Abstract
Honey is a very unusual food – a natural substance produced by bees from the nectar or secretion of plants and elaborated by the bees themselves. Differences in trace elements and rare earth elements (REEs) are related to botanical and geographical origin, and, as such, honey could be a suitable indicator for metal occurrence in the environment within the bee forage area. A total of 40 metals were analyzed by inductively coupled plasma-mass spectrometry in multi-floral honey samples from different geographical areas. The highest levels of trace elements and REEs were found in honey from Tanzania, while lower concentrations were recorded in Italian, Kazakhstan, the Balkans, and South American honeys. Tanzanian honey is of interest due to its different content of metals, related to the peculiar lithology and the mineral resources of this country. Rubidium and aluminum were the most represented nonessential elements, while manganese, iron, zinc, and copper registered the highest values of the essential elements. Very low concentrations were found for the toxic elements lead, cadmium, and arsenic. The present study showed differences in metal concentrations in multi-floral honey from different countries, confirming the strong influence of the area of origin on the chemical composition of honeys.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a natural product elaborated by bees (Apis mellifera) from the nectar of flowers, which is harvested and subsequently transformed. There are about 300 known different types of honey, the variety of which depends on the nectar of the different flowers. Honey consists mainly of carbohydrates, along with other compounds such as proteins, vitamins, amino acids, organic acids, and minerals.
The medicinal properties of honey have been known since antiquity: the use of bee products to treat diseases is attested by cave paintings dating back to 8000 years ago. Numerous documents such as Sumerian inscriptions, Egyptian papyri, Hindu scriptures, the Koran, the Bible, and the writings of Hippocrates and Aristotle have illustrated how honey has always been used by man in a medicinal capacity. Several investigations have reported antioxidant, antimicrobial, anti-inflammatory, antiproliferative, antitumoral, and antimetastatic effects of honey, as recently reviewed by Samarghandian et al. (2017) and by Eteraf-Oskouei and Najafi (2013).
Honey is a natural product that, in recent years, has undergone the effects of industrialization and the evolution of agricultural production, from which it may become contaminated by anthropogenic pollutants such as metals. A hive is, in fact, a potential natural accumulator of contaminants that the bees collect from the foraging area. Bees seal the nectar obtained from flowers, pick propolis from the gems, collect the pollen, and drink the water present in the environment; as a result, the insects are exposed to contamination of particles suspended in the air and deposited on the flowers, herbs, and leaves or collected with their hairy bodies (Kacaniova´ et al. 2009). In the case of metal contamination, the mortality rate of bees is not a suitable environmental contamination index. Bees are not killed by metal contamination, at least at concentrations that are of concern from an ecological environmental point of view; the most sensitive signal of environmental pollution is, instead, the presence of contaminants found in the products of the hive, and among these, honey is the most sensitive indicator and the most readily available.
There are several metals that can contaminate plants and subsequently honey. These include lead, which is a ubiquitous element, naturally present but further introduced into the environment in massive quantities as a result of various industrial activities (e.g., mining, mergers, welding) and, in the past, as a result of the use of paints and leaded gasoline. Several studies on animal models have been carried out to evaluate the carcinogenicity of lead-containing inorganic compounds; in 2006 the International Cancer Research Agency (IARC 2006) classified these substances as “potentially carcinogenic for man” (group 2A). Accordingly, to ensure a high level of public health, it was considered appropriate to reduce dietary exposure to lead, and, for the first time in 2015, the European Community (Commission Regulation 2015/1005 UE) set a maximum limit for lead in honey (0.10 mg kg−1). To date, lead is the only metal for which a limit has been established in honey in Europe.
However, many other trace elements can be transferred from soil/water systems to flowers and therefore to honey; some of them are essential for living beings such as copper, manganese, and zinc, which are involved in several physiological processes and are cofactors of important enzymes, such as those involved in the cellular defense system against oxidative stress. Other elements have no biological functions and thus have a higher possibility of exerting cellular damage even at low levels of exposure, such as arsenic, cadmium, and vanadium.
Multi-elemental analysis was suggested to be a suitable tool to study the mineral composition of honey (Berriel et al. 2019). In addition to trace elements, further information about honey content can derive from analyzing rare earth elements (REEs). Indeed, in the last decade, the extensive use of REEs in the most advanced technologies has led to wide dissemination of these metals in the environment. REEs are now considered emerging contaminants worldwide; due to their lithophile properties, they have been used as geological tracers and, in recent years, in fraud prevention to assess food traceability and food authenticity (Bertoldi et al. 2009).
So far, investigations regarding REE concentrations in honey samples are scarce; Squadrone et al. (2019) analyzed REEs in Italian honey, De Oliveira et al. (2017) analyzed REEs in Brazilian honey, and Pellerano et al. (2012) analyzed cerium, lanthanum, and vanadium in Argentinian honey.
Since honey composition is closely associated with its botanical origin and may even constitute a fingerprint of the geographical area in which it originates (Rashed and Soltan 2004), the study of both trace elements and rare earth elements in honey could allow a more complete and reliable indication of the occurrence of environmental metals in honey in a particular location. Moreover, due to the potential synergic and cumulative effects that contaminants can exert on the health of humans and animals, this information is crucial from a food safety point of view.
The aims of this study were:
To assess the occurrence of trace elements and rare earth elements in honeys from the Balkans (Macedonia, Montenegro), Kazakhstan, Italy, South America and Tanzania
To determine the compliance of analyzed samples with EU Regulations of the maximum limit for lead in honey.
To evaluate if the total content of metals in honey is recommended for human consumption
Materials and methods
Sampling
Samples of multi-floral honeys from different countries (total n = 75; the Balkans n = 10, Kazakhstan n = 10, Italy n = 40, South America n = 10, Tanzania n = 5) were obtained in 2018 from samples sent to the chemical laboratory for legacy contaminants (lead) and collected in the context of official controls for food.
Determination of concentrations of trace elements and rare earth elements
Mineralization of samples was performed using a microwave digestion lab station (Ethos 1, Milestone, Shelton, CT, USA); 7 ml of HNO3 (70% v/v) and 1.5 ml of H2O2 (30% v/v) were added to 1.0–1.5 g of honey samples before the microwave digestion process.
An inductively coupled plasma-mass spectrometer (ICP-MS Xseries II, Thermo Scientific, Bremen, Germany) was utilized for the simultaneous detection of 24 trace elements (Ag, Al, As, Be, Bi, Cd, Co, Cr, Cu, Fe, Ga, In, Mo, Mn, Ni, Pb, Rb, Sb, Se, Sn, Tl, U, V, Zn) following the protocol previously described (Squadrone et al. 2016). In the absence of a certified reference material, the Standard Reference Material (1573a from the National Institute of Standards and Technology, Tomato Leaves) was used to check the accuracy of the method (Table S1).
LOD, LOQ, and precision were calculated according to the Commission Regulation (EU) No. 2016/582 amending Regulation (EC) No 333/2007 as regards the analysis of inorganic arsenic, lead, and polycyclic aromatic hydrocarbons and certain performance criteria for analysis. In particular, the limits of detection (LODs) and limits of quantification (LOQs) for the analyte elements were calculated with standard deviation (σ) of 20 independent measurements of a blank solution, where 3σ for LODs and 10σ for LOQs. The repeatability was calculated with the formula HORRATr where observed RSDr (relative standard deviation) were divided by the RSDr values estimated from the modified Horwitz equation; all the values must be smaller than 2.
Simultaneous determination of the presence of 16 REEs, lanthanum, (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), gadolinium (Gd), europium (Eu), terbium (Tb), dysprosium (Dy), thulium (Tm), ytterbium (Yb), holmium (Ho), erbium (Er), lutetium (Lu), yttrium (Y), and scandium (Sc), was performed using an ICP-MS (Xseries II, Thermo Scientific, Bremen, Germany), as previously described (Squadrone et al. 2017); promethium (Pm), which is radioactive and not naturally occurring, was not evaluated. Certified Reference Materials (BCR®-668, mussel tissue, Joint Research Center), blank reagents, and standard solutions were processed during each analytical session to verify performances of the methods (Table S2). The limit of quantification of the analyzed elements ranges between 0.0001 and 0.001 mg kg−1. LOQ was set at 0.001 for all elements to facilitate results interpretation.
Statistical analysis
One-way analysis of variance (ANOVA) was employed to compare the average contents between the different samples. Results were considered statistically significant at p values of < 0.05. GraphPad Statistics Software Version 6.0 (GraphPad Software, Inc., La Jolla California, USA) was used for statistical evaluations.
Principal component analysis (PCA) was employed to explore interrelationship among metals and the country of origin of honey. The data matrix for the analysis of trace elements was constituted by seventeen loadings: Al, As, Cd, Co, Cu, Cr, Fe, Mn, Mo, Ni, Pb, Rb, Se, Sn, V, Zn, and total metals content. The data matrix for the analysis of rare earth elements was constituted by seventeen loadings: La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, Sc, and ΣREE. Data analysis was performed using the software XLSTAT statistical and data analysis solution (New York, USA).
Results and discussion
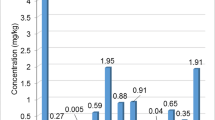
Concentrations of trace elements and rare earth elements expressed, respectively, in mg kg−1 and μg kg−1, are shown in Table 1 and in Table 2. The total content of metals, as well as the essential and nonessential contributors, is graphically represented in Fig. 1, while the sum of REEs (ΣREEs) is shown in Fig. 2; the percentage distributions of Light (L)REE and Heavy (H)REE are shown in Fig. 3.
The highest total metal level was found in honey from Tanzania (42 mg kg−1), followed by Italian honey (28 mg kg−1); honeys from Kazakhstan, the Balkans, and South America showed lower values (5.3, 9.3, and 8.6 mg kg−1, respectively). Regarding ΣREEs in honey, values were in the following decreasing order: Tanzania (65 μg kg−1), Italy (12 μg kg−1), South America (9.8 μg kg−1), Kazakhstan (9.3 μg kg−1), and the Balkans (9.0 μg kg−1).
In Figs. 4 and 5, the distributions of nonessential and essential elements are graphically represented. Finally, in Fig. 6, REE patterns are shown after normalization with the Leedey chondrite values reported by Masuda (1975).
Nonessential trace elements
Ag, Be, Bi, In, Ga, Sb, U, and Tl were < LOQ in all samples. The other nonessential elements were found in the decreasing range Rb > Al > As > Sn > V > Cd > Pb. In Fig. 4, nonessential elements are graphically represented with their statistical significance.
Rubidium is a nonessential element rarely investigated in the food chain; in nature, its abundance is comparable with zinc, since it is widely distributed in the earth’s crust, where it can be found in minerals together with potassium. The geological origins of the site significantly influence the Rb content of the plants (Folkeson et al. 1990) and may consequently affect the honey Rb content.
In honey samples from Italy and Tanzania, we found a value of 16 mg kg−1 of Rb, while in samples from South American and the Balkans, concentrations were an order of magnitude lower, and in Kazakhstan, the lowest value was found (Table 1). Rb values found in this study were lower than those found in the only other study to have investigated Rb in honey, performed by Hernández et al. (2005) in samples from the Canary Islands; the Rb concentration they found in honey was, in fact, an order of magnitude higher (mean value 534 mg kg−1).
Aluminum in honey samples was always detectable, ranging from 0.033 mg kg−1 (Tanzania) to 2.8 mg kg−1 (South America); values were consistent with the wide distribution of Al in the environment and agreed with the levels found by Conti et al. (2018) in Italian honeys (Table 3). Values reported by the review of Solayman et al. (2016) in honey from all over the world ranged, instead, from 1.4 to 11 mg kg−1.
Arsenic is a metalloid more commonly found in marine environments than in terrestrial ecosystems; however, in its inorganic form, As is highly toxic and should, therefore, be investigated in the context of food safety. As was detected in the range of 0.0061 mg kg−1 (Italy) to 0.020 mg kg−1 (South America), concentrations (Table 1) were very low and consistent with other findings worldwide (Bilandzˇic´ et al. 2017; Oroian et al. 2016; Solayman et al. 2016; Bilandzˇic´ et al. 2014; Saunier et al. 2013; Qiao-Mei et al. 2013; Batista et al. 2012; Pisani et al. 2008). The range reported by Solayman and coauthors (Table 3) for As was up to 0.10 mg kg−1.
Tin was found at low concentrations in honey (Table 1), with the highest level recovered in samples from Italy (0.044 mg kg−1), in the range of values previously found for Sn in Italian honey (Conti et al. 2018; Pisani et al. 2008); the lowest concentration was recorded in Kazakhstan samples (0.0015 mg kg−1).
Vanadium is another nonessential trace element recovered in honeys; the highest value (0.013 mg kg−1) was recorded in Tanzanian honey (Table 1), a level comparable with concentrations registered in Italian honey by Conti et al. (2018) and in honey from Croatia by Bilandzˇic´ et al. (2017); the lowest value (0.029 mg kg−1) was found in South American honey (Table 3).
Cadmium and lead are nonessential elements that can exert serious damage in human and animal organs; moreover, they are well-known environmental pollutants and can enter the food chain after contamination of flowering plants (Lodenius 2000). Polluted pollen results in higher levels of metals in honey, and pollen and honey were thus suggested as indicators of metal pollution in the area of origin (Lodenius 2000). Regulation 1005/2015 of the European Community set a maximum limit (0.10 mg kg−1), for the first time, for lead in honey, while cadmium is still unregulated.
Cadmium concentrations were very low in the analyzed honeys, the highest level was found in honey samples from Tanzania (0.011 mg kg−1), while in honeys from South America and the Balkans, Cd was an order of magnitude lower (Table 1); in Italian and in Kazakhstan honey, Cd was below the LOQ. Cd levels of this investigation were comparable to concentrations recovered in honeys from different countries (Table 3).
No sample exceeded the maximum limit set for lead that was below the LOQ in honeys from the Balkans, Tanzania, and South America, while in Italian honey, a mean level of 0.014 mg kg−1 was recorded, higher than in the Kazakhstan honey (0.0067 mg kg−1). Both of these concentrations were lower or comparable to those found for lead in honey by other investigations performed in Italy (Table 3), in European countries (Bilandzˇic´ et al. 2017; Oroian et al. 2016; Saunier et al. 2013), in South American countries (Conti et al. 2014; Batista et al. 2012), and in China (Qiao-Mei et al. 2013). In Table S3, significant statistical differences between countries are indicated for all the nonessential elements.
Considering the contribution of nonessential elements to the total concentration of trace elements (Fig. 1) in Italian and South American honeys, nonessential elements were, in fact, higher than the contribution of the essential elements, due to the concentrations of aluminum and rubidium.
Essential trace elements
The essential elements were found in honeys with the following decreasing trend: Mn > Fe > Zn > Cu > Mo > Co > Ni > Se > Cr. In Fig. 5, essential elements are graphically represented with their respective statistical significance.
Manganese is an essential trace element, part of metalloenzymes involved in amino acid, lipid, and carbohydrate metabolism; moreover, Mn is present in mitochondrial superoxide dismutase (SOD2) involved in defense against oxidative stress (EFSA 2013a). An adequate intake (AI) of manganese of 3 mg day−1 was proposed in adults by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). The lowest concentration of Mn was detected in honey from South America (0.59 mg kg−1), lower than values previously reported for Argentinian and Brazilian honeys (Conti et al. 2014; Batista et al. 2012), and from Kazakhstan (0.92 mg kg−1). The highest Mn concentration was found in Italian honey (6.1 mg kg−1), higher than those recorded (Table 3) in previous investigations in Italy (Conti et al. 2018; Pisani et al. 2008). The Balkans and Tanzanian honeys contained comparable values (Table 1).
Iron is a trace element required for essential functions in cells, such as oxygen transport, oxidase activities, and energy metabolism (EFSA 2015a). Iron in the body is mainly distributed in erythrocyte hemoglobin, muscle myoglobin, and liver ferritin. The AI of iron suggested by the EFSA Panel on NDA was 6 mg day−1 for men and 7 mg day−1 for women (EFSA 2015a). We registered almost the same mean values for iron in honey from the Balkans, Kazakhstan, Italy, and South America (Table 1), while in Tanzanian honey, Fe was higher (9.0 mg kg−1); however, a high variability of iron content in honey has been reported, as reviewed by Solayman et al. (2016), who recorded a range of 0.4–224 mg kg−1.
Zinc is involved in several physiological functions and is ubiquitous in the body; three general functional classes (catalytic, structural, and regulatory) define the role of zinc in biological processes (EFSA 2014a). Regulation of gene expression is a key biochemical role of zinc. The upper level (UL) proposed for zinc intake in adults is 25 mg day−1 (EFSA 2014a). In the analyzed samples, Italian, South American, and the Balkans honey registered comparable values (Table 1) that were also in agreement with previous investigations in the same areas (Conti et al. 2018; Bilandzˇic´ et al. 2017; Oroian et al. 2016; Conti et al. 2014; Batista et al. 2012; Pisani et al. 2008), while in Tanzanian honey, Zn was higher (8.9 mg kg−1), and in Kazakhstan honey, the lowest concentration was recorded (0.010 mg kg−1).
Copper is involved in several physiological processes such as carbohydrate metabolism and antioxidant defense mechanism, being part of the enzyme superoxide dismutase SOD1, Cu-Zn-SOD, together with zinc (ATSDR 2004). Exposure to excessive levels of copper can cause adverse health effects including liver and kidney damage and immunotoxicity. The AI of copper proposed by the EFSA Panel on NDA was 1.6 mg day−1 for men and 1.3 mg day−1 for women (EFSA 2015b). Copper was recorded in honey (Table 1) with values from 0.18 mg kg−1 (Kazakhstan) to 4 mg kg−1 (Tanzania). Copper content (0.91 mg day−1) in Italian honey was comparable with previous data (Conti et al. 2018; Pisani et al. 2008) reported from Italy; analogously, copper values in honey from the Balkans and South America were consistent with the literature (Bilandzˇic´ et al. 2017; Oroian et al. 2016; Conti et al. 2014; Batista et al. 2012; Table 3).
Molybdenum, due to its easy interconvertibility of different oxidation states (Mo4 +/Mo6 +), is involved in electron transfer pathways; Mo-enzymes that catalyze redox reactions are found in both plants and animal organisms (EFSA 2013b). An AI of molybdenum of 65 μg day−1 was proposed in adults by the EFSA Panel on NDA. Molybdenum has been rarely investigated in honey; in our study, the lowest concentration was found in honey from the Balkans and the highest in Tanzanian honey (Table 1); South American and the Italian honey recorded comparable values (Table 3).
Cobalt is an essential trace element being part of vitamin B12, necessary for folate and fatty acid metabolism (EFSA 2009); however, the Agency for Toxic Substances and Disease Registry (IARC 2006) concluded that genotoxicity assays clearly demonstrated the mutagenic potential of cobalt salts. In fact, like the other essential elements, high doses of cobalt could be toxic for living beings. A daily oral intake (DI) of 0.6 mg cobalt appears to be the minimum risk level for humans that can protect from the known threshold-related adverse effects (EFSA 2009).
We found Co in the range of 0.0027 mg kg−1 (South America) to 0.31 mg kg−1 (Tanzania), comparable to previous values recorded from Italy and other world regions (Conti et al. 2018; Conti et al. 2014; Solayman et al. 2016; Batista et al. 2012; Pisani et al. 2008).
Nickel is a naturally occurring trace element, widespread in all compartments of the environment and ubiquitous in the biosphere. A tolerable daily intake of 2.8 μg Ni kg−1 body weight (b.w.) was set by the EFSA (2015c). Ni levels in honey ranged from 0.0083 mg kg−1 (South America) to 0.47 mg kg−1 (Tanzania) in agreement with or lower than concentrations reported in previous investigation (Solayman et al. 2016). In Kazakhstan honey Ni was found below the LOQ.
Selenium is a cofactor of several proteins with a variety of functions, such as antioxidant effects, since glutathione peroxidases (GPxs) are part of the human antioxidant network that protect the organism from oxidative damage. An AI of 70 μg day−1 of Se for adults has been suggested (EFSA 2014b). Selenium levels were low in honey, and the highest value (Table 1) was recorded in Tanzania (0.026 mg kg−1) and the lowest in the Balkans honey (0.0018 mg kg−1), comparable with levels recorded by Bilandzˇic´ et al. (2017) in honey from Croatia. The concentration found in Italian honey was consistent with previous investigations on Italian honey performed by Conti et al. (2018); analogous values found in South American honey were comparable with those recorded by Batista and coauthors on honey from Brazil (Table 3).
Chromium, in its trivalent form, is an essential micronutrient and suggested to be necessary in the metabolism of carbohydrates, lipids, and proteins (EFSA 2014c). The tolerable daily intake calculated by the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) is 0.30 mg Cr (III)/kg body weight per day. Cr was below the limit of quantification in honey samples from the Balkans, South America, and Tanzania, while in Italian samples, there was a mean value of 0.019 mg kg−1 of Cr (Table 1), comparable to values found by Conti and coauthors in Italy (2018).
In Table S3, significant statistical differences between countries are highlighted for all the essential elements.
Considering the contribution of essential elements to the total concentration of trace elements (Fig. 1) in the Balkans and Tanzanian honeys, essential elements were higher than the contribution of nonessential elements, due to the concentrations of iron, copper, manganese, and zinc. In Kazakhstan honey essential and nonessential elements contribute equally to the total metal content.
Rare earth elements
REEs are concentrated in different geological environments, with endogenous deposits derived from processes within the earth’s crust and exogenous deposits, formed during weathering and/or sedimentary processes (Paulick and Machacek 2017). REEs in honey are found to be related to geographic origin and geochemical soil composition (Batista et al. 2012).
In our investigations, ΣREEs in multi-floral honey (Table 2) were found with the following trend: Tanzania (65 μg kg−1) > Italy (11 μg kg−1) > South America (9.8 μg kg−1) > the Balkans (9.3 μg kg−1) > and Kazakhstan (9.1 μg kg−1). REE levels in honey have been rarely investigated, and to our knowledge, there have been two studies performed on Italian honey (Squadrone et al. 2019) and on Brazilian honey (De Oliveira et al. 2017), respectively, reporting a mean total content (ΣREEs) of 7.8 μg kg−1 and 11 μg kg−1, in agreement with the present study.
LREEs were lower than HREEs (Table 2 and Fig. 3) in all samples apart from Tanzanian honey. REEs are extracted from minerals containing REE fluoro-carbonate, the bastnaesite, which is strongly enriched in LREEs compared to HREEs (Chakhmouradian and Wall 2012), while minerals such as xenotime and monazite are enriched in HREEs (Paulick and Machacek 2017). The LREE/HREE ratio (Table 2) ranged from 0.28 (Italy) to 2.5 (Tanzania). The highest value of REEs recorded in Tanzanian honey was consistent with the fact that, outside of China, Tanzania is one of the countries with the highest concentration of resources (Paulick and Machacek 2017); the higher levels of LREEs in Tanzanian honey are consistent with the presence of the weathered bastnaesite zone REE ore (Witt et al. 2019).
Chondrite-normalized REE pattern (Leedey Oklahoma chondrite)
To study the REE pattern, we used the normalized values reported by Masuda (1975) for the Leedey chondrite, which is thought to be the most primitive chondrite (Sakamoto et al. 2008). In fact, chondrite meteorites represent a reference for the normalization of lanthanides, since they are assumed to reflect the original composition of the earth’s crust (Masuda 1975). REE patterns for honey samples are shown in Fig. 6.
The chondrite normalized patterns were similar between countries, except for a Ce negative anomaly in Italian honey, consistent with a previous study (Squadrone et al. 2019), and an Eu positive anomaly registered in Italian and South American samples.
Ce and Eu possess additional valence electrons compared to the other REEs, and when their concentrations are enriched or depleted compared to chondrite, this phenomenon is called a Ce or an Eu (positive or negative) anomaly, which is strictly dependent on the country lithology.
Principal component analysis
The principal component analysis loading plots of the metal content of honeys from the considered countries are presented in Fig. 7, and the results of the PCA are summarized in Table S4. According to the results of the initial eigenvalues, two principal components were considered and grouped metals into two-component model accounting for 84.52% of the total variance. All of the trace elements are consequently well represented by these two principal components. The initial component matrix indicated that Al, Cd, Co, Cu, Zn, Fe, Mn, Ni, Se, V, and Zn are tightly associated with the first principal component (PC1), which explained 58.78% of the total variance; Al was negatively correlated, and Cd, Co, Cu, Zn, Fe, Mn, Ni, Se, V, and Zn are positively correlated with PC1.
As, Pb, Mo, and Sn are distributed with the second component (PC2) with 25.74% of the total variance (positively correlated with PC2); Cr (negatively correlated) and Rb (positively correlated) were mainly associated with PC2 and partially with PC1.
The PCA loading plots of the REEs content of honeys from the different countries are presented in Fig. 8, and the results of the PCA are summarized in Table S5. According to the results of the initial eigenvalues, two principal components were considered and grouped REEs into two-component model accounting for 99.37% of the total variance. All REEs are well represented by these two principal components. The initial component matrix indicated that the lanthanides La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu are tightly associated with the first principal component (positively correlated to PC1), which explained 90.66% of the total variance, while Y and Sc that are rare earth elements since their chemical behavior is very similar to that of the lanthanide group are distributed with the second component (positively correlated to PC2) with 8.71% of the total variance.
The biplots of loadings (variables) and score (observations) have shown (Fig. 7) that total metal content was strongly correlated to PC1 for honey from Tanzania (positive correlation), Kazakhstan, and the Balkans (negative correlation), while honey from Italy (positive correlation) and South America (negative correlation) was correlated to PC2. Regarding rare earth elements (Fig. 8), ΣREE was strongly positively correlated to PC1 for honey from Tanzania, while honey from the Balkans, Kazakhstan (negative correlation), Italy, and South America (positive correlation) was related to PC2.
As stated by ANOVA, PCA confirmed that metal concentrations in honey was strongly influenced by geographical origin and that honey from Tanzania is really peculiar regarding both trace elements and REEs levels.
Final considerations and conclusions
Our investigation found significant differences in trace and rare earth element concentrations in honey, related to the country of origin. The analyzed samples contained very low or negligible concentrations of elements known to exert toxicity for living beings, such as arsenic, cadmium, chromium, and lead. No samples exceeded the maximum limits set for lead by EU Regulations. The contribution of essential trace elements varied greatly between the four areas, according to differences in soil composition, floral type, and floral density. Tanzanian honeys revealed the highest content of both trace elements and REEs, while Kazakhstan, the Balkans, and South American honeys had the lowest values, with Italian honey having an intermediate content. Further investigations regarding REEs and other rarely studied elements such as Al, Bi, Be, Tl, and U are recommended since they could be potential markers of geographical origin. Data about the metal content in honey is helpful both for consumers and for beekeepers to assess the food safety of this unusual product; moreover, honey can signal the possible contamination of an area allowing the authorities responsible for food safety and environmental protection to take suitable measures.
References
ATSDR (Agency for Toxic Substances and Disease Registry) (2004) Toxicological profile for copper. U.S. Public Health Service. Agency for Toxic Substances and Disease Registry, Atlanta, GA
Batista BL, da Silva LRS, Rocha BA, Rodrigues JL, Berretta-Silva AA, Bonates TO, Gomes VSD, Barbosa RM, Barbosa F (2012) Multi-element determination in Brazilian honey samples by inductively coupled plasma mass spectrometry and estimation of geographic origin with data mining techniques. Food Res Int 49:209–215
Berriel V, Barreto P, Perdomo C (2019) Characterisation of Uruguayan honeys by multi-elemental analyses as a basis to assess their geographical origin. Foods 8:24
Bertoldi D, Larcher R, Nicolini G, Bertamini M, Concheri G (2009) Distribution of rare earth elements in Vitis vinifera L. ‘chardonnay’ berries. Vitis 48(1):49–51
Bilandzˇic´ N, Gacˇic´ M, Ðokic´ M, Sedak Ivanec Šipušic´ Ð, Koncˇurat A, Tlak Gajger I (2014) Major and trace elements levels in multifloral and unifloral honeys in Croatia. J Food Compos Anal 33:132–138
Bilandzˇic´ N, Tlak Gajger I, Kosanovic´ M, Cˇalopek B, Sedak M, Solomun Kolanovic´ B, Varenina I, Bozˇic´ Luburic´ D, Varga I, Ðokic M (2017) Essential and toxic element concentrations in monofloral honeys from southern Croatia. Food Chem 234:245–253
Chakhmouradian AR, Wall F (2012) Rare earth elements. Elements 8(5):321–400
Conti ME, Finoia MG, Fontana L, Mele G, Botre F, Iavicoli I (2014) Characterization of argentine honeys on the basis of their mineral content and some typical quality parameters. Chemistry Central Journal 8:44
Conti ME, Canepari S, Finoia MG, Mele G, Astolfi ML (2018) Characterization of Italian multifloral honeys on the basis of their mineral content and some typical quality parameters. J Food Compos Anal 74:102–113
De Oliveira FA, de Abreu AT, de Oliveira Nascimento N, Froes-Silva RES, Antonini Y, Nalini HA Jr, de Lena JC (2017) Evaluation of matrix effects on the determination of rare earth elements and As, Bi, Cd, Pb, Se and in honey and pollen of native Brazilian bees (Tetragonisca angustula e Jatai) by Q-ICP-MS. Talanta 162:488–494
EFSA (2009) Assessment of the safety of cobalt (II) chloride hexahydrate added for nutritional purposes as a source of cobalt in food supplements and the bioavailability of cobalt from this source. EFSA J 1066:6–8
EFSA (2013a) Scientific opinion on dietary reference values for manganese. EFSA J 11(11):3419
EFSA (2013b) Scientific opinion on dietary reference values for molybdenum. EFSA J 11(8):3333
EFSA (2014a) Scientific opinion on dietary reference values for zinc. EFSA J EFSA 12(10):3844
EFSA (2014b) Scientific opinion on dietary reference values for selenium. EFSA J 12(10):3846
EFSA (2014c) Scientific opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J 12(3):3595
EFSA (2015a) Scientific opinion on dietary reference values for iron. EFSA J 13(10):4254
EFSA (2015b) Scientific opinion on dietary reference values for copper. EFSA J 13(10):4253
EFSA (2015c) Scientific opinion on the risks to public health related to the presence of nickel 473 in food and drinking water. EFSA Journal 13(2):4002, 202
Eteraf-Oskouei T, Najafi M (2013) Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci 16:731–742
Folkeson L, Nyholm NEI, Tyler G (1990) Influence of acidity and other soil properties on metal concentrations in forest plants and animals. Sci Total Environ 96:211–233
Hernández O, Fraga J, Jimenez A, Jimenez F, Arias J (2005) Characterisation of honey from the Canary Islands: determination of the mineral content by atomic absorption spectrometry. Food Chem 93:449–458
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2006) Cobalt in hard metals and cobalt sulfate, gallium arsenide. Indium Phosphide and Vanadium Pentoxide Metallic Cobalt Particles (with or without Tungsten Carbide)
Kacaniova´ M, Knazovicka V, Melich M, Fikselova M, Massanyi P, Stawarz R, Hascik P, Pechociak T, Kuczkowska A, Putala A (2009) Environmental concentration of selected elements and relation to physicochemical parameters in honey. Journal of Environmental Science & Health part A 44:414–422
Lodenius M (2000) Honey, pollen and bees as indicators for metal pollution. Acta Universitatis Carolinae Environmentalica 14:13–20
Masuda A (1975) Abundance of monoisotopic REE, consistent with Leedey chondrite values. Geochem J 9:183–184
Oroian M, Prisacaru A, Hretcanu EC, Stroe S-G, Leahu A, Buculei A (2016) Heavy metals profile in honey as a potential indicator of botanical and geographical origin. International Journal Food Properties 19:1825–1836
Paulick H, Machacek E (2017) The global rare earth element exploration boom: an analysis of resources outside of China and discussion of development perspectives. Resources Policy 52:134–153
Pellerano RG, Uñates MA, Cantarelli MA, Camiña JM, Marchevsky EJ (2012) Analysis of trace elements in multifloral Argentine honeys and their classification according to provenance. Food Chemistry 134 (1):578–582
Pisani A, Protano G, Riccobono F (2008) Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem 107:1553–1560
Qiao-Mei R, Qiang F, Jin-Zhe H (2013) Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chemistry Toxicology 53:256–262
Rashed MN, Soltan ME (2004) Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J Food Composition Anal 17(6):725–735
Sakamoto N, Kano N, Imaizumi H (2008) Determination of rare earth elements, thorium and uranium in seaweed samples on the coast in Niigata Prefecture by inductively coupled plasma mass spectrometry. Applied Geochemistry 23(10):2955–2960
Samarghandian S, Farkhondeh T, Samini F (2017) Honey and health: a review of recent clinical research. Pharm Res 9:121–127
Saunier J-B, Losfeld G, Freydier R, Grison C (2013) Trace elements biomonitoring in a historical mining district (les Malines, France). Chemosphere. 93:2016–2023
Solayman M, Islam M, Paul S, Ali Y, Khalil M, Alam N, Gan SH (2016) Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: a comprehensive review. Compr Rev Food Sci Food Saf 15(1):219–233
Squadrone S, Brizio P, Stella C, Prearo M, Pastorino P, Serracca L, Ercolini C, Abete MC (2016) Presence of trace metals in aquaculture marine ecosystems of the northwestern Mediterranean Sea (Italy). Environ Pollut 215:77–83
Squadrone S, Brizio P, Battuello M, Nurra N, Mussat Sartor R, Benedetto A, Pessani D, Abete MC (2017) A first report of rare earth elements in northwestern Mediterranean seaweeds. Mar Pollut Bull 122:236–242
Squadrone S, Brizio P, Stella C, Mantia M, Battuello M, Nurra N, Mussat Sartor R, Orusa R, Robetto S, Brusa F, Mogliotti P, Garrone A, Abete MC (2019) Rare earth elements in marine and terrestrial matrices of northwestern Italy: implications for food safety and human health. Sci Total Environ 660:1383–1391
Witt WK, Hammond DP, Hughes M (2019) Geology of the Ngualla carbonatite complex, Tanzania, and origin of the weathered Bastnaesite zone REE ore. Ore Geol Rev 105:28–54
Acknowledgements
The authors thank the editor and the anonymous reviewers that greatly improved the manuscript quality.
Funding
This research was funded by the Italian Health Ministry Research Grants (Project n. IZS PLV 20/16RC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Squadrone, S., Brizio, P., Stella, C. et al. Trace elements and rare earth elements in honeys from the Balkans, Kazakhstan, Italy, South America, and Tanzania. Environ Sci Pollut Res 27, 12646–12657 (2020). https://doi.org/10.1007/s11356-020-07792-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07792-7