eutrophic lake, Lake Taihu, China. Environ Pollut 157:2507–2513
Baldwin DS (2013) Organic phosphorus in the aquatic environment. Environ Chem 10:439–454
Boland KT (1993) A comparison of two lakes in north western Queensland. Ph. D. Thesis. James Cook University of North Queensland, Townsville
Bonilla S, Aubriot L, Soares MCS, González-Piana M, Fabre A, Huszar VLM, Lürling M, Antoniades D, Padisák J, Kruk C (2011) What drives the distribution of the bloom forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol Ecol 3:594–607
Bourke ATC, Hawes RB, Nellson A, Stallman ND (1983) An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon 3:45–48
Briand J-F, Leboulanger C, Humbert J-F, Bernard C, Dufour P (2004) Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? J Phycol 2:231–238
Burford MA, Beardall J, Willis A, Orr PT, Magalhaes VF, Rangel LM, Azevedo SM, Neilan BA (2016) Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 54:44–53
Burford MA, O’Donohue MJ (2006) A comparison of phytoplankton community assemblages in artificially and naturally mixed subtropical water reservoirs. Freshw Biol 51:973–982
Byth S (1980) Palm island mystery disease. Med J Aust 2:40–42
Clark LL, Ingall ED, Benner R (1998) Marine phosphorus is selectively remineralized. Nature 393:426–429
Coles JF, Jones R (2000) Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J Phycol 16:7–16
Dantas EW, Moura AN, Bittencourt-Oliveira Mdo C (2011) Cyanobacterial blooms in stratified and destratified eutrophic reservoirs in semi-arid region of Brazil. An Acad Bras Cienc 4:1327–1338
De Nobel WT, Huisman J, Snoep JL, Mur LR (1997) Competition for phosphorus between the nitrogen-fixing cyanobacteria Anabaena and Aphanizomenon. FEMS Microbiol Ecol 24:259–267
Diaz J, Ingall E, Benitez-Nelson C, Paterson D, de Jonge MD, McNulty I, Brandes JA (2008) Marine polyphosphate: a key player in geologic phosphorus sequestration. Science 320:652–655
Dyhrman ST, Ammerman JW, Van Mooy BAS (2007) Microbes and the marine phosphorus cycle. Oceanography 20:110–116
Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury J, Webb EA (2006) Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439:68–71
Dyhrman ST, Webb E, Anderson DM, Moffett J, Waterbury J (2002) Cell specific detection of phosphorus stress in Trichodesmium from the Western North Atlantic. Limnol Oceanogr 6:1823–1836
Dyhrman ST, Ruttenberg KC (2006) Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnol Oceanogr 3:1381–1390
Finlayson CM, Farrell TP, Griffiths DJ (1985) The hydrobiology of five man-made lakes in northwestern Queensland. Proc R Soc Queensland 95:29–40
Forlani G, Pavan M, Gramek M, Kafarski P, Lipok J (2008) Biochemical bases for a widespread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant Cell Physiol 49:443–456
Fujimoto N, Sudo R (1997) Nutrient-limited growth of Microcystis aeruginosa and Phormidium tenue and competition under various N:P supply ratios and temperatures. Limnol Oceanogr 2:250–256
Gillor O, Hadas O, Post AF, Belkin S (2002) Phosphorus bioavailability monitoring by a bioluminescent cyanobacterial sensor strain. J Phycol 38:107–115
Gomes LC, Miranda LE (2001) Hydrological and climatic factors limit phytoplankton biomass in the upper Parana River Basin, Brazil. Hydrobiologia 457:205–214
Hamilton PB, Ley LM, Dean S, Pick FR (2005) The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: an exotic cyanoprokaryote new to Canada. Phycologia 44:17–25
Hlaili AS, Chikhaoui MA, Grami BE, Mabrouk HH (2006) Effects of N and P supply on phytoplankton in Bizerte Lagoon (western Mediterranean). J Exp Mar Biol Ecol 333:79–96
Ichimura T (1979) Isolation and culture methods of algae (Sôrui no bunri to baiôyhô. 2.5.B. Tansui sôrui). In: Nishizawa K, Chihara M (eds) Methods in Phycological Studies (Sôrui Kenkyûhô). Kyoritu Shuppan, Tokyo, (in Japanese without English title), pp 294–305
Ilikchyan N, Mckay RML, Zehr JP, Dyhrman ST, Bullerjahn GS (2009) Detection and expression of the phosphonate transporter gene phnD, in marine and freshwater picocyanobacteria. Environ Microbiol 11:1314–1324
Isvánovics V, Shafik HM, Présing M, Juhos S (2000) Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in through flow cultures. Freshw Biol 2:257–275
Jacobson L, Halmann M (1982) Polyphosphate metabolism in the blue-green alga Microcystis aeruginosa. J Plankton Res 3:481–488
Karadžić V, Simić GS, Natić D, Ržaničanin A, Ćirić M, Gačic Z (2013) Changes in the phytoplankton community and dominance of Cylindrospermopsis raciborskii (Wolosz.) Subba Raju in a temperate lowland river (Ponjavica, Serbia). Hydrobiologia 1:43–60
Kolowith LC, Ingall ED, Benner R (2001) Composition and cycling of marine organic phosphorus. Limnol Oceanogr 46:309–320
Lagos N, Onodera H, Zagatto PA, Andrinolo D, Azevedo SMFQ, Oshima Y (1999) The first evidence of paralytic shellfish toxin in the freshwater cyanobacterium Cylindrospermopsis raciborskii isolated from Brazil. Toxicon 37:1359–1373
Laws EA, Pei S, Bienfang P, Grant S, Sunda WG (2011) Phosphate-limited growth of Pavlova lutheri (Prymnesiophyceae) in continuous culture: determination of growth-rate-limiting substrate concentrations with a sensitive bioassay procedure. J Phycol 5:1089–1097
Li B, Brett MT (2013) The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ Pollut 182:37–44
Li JH, Wang ZW, Cao X, Wang ZF, Zheng Z (2015) Effect of orthophosphate and bioavailability of dissolved organic phosphorous compounds to typically harmful cyanobacterium Microcystis aeruginosa. Mar Pollut Bull 92:52–58
Marinho MM, Huszar VL (2000) Nutrient availability and physical conditions as controlling factors of phytoplankton composition and biomass in a tropical reservoir (Southeastern Brazil). Arch Hydrobiol 3:443–468
Marinho MM, Souza MBG, Lürling M (2013) Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Microb Ecol 3:479–488
Martin JB, Doty DM (1949) Determination of inorganic phosphate. Anal Chem 21:965–967
Mello MME, Soares MCS, Roland F, Lurling M (2012) Growth inhibition and colony formation in the cyanobacterium Microcystis aeruginosa induced by the cyanobacterium Cylindrospermopsis raciborskii. J Plankton Res 34(7):987–994
Moisan JR, Moisan TA, Abbott MR (2002) Modeling the effect of temperature on the maximum growth rates of phytoplankton populations. Ecol Model 153:197–215
Moisander PH, Cheshire LA, Braddy J, Calandrino ES, Hoffman M, Piehler MF, Parel HW (2012) Facultative diazotrophy increases Cylindrospermopsis raciborskii competitiveness under fluctuating nitrogen availability. FEMS Microbiol Ecol 3:800–811
Molica RJR, Oliveira EJA, Carvalho PVVC, Costa ANSF, Cunhaa MCC, Melo GL, Azevedo SMFO (2005) Occurrence of saxitoxins and an anatoxin-a(s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae 4:743–753
Moreira C, Fathalli A, Vasconcelos V, Antunes A (2015) Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Arch Mikrobiol 197:47–52
Nixdorf B, Mischke U, Rücker J (2003) Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes-an approach to differentiate the habitat properties of Oscillatoriales. Hydrobiologia 172:111–132
Padisák J (1997) Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology. Arch Hydrobiol Suppl 107:563–593
Padisák J, Reynolds RC (1998) Selection of phytoplankton associations in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia 384:41–53
Paerl HW, Huisman J (2009) Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep 1:27–37
Piehler MF, Twomey LJ, Hall NS, Paerl HW (2004) Impacts of inorganic nutrient enrichment on the phytoplankton community structure and function in Pamlico Sound, NC USA. Estuar Coast Shelf Sci 2:197–209
Posselt AJ, Burford MA, Shaw G (2009) Pulses of phosphate promote dominance of the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. J Phycol 45:540–546
Prentice MJ, Hamilton DP, Willis A, O'Brien KR, Burford MA (2019) Quantifying the role of organic phosphorus mineralisation on phytoplankton communities in a warm-monomictic lake. Inland Waters 9:10–24
Qiu H, Geng J, Ren H, Xia X, Wang X, Yu Y (2013) Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J Hazard Mater 248-249:172–176
Ren LX, Wang PF, Wang C, Chen J, Hou J, Qian J (2017) Algal growth and utilization of phosphorus studied by combined mono-culture and co-culture experiments. Environ Pollut 220:274–285
Reynolds CS (1997) Vegetation processes in the pelagic: a model for ecosystem theory. Ecology Institute, Berlin, p 371
Rinker KR, Powell RT (2006) Dissolved organic phosphorus in the Mississippi River plume during spring and fall 2002. Mar Chem 102:170–179
Robarts RB, Zohary T (1992) The influence of temperature and light on the upper limit of Microcystis aeruginosa production in a hypertrophic reservoir. J Plankton Res 14:235–247
Rzymski P, Poniedziaek B, Kokociński M, Jurczak T, Lipski D, Wiktorowicza K (2014) Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 35:1–8
Saker ML, Griffiths DJ (2001) Occurrence of blooms of the cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya and Subba Raju in a North Queensland domestic water supply. Mar Freshw Res 6:907–915
Shen H, Song L (2007) Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 1:475–486
Shi X, Qian S, Kong F, Zhang M, Yu Y (2011) Differences in growth and alkaline phosphatase activity between Microcystis aeruginosa and Chlorella pyrenoidosa in response to media with different organic phosphorus. J Limnol 1:21–25
Smith REH, Kalff J (1982) Size-dependent phosphorus uptaken kinetics and cell quota in phytoplankton. J Phycol 18:275–284
Soares MC, Rocha MIA, Marinho MM, Azevedo SMFO, Branco CWC, Huszar VLM (2009) Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: physical factors, nutrients and grazing effects. Aquat Microb Ecol 57:137–149
Stihl A, Sommer U, Post AF (2001) Alkaline phosphatase activities among populations of the colony-forming diazotrophic cyanobacterium Trichodesmium spp. (cyanobacteria) in the Red Sea. J Phycol 37:310–317
Stüken A, Rücker J, Endrulat T, Preussel K, Hemm M, Nixdorf B, Karsten U, Wiedner C (2006) Distribution of three alien cyanobacterial species (Nostocales) in Northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia 6:696–703
Tan X, Shen H, Song L (2007) Comparative studies on physiological responses at phosphorus stress of three waterbloom-forming cyanobacteria. Acta Hydrobiol Sin 5:693–699 (in Chinese)
Tomioka N, Imai A, Komatsu K (2011) Effect of light availability on Microcystis aeruginosa blooms in shallow hypereutrophic Lake Kasumigaura. J Plankton Res 8:1263–1273
Tsui MTK, Chu LM (2008) Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 3:439–446
Van Moorleghem C, De Schutter N, Smolders E, Merckx R (2013) Bioavailability of organic phosphorus to Pseudokirchneriella subcapitata as affected by phosphorus starvation: an isotope dilution study. Water Res 9:3047–3056
Wan LL, Chen XY, Deng QH, Yang L, Li XW, Zhang JY, Song CL, Zhou YY, Cao XY (2019) Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 84:46–55
Wang ZH, Liang Y, Kang W (2011) Utilization of dissolved organic phosphorus by different groups of phytoplankton taxa. Harmful Algae 12:113–118
White AK, Metcalf WW (2007) Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61:379–400
Willis A, Adams MP, Chuang AW, Orr PT, O’Brien KR (2015) Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Wolsoszyńska) Seenayya et Subba Raju). Harmful Algae 47:27–34
Willis A, Posselt AJ, Burford MA (2017) Variations in carbon-to-phosphorus ratios of two Australian strains of Cylindrospermopsis raciborskii. Eur J Phycol 52:303–310
Wong PK (2000) Effect of 2, 4-D, glyphosate and paraquat on growth, photosynthesis and chlorophyll-a synthesis of Scenedesmus quadricauda Berb. Chemosphere 41:177–182
Wu Z, Shi J, Li R (2009) Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon flos-aquae. Harmful Algae 8:910–915
Wu Z, Zeng B, Li R, Song L (2012) Physiological regulation of Cylindrospermopsis raciborskii (Nostocales, cyanobacteria) in response to inorganic phosphorus limitation. Harmful Algae 15:53–58
Xiao M, Adams MP, Willis A, Burford MA, O’Brien KR (2017a) Variation within and between cyanobacterial species and strains affects competition: implications for phytoplankton modeling. Harmful Algae 69:38–47
Xiao M, Willis A, Burford MA (2017b) Differences in cyanobacterial strain responses to light and temperature reflect species plasticity. Harmful Algae 62:84–93
Yatigammana SK, Perera MBU (2017) Distribution of Cylindrospermopsis raciborskii (Cyanobacteria) in Sri Lanka. Ceylon J Sci 3:65–80
Funding
The study was supported by the National Natural Science Foundation of China (41877410) and the Fundamental Research Funds for the Central Universities (XDJK2019C044).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vitor Manuel Oliveira Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, F., Shi, J., Yang, S. et al. Interspecific competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa on different phosphorus substrates. Environ Sci Pollut Res 27, 42264–42275 (2020). https://doi.org/10.1007/s11356-020-08652-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08652-0