Abstract
The interactions between microplastics (MPs) and aquatic organisms are becoming increasingly frequent due to the extensive distribution of MPs in aquatic environments. MPs from the aquatic environment tend to accumulate and move through living organisms. Therefore, MPs can affect human health though the food chain and human consumption. In this brief review, the environmental distribution, sources, and transport of MPs are reviewed, and the potential consequences of the presence of MPs in the aquatic environment to human food are discussed. This review also summarized the toxicity effects and toxicity mechanisms of MPs based on various environmentally relevant test species and discussed the combined toxicity effects of MPs and various pollutants in aquatic ecosystems. The knowledge of the adverse effects on combined toxicity and the mechanism of MPs toxicity are very limited. Thus, a systematic assessment of the aquatic environmental risk in various species from MPs is challenging. In the end, we identify the knowledge gaps that need to be filled and provide suggestions for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are widely used to manufacture industrial and daily life products due to their unique properties, such as low production costs, light weight, versatility, and durability (Wang et al. 2016). The amount of plastic in diverse environments has increased dramatically over the last few years since global production has increased drastically (Derraik 2002). Jambeck et al. (2015) reported that over 10 million metric tons of plastic waste enters the oceans each year. Microplastics (MPs) are defined as tiny plastic particles smaller than 5 mm in size that have environmental persistence, bioavailability and harmful effects on the ecosystem (Scheurer and Bigalke 2018). Polyethylene (PE), polypropylene (PP), and polystyrene (PS) particles in cosmetic and medical products are the main sources of MPs in the environment. MPs can enter the aquatic environment through household sewage discharge or runoff from landfills. In addition, anthropogenic activities, such as littering, municipal solid waste collection and disposal processes, are also secondary sources of MPs in the environment (Horton et al. 2017). Different forms of MPs, such as pellets, fibers, and fragments, have been found in environmental samples. The amount of research on MPs has also been growing rapidly in marine and freshwater environments. Studies on plastic waste in aquatic environments have attracted attention since the early 2000s. MPs undoubtedly created enormous threats to human health and ecological balance. MPs pose a great threat to the aquatic environment due to their permanence and toxic potential (Barletta et al. 2019). Hidalgo-Ruz et al. (2012) reported that MPs were detected in various aquatic environments, such as surface water, shorelines, deep water, sediment, beaches, and benthic zones. Wright et al. (2013) found that MPs could be ingested and accumulate in different marine species, such as barnacles, mussels, lugworms, crustaceans and fishes, turtles, and seals. The continuous ingestion and accumulation of MPs in marine environments are unavoidable and exacerbating potential threats to organism survival and human health. Abbasi et al. (2018) suggested that MPs could accumulate in the human body though the consumption of seafood. MPs also cause physiological damage (internal scratches and obstructions) to organisms via ingested exposure. In addition, the toxicity to organisms could be changed through the sorption of chemical contaminants to MPs. Watts et al. (2014) found that the contamination sported by MPs might also affect marine food chains from the smallest planktivorous organisms to large fish and mammals.
Some research has been focus on in vitro and in vivo study to analyze MPs toxic effects on aquatic organisms. In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism. It facilitates probing the experimental effects of MPs on living subject. An in vivo test is considered a good method with which to investigate long-term toxicity and can better reveal in vivo organism toxicity. However, it is time and money consuming as its disadvantages. In vitro studies in experimental biology are those that are conducted using components of an organism that have been isolated from their usual biological surroundings in order to permit a more detailed or more convenient analysis than can be done with whole organisms, such as microorganisms, cells, or biological molecules. The primary advantage of in vitro work is that it permits an enormous level of simplification of the system under study, so that the investigator can focus on a small number of components. Using in vitro study can also avoid ethical issues by using animals. In vitro techniques allow specific biological and mechanistic pathways to be isolated and tested under controlled conditions in ways that are not feasible in in vivo tests. The toxic effects of MPs described in the in vivo and in vitro studies have mainly included cytotoxicity (altered metabolism, decreased growth, and lytic or apoptotic cell death), proliferation, genotoxicity, altered gene expression, oxidative stress, growth inhibition, and neurotoxicity. In aquatic toxicity studies of MPs are concerned more and more on many kinds of species including fish, algae, shellfish, shrimp, and marine mollusks. In addition, organisms reared in aquaculture can also ingest MPs similar to wild populations, which are consumed by humans as food (Bessa et al. 2018). As a result, the concern about the potential threat of MPs to human health has increased. In this review, we summarized the biotoxicity of MPs on aquatic organisms and discussed the toxicological impacts of MPs on organisms. We also discussed the distribution and transport of MPs in the environment. The sorption behavior of MPs with changing joint toxicity effects of other chemicals has been summarized in this review. Furthermore, the present shortage of research, future challenges, and potential hazards of MPs to human health are discussed in depth.
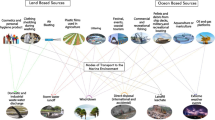
Environmental distribution, source, and transport of MPs in food chain
MPs are widespread in global environments and are highly persistent in different aquatic environments, such as marine water, freshwater (rivers and lakes), groundwater, ocean sediments, and river sediments (Klein et al. 2015). Frere et al. (2017) found that the most polluted regions of MPs were ocean estuaries and other coastal areas in regions heavily impacted by anthropogenic factors. Most MPs come from continental sources entering the marine environment mainly through rivers, soil and beach sediment runoff, rainfall, and industrial and urban effluents (Lebreton et al. 2017).
Riverine transport to the marine environment is an important pollutant transfer behavior for MPs. Andrady (2011) also discussed the degradation of plastics via weathering on the beaches, which results in surface embrittlement and microcracking, yielding MPs that are carried into the water by wind or wave action. Zylstra (2013) found that MPs with higher densities were susceptible to wind and surface runoff and were more likely to enter surface aquatic and terrestrial systems. The other sources of MPs entering the marine environment are direct inputs, including oil and gas extraction, aquaculture, and litter released during human activities. Retama et al. (2016) found that 60–80% of marine and ocean litters are various types of plastic particles, such as polystyrene, polyvinyl chloride, polyethylene, nylon, and polycarbonates. Gewert et al. (2017) suggested that marine MPs are divided into primary MPs, which are originally formed in MPs size range (from cosmetics, cleaning products, industries, or industrial wastewater) and secondary microplastics, produced by breakdown of larger size plastic debris through physical, sunlight radiation, and biological degradation processes. A considerable amount of such plastic debris has been found worldwide and is highly persistent in the Atlantic and Pacific oceans. Moreover, some researchers reported that MPs were also detected in the open ocean and abyssal and polar regions, ranging from the Caribbean Sea to the Mediterranean Sea in terms of enclosed oceans (Barnes et al. 2009; Welden and Lusher 2017). Obbard et al. (2014) found that MPs have accumulated far from population centers and that polar sea ice represents a major historic global sink of man-made particulates. Moreover, MPs are one of the most significant organic pollutants in freshwater. Mani et al. (2015) reported that a peak concentration of 3.9 million particles/km2 was measured in the Rhine-Ruhr (Rhine, one of the largest rivers in Europe) metropolitan area. The concentrations of MPs were diverse along and across the river, reflecting various sources and sinks, such as wastewater treatment plants, tributaries, and weirs. In addition, MPs concentrations demonstrated statistically significant positive correlations with population density and proportion of urban/suburban development within watersheds. The greatest MPs concentrations also occurred after major rain events in estuarine rivers (Chesapeake Bay) (Yonkos et al. 2014). Lusher et al. (2015) indicated that the concentration of MPs ranged from 0.34 to 102,000 particles/m3 in aquatic environments. Klein et al. (2015) suggested the importance of rivers as vectors of transport of MPs into the ocean. In addition, large rivers are the major pathways for the transport of land-based plastic litter into the ocean (Lechner et al. 2014). For example, approximately 4.2 t of MPs flow into the Black Sea via the Danube each day (Lechner et al. 2014). Freshwater sediments have also attracted attention during the investigation of sediments in aquatic environments. MPs have been detected in river shore sediments of the Rhine and Main rivers in the Rhine-Main area in Germany (Klein et al. 2015). MPs ultimately accumulate in sediments worldwide due to their properties, such as buoyancy, extreme durability, and synthetic polymers. It has been reported that 4.8 to 12.7 million tons of MPs were estimated to be released into the marine environment every year (Jung et al. 2018). A lot of MPs accumulate on beaches and coastal areas, float on the oceans, sink, and remain in the sediment at the bottom of the ocean (Qiu et al. 2016). Browne et al. (2011) showed that MPs contaminated the shorelines at 18 sites worldwide representing six continents from the poles to the equator, with more material in densely populated areas. MPs from coastal sediments of the Algarve region and Southern Portuguese shelf waters were retrieved from sediment samples, and the concentration of MPs ranged from 10 to 3320 items/kg (Frias et al. 2016). Imhof et al. (2013) reported that a high concentration of MPs was detected in the beach sediments of a subalpine lake. Di and Wang (2018) found that the MPs pollution in the surface waters and sediments from the Three Gorges Reservoir reached levels from 25 to 300 n/kg wet weight (WW). With the large production and rapid expansion of MPs, there is a crucial need for a critical review of health risk assessments of MPs in the environment.
Lebreton et al. (2017) found that MPs come from continental sources entering the marine environment mainly through rivers, industrial and urban effluents, and runoff of beach sediments and neighbor fields. River hydrodynamics, water surface area, depth, prevailing wind, and surface current affected MPs distributions, with profound implications for emissions to marine systems. Andrady (2011) explained that surface runoff was the main mechanism for MPs transported in water along rivers and into oceans. Zylstra (2013) found that wind could disperse substantial amounts of trash > 2 km into protected natural areas and suggested that the accumulation of trash poses a potential threat to desert ecosystems. This research also found that MPs with higher densities were more susceptible to wind and surface runoffs and, furthermore, were more likely to reach surface aquatic systems. In addition, the organisms in the soil also act as a good medium for the transport of MPs in the water through surface runoff. There is an increasing tendency for global MPs abundance and dispersion with the wide use of MPs. MPs in the environment also have the potential to absorb various pollutants, including heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, perfluoroalkyl acids, and other toxic chemicals. The different chemicals are always adsorbed to the surface of the plastics. Hydrophobic and electrostatic forces in the solution are suggested to be another mechanism of MPs toxicity, which refers to the interaction of different pollutants in the water environment. Wu et al. (2019a) suggested that hydrophobic and electrostatic interactions were the main reasons for the sorption of other contaminants. Hydrophobic interactions relate to the attraction of non-polar molecules to the non-polar MPs surface, which are considered to be the main reason for the sorption of hydrophobic organic chemicals to MPs. Electrostatic interactions are caused by the attraction of oppositely charged molecules or repulsion of similarly charged molecules. In addition, size and shape may also affect adsorption, but absorption does not depend on the availability of sorption sites on the surface (Tourinho et al. 2019). The adsorption characteristics depend on the physicochemical characteristics of MPs, as well as their composition, size, shape, and color. Particles usually present a high area-to-volume ratio, which is expected to have high adsorption capacity, such as the particles with smaller sizes and irregular shapes. The contaminants can be released from the surface of MPs during their transport in the environment or inside organisms. Pollutants adsorbed by MPs can penetrate cells, disrupt molecules, and affect biological systems (Rochman et al. 2014). Hydrogen ions (H+) also affect the sorption of pollutants onto MPs when proton donor and proton acceptor groups are involved. For example, polyamide had a greater sorption capacity for a group of antibiotics than other MPs due to the hydrogen bond caused by the proton donor characteristic of the amide group in polyamide (Li et al. 2018).
Aquatic organisms can be exposed to MPs by different processes. Ingestion is an important absorption route for marine species exposure to pollution. After ingestion, MPs are transported through the circulatory system of an organism and transferred into different tissues and cells, potentially resulting in several types of adverse effects, such as pathological damage, lysosomal membrane destabilization, cell death, inflammatory response, development inhibition, and muscle scratches (von Moos et al. 2012). These adverse effects in organisms may be caused by the physical damage or biological toxicity of MPs. MPs can biologically accumulate in aquatic animal tissue (including wild populations and aquaculture) via the ingesting process, and this tissue is ultimately consumed by humans as food. Lusher et al. (2017) suggested that bivalves cultured in estuaries and coastal lagoons were more inclined to ingest MPs due to contamination with these particles in such areas. MPs have also been detected in seafood sold from markets, including in fish, bivalves, crustaceans, and shellfish sold for human consumption (Rochman et al. 2015). Furthermore, fish, shrimp or other farmed species could ingest MPs through the food chain, which also enhances the potential effects on human health. Abbasi et al. (2018) suggested that regardless of the mode of accumulation, the presence of MPs in heavily fished species of fish and crustaceans raised concerns about the potential transfer of synthetic materials into humans. Akhbarizadeh et al. (2018) suggested that consumption of meal ratios of 300 and < 100 g/week for adults and children, respectively, was recommended with no human health risk. In addition, the trophic transfer of MPs between species (Mateos-Cardenas et al. 2019) has been reported. The information about the toxicity effects of MPs on the human health through the food chain behavior is still lack. This review was to provide an overview of MPs potential risks in aquatic environment for human food security and health. The influence of physical characteristics of MPs on distribution and transport in the environmental was also need future investigation.
MPs research in the aquatic environment
In vivo toxicology studies
Aquatic organisms are directly or indirectly exposed to MPs due to their widespread water environments. Direct exposure is when pollutants come into direct contact with organisms through a medium such as water, soil, or air. However, indirect exposure is a form of exposure in which pollutants are toxic to organisms through the food chain or in combination with other compounds. Direct exposure generally causes short-term acute toxicity, while indirect exposure causes chronic organ toxicity. MPs can exposure to aquatic organisms by different exposure processes (intravenous, transdermal, subcutaneous, inhalation, intraperitoneal, and oral) and finally accumulate in the tissues and organs. The different exposure processes tend to different environmental condition. In vivo tests tend to focus on intravenous, subcutaneous, intraperitoneal, oral, and skin exposure. While in aquatic environmental tend to focus on inhalation, oral, and skin exposure. In addition, the adverse effects of MPs on organisms can be physical and biological. The physical effect of MPs causes the mechanical death, organ, tissue, or skin damage of aquatic organisms. The biochemical effect of MPs induces oxidative stress, inflammation, growth inhibition, accumulation distribution, and transportation in organs and impacts to lipid metabolism in aquatic organisms.
Ingestion is one type of common physical impacts on aquatic organisms. The ingestion of MPs could reduce digestion and feeding capacity and increase undernutrition and the disease states of aquatic animals due to blockages of the digestive tract by MPs (Van Franeker 1985). Small sizes of the MPs enhance their translocation across the gastro-intestinal membranes via endocytosis-like mechanisms and distribution into tissues and organs (Alimba and Faggio 2019). Since the last century, the ingestion of MPs by seabirds and turtles has been widely reported and reviewed (Tourinho et al. 2010). Fish as one of the largest and most diverse animal groups in the aquatic environmental and also be affected by plastic MPs. For example, in a tropical estuary of the Brazilian Northeast, Cathorops spixii and Cathorops agassizii, 18% and 33% of individuals had ingested plastic debris in their stomachs, respectively. These plastics were threat to feeding resource in southwestern Atlantic estuaries (Possatto et al. 2011). Sharks and rays were also reported as being physical affected by MPs ingestion (Boerger et al. 2010). MPs are thought to be ingested by a wide range of marine organisms and be translocated in other organs before being excreted (Collard et al. 2017).
The adverse biological effects of MPs on vertebrates include accumulation, distribution, and transportation in organs. For example, polystyrene MPs could induce microbiota dysbiosis and inflammation in the gut of adult zebrafish (Jin et al. 2018). Lei et al. (2018) found that the exposure of zebrafish to ~ 70 μm MPs caused intestinal damage, including cracking of villi and splitting of enterocytes. Exposure to MPs also induced alterations of metabolic profiles in zebrafish liver and disturbed lipid and energy metabolism (Lu et al. 2016). Furthermore, 7 days of exposure to 5 μm diameter MPs resulted in accumulation in the gills, livers, and guts of fish, while 20 μm diameter MPs accumulated in only the gills and guts of fish (Lu et al. 2016). Collard et al. (2017) suggested that there was one translocation pathway of MPs between organs: smaller pieces agglomerated into large particles and passed through the intestinal barrier to other organs. In addition, the muscle, gut, and gill of fish are also considered common organs for MPs accumulation and distribution. Akhbarizadeh et al. (2018) suggested that MPs accumulated in the muscles of pelagic fish species, including Platycephalus indicus, Epinephelus coioides, Alepes djedaba, and Sphyraena jello. MPs could be transported into the circulatory system of the mussel Mytilus edulis and accumulated in the gut (Browne et al. 2008). Watts et al. (2014) found that shore crab (Carcinus maenas) could take up MPs through transport across the gills as well as ingestion of pre-exposed food (common mussel Mytilus edulis). Moreover, the adverse effects of MPs also include oxidative stress, inflammation, growth inhibition, and impacts to lipid metabolism in aquatic organisms. Inflammation and oxidative stress were observed in the gut of zebrafish after exposure to MPs (5-pm beads; 50 vig/L and 500 irg/L) for 21 days (Qiao et al. 2019). Oliviero et al. (2019) showed a decrease in larval length in plutei exposed to low concentrations of MPs and a blockage of larval development in sea urchin embryos exposed to the highest dose. In addition, MPs elicited immunological responses and induced oxidative stress in aquatic organisms via altered gene expression and free radical generation. For example, Zhang et al. (2019) found that 48-h exposure to MPs (0.1 mg/L) activated the activities of catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) in Daphnia magna. Acute exposure of MPs could activate the stress response of the scleractinian coral Pocillopora damicornis and repress its detoxification and immune system through the JNK and ERK signal pathways (Tang et al. 2018).
The research on MPs toxicity in invertebrates has mostly focused on amphipods. Some research found that MPs could decrease the growth and reproduction of Hyalella azteca (Au et al. 2015; Syakti et al. 2019) and increase the mortality of starved daphnids (Aljaibachi and Callaghan 2018). However, ingestion of MPs had no apparent impact on amphipod mortality or mobility after 24 or 48 h of exposure (Mateos-Cardenas et al. 2019). Bruck and Ford (2018) reported that ingesting low concentrations of 8 mm MPs did not impair the feeding or growth of amphipods during the exposure period. In addition, after chronic exposure over 48 days, MPs did not significantly affect the survival, development (molting), metabolism (glycogen, lipid storage), or feeding activity of Gammarus pulex (Weber et al. 2018). Some studies found that MPs could inhibit the root growth of Lemna minor (Kalcikova et al. 2017), inhibit the growth of Skeletonema costatum (Zhang et al. 2017), inhibit the shoot length of Myriophyllum spicatum (van Weert et al. 2019), inhibit the growth of the freshwater algae Chlorella pyrenoidosa (Mao et al. 2018), affect the growth of Chlorella pyrenoidosa and Microcystis, (Wu et al. 2019a, b) and decrease the growth rate of Tetraselmis chuii (Davarpanah and Guilhermino 2019). In addition, MPs also caused reproductive toxicity in Daphnia magna, Daphnia pulex, and Ceriodaphnia dubia (Jaikumar et al. 2019) after chronic exposure and reduced photosynthetic activity in the freshwater algae Chlorella pyrenoidosa (Mao et al. 2018).
Furthermore, the potential for MPs to biologically accumulate in organisms and the fate of biological chain transfer are largely unexplored. This review presents the trend of aquatic MPs pollution with a focus on its current toxicological consequences in mammals, fish, and plants.
In vitro toxicology studies
The toxic effects of MPs on aquatic species at the molecular level have always been evaluated in vitro, but the research is very rare. MPs can be taken up into cells and induce cell-level toxicity. For example, Espinosa et al. (2018) suggested that continued exposure of fish to polyvinylchloride (PVC) and PE MPs could impair fish immune parameters, probably due to the oxidative stress produced in the fish leukocytes. However, there was no evidence of oxidative stress or cellular damage in the liver of fish that had ingested MPs (Alomar et al. 2017). von Moos et al. (2012) found notable histological changes and a strong inflammatory response demonstrated by the formation of granulocytomas after 6 h of MPs exposure in the blue mussel Mytilus edulis L. Pannetier et al. (2019) suggested that DNA damage was observed after exposure to four microplastic samples in six tested rainbow trout liver cell lines. Aquatic plant cells are also an important index in the assessment of in vitro MPs toxicity. MPs-exposed cells could modulate their energy metabolism to properly acclimate to the stress conditions. Seoane et al. (2019) found a significant decrease in cellular esterase activity and neutral lipid content in Chaetoceros neogracile after exposure to MPs. In addition, exposure of Chlorella vulgaris to MPs exerted a significant inhibitory influence on cell photosynthesis (Luo et al. 2019). Although in vitro studies can explain the toxicity effects of MPs at the molecular level, unfortunately, at present, the available in vitro studies on aquatic species are very rare. This review presents the urgency and importance of future research on the influence of in vitro MPs. The studies demonstrating the toxicity effects in aquatic species exposed to MPs are reviewed in Table 1.
Toxicity mechanisms of MPs in aquatic environment
Oxidative stress is summarized as the main proposed mechanisms for the environmental toxicity of pollutants and ecotoxicity in organisms (Du et al. 2018a; Du et al. 2018b). Oxidative stress can disturb the balance of the capacity of an organism to deal with excessive reactive oxygen species (ROS), which can induce the antioxidant defense of organisms and cause oxidative damage to molecules (Prokic et al. 2018). ROS were necessary for organisms at moderate levels (Du et al. 2018a). Excessive ROS caused peroxidation of polyunsaturated fatty acids, damaged the cell membrane permeability, and damaged the structure of other molecules, such as DNA, proteins, and lipids. The damage of biomolecules triggers a series of reactions, such as inflammatory response, cell death, tissue damage, and DNA damage (Bartoskova et al. 2013). Oxidative stress can be adjusted through free radical production (non-enzymatic antioxidants) and antioxidant defenses, which balance excessive ROS and repair damaged cells (Sayre et al. 2005).
The toxicity mechanisms underlying the oxidative stress (free radical production, antioxidant defense regulation, some signal pathways, and gene expression) that is induced in aquatic organisms by MPs are summarized in this review. Several studies have indicated that antioxidant defenses are generated after exposure to MPs. For example, Ribeiro et al. (2017) found that exposure of Scrobicularia plana to 1 mg/L (20 mm) of MPs for 14 days and depuration for 7 days caused DNA damage, neurotoxicity, and oxidative damage. Oxidative stress biomarkers were detected in the tissues of organisms. In addition, MPs could induce significantly increased activities of SOD and CAT, indicating that oxidative stress was induced after treatment with MPs (Lu et al. 2016). MPs significantly increased the activities of SOD and CAT and decreased GPx and alkaline phosphatase after scleractinian corals were exposed to MPs for 6, 12, and 24 h (Tang et al. 2018). Lei et al. (2018) suggested that MPs caused oxidative stress in Danio rerio and Caenorhabditis elegans through free radical formation, as an overproduction could alter physiological homeostasis of cellular components via suppressing the activity of antioxidant systems. Ribeiro et al. (2017) found that exposure of Scrobicularia plana to MPs induced a reduction in lipid oxidative damage, inflammation, and destabilization of lysosomal membranes, which was likely due to sufficient neutralization of ROS by the activation of antioxidative defense enzymes (Ribeiro et al. 2017). Some researchers found that the activation of the stress response was related to some signal pathways or gene expression. For example, Qu et al. (2018) found that nanopolystyrene particles (1 mu g/L) dysregulated the expression of some genes required for the control of oxidative stress and activated the expression of the Nrf signaling pathway after prolonged exposure in Caenorhabditis elegans. In a previous study, MPs activated oxidative stress by repressing the detoxification and immune system through the JNK and ERK signaling pathways after acute exposure in P. damicornis for 12 h (Tang et al. 2018). Additionally, Lei et al. (2018) suggested that 0.1, 1.0, and 5.0 μm sizes of MPs elicited oxidative stress by activating the expression of glutathione S-transferase 4 in nematodes. The production of ROS can cause DNA damage resulting in DNA strand breaks in response to MP exposure of organisms. Avio et al. (2015) indicated that MPs could induce irreversible loss of DNA integrity (nuclear alterations) and increased frequency of micronuclei in marine mussels. However, MPs did not produce oxidative stress and genetic damage, with the exception of a significant modulation of CAT and GSH activities in the freshwater zebra mussel Dreissena polymorpha exposed to MPs for 6 days (Magni et al. 2018). Other molecular mechanisms were proposed based on cytotoxicity. For example, both decrease in cellular esterase activity and inhibitory influence on cell photosynthesis after exposure to MPs could inhibit plant growth (Luo et al. 2019; Seoane et al. 2019). But the research on cytotoxicity of MPs is very rare in aquatic environment. Non-oxidative stress-related mechanism of MPs toxicity are required to fill knowledge gaps. The toxicity mechanisms of MPs are very important for understanding the harmful effects on aquatic organisms. The information on mechanisms is necessary to design more safe MPs products for human use and to analyze environmental risk assessment.
MPs influence on other chemical contaminant toxicity in aquatic environment
The pollutants attached to the MPs surface could improve some MPs properties and change the toxicity effects in organisms. MPs can be a vector for pollutants to enter an organism due to the sorption characteristics. For example, the solubility and Kow of polybrominated diphenyl are two important factors for determining pollutant sorption to MPs (Chua et al. 2014). Rochman et al. (2014) suggested that plastic debris associated with several chemical pollutants could disrupt the functioning of the endocrine system, alter gene expression, and affect the proliferation of germ cells. The interaction of MPs and compounds alters the toxicity effect of individual chemicals. It is important to determine the bioavailability of MPs and their coexisting pollution. Further investigations are needed to evaluate the potential risk of MPs and their coexisting characteristics in aquatic species.
There are usually three different complex toxicity effects on combined pollutants for aquatic species, including antagonistic, additive, and synergistic. The common aquatic species used in toxicity studies are microalgae, Daphnia magna, and fish. For example, the pesticide chlorpyrifos was found to adsorb onto MPs surfaces, which decreased bioavailability in the algal cells and inhibited microalgae growth (Garrido et al. 2019). Davarpanah and Guilhermino (2019) also found that a mixture containing 3 mg/L gold nanoparticles + 4 mg/L MPs significantly reduced the average specific growth rate of Tetraselmis chuii. This study also indicated that the tested MPs and gold nanoparticles had a relatively low toxicity to T. chuii, but the toxicity increased when they were in mixtures containing high concentrations of both substances. However, the joint toxicity of two types of MPs in combination with triclosan decreased more than that of single MPs when exposed to Skeletonema costatum. The increased adsorption capacity of triclosan on MPs is one possible reason for the greater reduction in co-exposure toxicity (Zhu et al. 2019). Bellingeri et al. (2019) found that Cu in combination with MPs exposure caused no significant difference in algal growth inhibition compared with a single exposure in short-term as well as long-term tests. Zhang et al. (2019) suggested that co-exposure of Daphnia magna to MPs and roxithromycin could activate the activities of CAT and GST as well as the MDA levels. The results showed that the responses of GPx and MDA in D. magna co-exposed to 1-μm MPs were significantly decreased, while co-exposure to 10-μm MPs significantly decreased the responses of GST and MDA compared with exposure to ROX alone. Rainieri et al. (2018) found that the combined effect of MPs and methylmercury significantly altered organ homeostasis, such as in the liver, intestine, muscular tissue, and brain, compared with MPs exposure alone. However, the adsorption characteristics were also dependent on the physicochemical characteristics of pollution (composition, size, shape, and color) and the endpoint analyzed. Zhang et al. (2019) also suggested that the effect of combined toxicity was largely dependent on the experimental method. For example, the presence of MPs did not influence the toxicity effects of gold nanoparticles on juveniles (Ferreira et al. 2016), but the presence of MPs caused significantly reduced predatory performance at high concentrations of chromium (VI) in Pomatoschistus microps in Lima but not in Minho Pomatoschistus microps (Luis et al. 2015). The studies demonstrating the combined toxicity effects in aquatic species exposed to MPs are reviewed in Table 2. Some researchers have noted that the affinity between pollutants affects the characteristics of sorption and that the toxicity effect of MPs interacts with other pollution (Tourinho et al. 2019). At present, the combined toxicity study of MPs is still scarce. The mechanism research of combined toxicity requires filling the gap in the aquatic environmental system.
Conclusions
The database Web of science was searched on November 1, 2019, for research that focused on MPs aquatic environmental toxicity, and the articles were selected published between 2005 and 2020. After systematic analysis, 89 pertinent articles were isolated. A summary of the search results is detailed in Fig. 1. This review was to provide an overview of the potential ecological risks associated with the presence of MPs in the aquatic environment. The environmental occurrence, transport, human food security, toxicity mechanism, toxicity effect, and ecological compatibility of MPs were reviewed and discussed in this review, as shown in Fig. 2. It is necessary to understand the interaction toxicity of pollutants and MPs in aquatic environmental. In addition, more studies are needed to be done to investigate the potential consequences for sustainability and human food safety with environmental accumulation of MPs in the future. The transport behaviors of MPs in aquatic environmental pathways are the main factor in affecting the human health. Therefore, there is urgent need to study the toxicity effect transfer with other pollutants though food chain and environmental behavior. A systematic understanding of the toxicity of MPs is important for restricting use. In the end, we indicated the current research gaps and proposed further research interests as follows:
- 1.
The biological coverage of MPs toxicity research is not broad. In vitro research on animals mostly focuses on cell of aquatic organisms (fish), while the available in vitro studies on aquatic species are very rare.
- 2.
The potential toxicity effects of MPs on other compounds should be highlighted. Biocompatibility is an important design criterion for MPs use in human product and release into the environment. The ecological toxicology risk assessment of MPs is required for the government formulating policy coordination.
References
Abbasi S, Soltani N, Keshavarzi B, Moore F, Turner A, Hassanaghaei M (2018) Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 205:80–87
Akhbarizadeh R, Moore F, Keshavarzi B (2018) Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163
Alimba CG, Faggio C (2019) Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol 68:61–74
Aljaibachi R, Callaghan A (2018) Impact of polystyrene microplastics on Daphnia magna mortality and reproduction in relation to food availability. Peer J 6:e4601
Alomar C, Sureda A, Capo X, Guijarro B, Tejada S, Deudero S (2017) Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ Res 159:135–142
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605
Au SY, Bruce TF, Bridges WC, Klaine SJ (2015) Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ Toxicol Chem 34:2564–2572
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222
Barletta M, Lima ARA, Costa MF (2019) Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci Total Environ 651:1199–1218
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond B Biol Sci 364:1985–1998
Bartoskova M, Dobsikova R, Stancova V, Zivna D, Blahova J, Marsalek P, Zelnickova L, Bartos M, di Tocco FC, Faggio C (2013) Evaluation of ibuprofen toxicity for zebrafish (Danio rerio) targeting on selected biomarkers of oxidative stress. Neuro Endocrinol Lett 34(Suppl 2):102–108
Bellingeri A, Bergami E, Grassi G, Faleri C, Redondo-Hasselerharm P, Koelmans AA, Corsi I (2019) Combined effects of nanoplastics and copper on the freshwater alga Raphidocelis subcapitata. Aquat Toxicol 210:179–187
Bessa F, Barria P, Neto JM, Frias J, Otero V, Sobral P, Marques JC (2018) Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar Pollut Bull 128:575–584
Boerger CM, Lattin GL, Moore SL, Moore CJ (2010) Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar Pollut Bull 60:2275–2278
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ Sci Technol 42:5026–5031
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45:9175–9179
Bruck S, Ford AT (2018) Chronic ingestion of polystyrene microparticles in low doses has no effect on food consumption and growth to the intertidal amphipod Echinogammarus marinus? Environ Pollut 233:1125–1130
Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes Compressa. Environ Sci Technol 48:8127–8134
Collard F, Gilbert B, Compere P, Eppe G, Das K, Jauniaux T, Parmentier E (2017) Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ Pollut 229:1000–1005
Davarpanah E, Guilhermino L (2019) Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicol Environ Saf 181:60–68
Derraik JG (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852
Di M, Wang J (2018) Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci Total Environ 616-617:1620–1627
Du J, Tang J, Xu S, Ge J, Dong Y, Li H, Jin M (2018a) A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul Toxicol Pharmacol 98:231–239
Du J, Tang J, Xu S, Ge J, Dong Y, Li H, Jin M (2018b) ZnO nanoparticles: recent advances in ecotoxicity and risk assessment. Drug Chem Toxicol:1–12
Espinosa C, Garcia Beltran JM, Esteban MA, Cuesta A (2018) In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environ Pollut 235:30–38
Ferreira P, Fonte E, Soares ME, Carvalho F, Guilhermino L (2016) Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat Toxicol 170:89–103
Frere L, Paul-Pont I, Rinnert E, Petton S, Jaffre J, Bihannic I, Soudant P, Lambert C, Huvet A (2017) Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: a case study of the Bay of Brest (Brittany, France). Environ Pollut 225:211–222
Frias JP, Gago J, Otero V, Sobral P (2016) Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar Environ Res 114:24–30
Garrido S, Linares M, Campillo JA, Albentosa M (2019) Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol Environ Saf 173:103–109
Gewert B, Ogonowski M, Barth A, MacLeod M (2017) Abundance and composition of near surface microplastics and plastic debris in the Stockholm Archipelago, Baltic Sea. Mar Pollut Bull 120:292–302
Grigorakis S, Mason SA, Drouillard KG (2017) Determination of the gut retention of plastic microbeads and microfibers in goldfish (Carassius auratus). Chemosphere 169, 233-238
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141
Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23:R867–R868
Jaikumar G, Brun NR, Vijver MG, Bosker T (2019) Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ Pollut 249:638–646
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771
Jin YX, Xia JZ, Pan ZH, Yang JJ, Wang WC, Fu ZW (2018) Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ Pollut 235:322–329
Jung MR, Horgen FD, Orski SV, Rodriguez CV, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer S, Hyrenbach D, Jensen BA, Lynch JM (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull 127:704–716
Kalcikova G, Zgajnar Gotvajn A, Kladnik A, Jemec A (2017) Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ Pollut 230:1108–1115
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ Sci Technol 49:6070–6076
Lebreton LCM, Van der Zwet J, Damsteeg JW, Slat B, Andrady A, Reisser J (2017) River plastic emissions to the world’s oceans. Nat Commun 8
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181
Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D (2018) Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 619-620:1–8
Li J, Zhang KN, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467
Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50:4054–4060
Luis LG, Ferreira P, Fonte E, Oliveira M, Guilhermino L (2015) Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat Toxicol 164:163–174
Luo H, Xiang Y, He D, Li Y, Zhao Y, Wang S, Pan X (2019) Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci Total Environ 678:1–9
Lusher AL, Tirelli V, O'Connor I, Officer R (2015) Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci Rep 5:14947
Lusher AL, Hollman PCH, Mendoza-Hill JJ (2017) Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety. In: FAO Fisheries and Aquaculture Technical Paper. No. 615. Rome, Italy
Magni S, Gagne F, Andre C, Della Torre C, Auclair J, Hanana H, Parenti CC, Bonasoro F, Binelli A (2018) Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci Total Environ 631-632:778–788
Mani T, Hauk A, Walter U, Burkhardt-Holm P (2015) Microplastics profile along the Rhine River. Sci Rep 5:17988
Mao YF, Ai HN, Chen Y, Zhang ZY, Zeng P, Kang L, Li W, Gu WK, He Q, Li H (2018) Phytoplankton response to polystyrene microplastics: perspective from an entire growth period. Chemosphere 208:59–68
Mateos-Cardenas A, Scott DT, Seitmaganbetova G, Frank NAMV, John O, Marcel AKJ (2019) Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci Total Environ 689:413–421
Obbard RW, Sadri S, Wong YQ, Khitun AA, Baker I, Thompson RC (2014) Global warming releases microplastic legacy frozen in Arctic Sea ice. Earths Future 2:315–320
Oliviero M, Tato T, Schiavo S, Fernandez V, Manzo S, Beiras R (2019) Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus. Environ Pollut 247:706–715
Pannetier P, Cachot J, Clerandeau C, Faure F, Van Arkel K, de Alencastro LF, Levasseur C, Sciacca F, Bourgeois JP, Morin B (2019) Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: part I—adverse effects on fish cell line. Environ Pollut
Possatto FE, Barletta M, Costa MF, do Sul JAI, Dantas DV (2011) Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Mar Pollut Bull 62:1098–1102
Prokic MD, Petrovic TG, Gavric JP, Despotovic SG, Gavrilovic BR, Radovanovic TB, Faggio C, Saicic ZS (2018) Comparative assessment of the antioxidative defense system in subadult and adult anurans: a lesson from the Bufotes viridis toad. Zoology 130:30–37
Qiao RX, Sheng C, Lu YF, Zhang Y, Ren HQ, Lemos B (2019) Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci Total Environ 662:246–253
Qiu Q, Tan Z, Wang J, Peng J, Li M, Zhang Z (2016) Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar Coast Shelf Sci 176:102–109
Qu M, Xu KN, Li YH, Wong G, Wang DY (2018) Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci Total Environ 643:119–126
Rainieri S, Conlledo N, Larsen BK, Granby K, Barranco A (2018) Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ Res 162:135–143
Retama I, Jonathan MP, Shruti VC, Velumani S, Sarkar SK, Roy PD, RodríguezEspinosa PF (2016) Microplastics in tourist beaches of Huatulco Bay, Pacific coast of southern Mexico. Mar Pollut Bull 113:530–535
Ribeiro F, Garcia AR, Pereira BP, Fonseca M, Mestre NC, Fonseca TG, Ilharco LM, Bebianno MJ (2017) Microplastics effects in Scrobicularia plana. Mar Pollut Bull 122:379–391
Rochman CM, Kurobe T, Flores I, Teh SJ (2014) Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ 493:656–661
Rochman CM, Tahir A, Williams SL, Baxa DV, Lam R, Miller JT, Teh FC, Werorilangi S, Teh SJ (2015) Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep 5:14340
Sayre LM, Moreira PI, Smith MA, Perry G (2005) Metal ions and oxidative protein modification in neurological disease. Ann Ist Super Sanita 41:143–164
Scheurer M, Bigalke M (2018) Microplastics in Swiss floodplain soils. Environ Sci Technol 52:3591–3598
Seoane M, Gonzalez-Fernandez C, Soudant P, Huvet A, Esperanza M, Cid A, Paul-Pont I (2019) Polystyrene microbeads modulate the energy metabolism of the marine diatom Chaetoceros neogracile. Environ Pollut 251:363–371
Syakti AD, Jaya JV, Rahman A, Hidayati NV, Raza'i TS, Idris F, Trenggono M, Doumenq P, Chou LM (2019) Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: immediate impact of LDPE microplastics. Chemosphere 228:528–535
Tang J, Ni X, Zhou Z, Wang L, Lin S (2018) Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ Pollut 243:66–74
Tourinho PS, do Sul JAI, Fillrnann G (2010) Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Mar Pollut Bull 60:396–401
Tourinho PS, Koci V, Loureiro S, van Gestel CAM (2019) Partitioning of chemical contaminants to microplastics: sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ Pollut 252:1246–1256
Van Franeker JA (1985) Plastic ingestion in the North Atlantic fulmar. Mar Pollut Bull 16:367–369
van Weert S, Redondo-Hasselerharm PE, Diepens NJ, Koelmans AA (2019) Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes. Sci Total Environ 654:1040–1047
von Moos N, Burkhardt-Holm P, Kohler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46:11327–11335
Wang JD, Tan Z, Peng JP, Qiu QX, Li MM (2016) The behaviors of microplastics in the marine environment. Mar Environ Res 113:7–17
Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS (2014) Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol 48:8823–8830
Weber A, Scherer C, Brennholt N, Reifferscheid G, Wagner M (2018) PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ Pollut 234:181–189
Welden NA, Lusher AL (2017) Impacts of changing ocean circulation on the distribution of marine microplastic litter. Integr Environ Assess Manag 13:483–487
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492
Wu P, Cai Z, Jin H, Tang Y (2019a) Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci Total Environ 650:671–678
Wu Y, Guo P, Zhang X, Zhang Y, Xie S, Deng J (2019b) Effect of microplastics exposure on the photosynthesis system of freshwater algae. J Hazard Mater 374:219–227
Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD (2014) Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ Sci Technol 48:14195–14202
Zhang C, Chen X, Wang J, Tan L (2017) Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ Pollut 220:1282–1288
Zhang P, Yan Z, Lu G, Ji Y (2019) Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ Sci Pollut Res Int
Zhu ZL, Wang SC, Zhao FF, Wang SG, Liu FF, Liu GZ (2019) Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ Pollut 246:509–517
Zylstra ER (2013) Accumulation of wind-dispersed trash in desert environments. J Arid Environ 89:13–15
Acknowledgments
We acknowledge the help from Dor. Zhen Zhang.
Funding
This work was financially supported by a scientific research project of the Zhejiang Education Department (Y201942068).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Presented the possible ecotoxicity related to MPs exposure.
2. Summarized the abundance, sources, and transport of MPs in aquatic environments.
3. Summarized the compound toxicity of MPs in aquatic environments.
4. Identified the current research deficiencies in this area.
Rights and permissions
About this article
Cite this article
Du, J., Xu, S., Zhou, Q. et al. A review of microplastics in the aquatic environmental: distribution, transport, ecotoxicology, and toxicological mechanisms. Environ Sci Pollut Res 27, 11494–11505 (2020). https://doi.org/10.1007/s11356-020-08104-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08104-9