Abstract
The Yungui Plateau lakes, which are characterized by a highly endemic biodiversity, have been suffering severely from anthropogenic intervention in the recent decades. Studies on the response of these biodiversity to human-mediated effects are still limited. Here, we selected the typical Lake Dianchi to investigate the correlation between macroinvertebrate spatiotemporal dynamics and human-induced eutrophication across a 2-year span (2009–2010). A total of 26 taxa were recorded, and the assemblage pattern of the macroinvertebrate community was mainly controlled by the spatiotemporal (region, season, and year) density fluctuations of some pollution-tolerant species (Limnodrilus hoffmeisteri, Tubifex tubifex, Branchiura sowerbyi, and Chironomus plumosus). Taxon richness, total density, biomass, and the abundance of Oligochaeta and Chironomidae decreased from the north to the south of the lake but were much higher in 2009 than in 2010. Moreover, the high densities of total assemblages and oligochaete occurred during spring and/or autumn, whereas that of chironomids was only high during summer. The contributions of important factors varied in different seasons, but the community variations were mainly shaped by eutrophication-related factors (e.g., Chla, N, and P). Variance partitioning analyses showed that aquatic factors were able to explain more community variations than sediment (6.9–36.6 vs. 5.3–14.7%) across seasons, but their interactive effects were negligible. The results of this study will be beneficial for restoring and managing hypereutrophic lakes in the Yungui Plateau and imply the necessity of long-term monitoring in bioassessment projects involving intensively disturbed lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accelerating lake eutrophication has become one of the most serious environmental problems worldwide (Liu et al. 2012a; Søndergaard et al. 2007; Ye et al. 2015), resulting in consequences, such as deterioration of water quality, loss of biodiversity, and destruction of ecosystem structure and multifunctions (Gong et al. 2009; Levin 1992; Wang et al. 2011). As for biota, for example, human-induced eutrophication has profoundly altered the richness and composition of local assemblages from sensitive and indigenous species to pollution-tolerant or invasive species in many lakes, leading to species extinction and biotic homogenization (Cai et al. 2011; Olden and Poff 2004; Rahel 2000; Strayer 2006; Toussaint et al. 2014; Villéger et al. 2011).
Benthic macroinvertebrates are taxonomically and functionally diverse and play crucial roles in the lake–ecosystem structure and functions (Covich et al. 1999; Fontanarrosa et al. 2013). These macroinvertebrates are also good bioindicators for ecological monitoring and assessment due to their sensitivities to different environmental conditions (Bazzanti et al. 2012; Donohue et al. 2009; Li et al. 2016). However, based on their short-term life history and complicated population behavior in the aquatic ecosystems, the macroinvertebrates’ spatiotemporal variation is quite elusive in nature and in human-induced conditions (Levin 1992). Therefore, systematically clarifying the response of macroinvertebrate assemblages to eutrophications at spatiotemporal scales can enhance our understanding of the driving mechanisms of macroinvertebrate communities under anthropogenic disturbances and contribute to environmental monitoring and ecological restoration. The spatial dynamics in eutrophic lakes are community-specific across different lake types and mainly affected by environmental filtering (Zhong et al. 2008; Takamura et al. 2009; Cai et al. 2011) and biotic inter-reactions (Cai et al. 2016; Céréghino et al. 2008; Żbikowski and Kobak 2007; Żbikowski et al. 2010). At the temporal scales, numerous previous studies have focused on successions and interannual community variations in coarse scale (Burlakova et al. 2014; Cai et al. 2015; French et al. 2009; Jimenez et al. 2011). Carefully examining the spatiotemporal response of macroinvertebrate assemblages to eutrophication in the fine scales (daily, seasonally, or annually) is warranted to provide detailed information for generalizing the dynamics between macroinvertebrates and environment conditions. The information should be imperative to restore and manage degenerated lake ecosystems (Jackson and Füreder 2006). However, detailed information about macroinvertebrates in eutrophic lakes is still limited.
The patterns of macroinvertebrates in plain eutrophic lakes have been extensively investigated in China, but such studies have seldom been carried out in plateau lakes (Cai et al. 2016; Meng et al. 2016). As one global hotspot for biodiversity conservation, plateau lake ecosystems breed some special and important species, such as Margarya melanioides, Margarya mansuyi, and Cipangopaludina dianchiensis (Kang et al. 2010; Olson and Dinerstein 1998; Zhang et al. 2012; Wang et al. 2013). The Yunnan Plateau lakes are situated in southwestern China and listed as one of the Palearctic Lake ecosystems of the Global 200 Priority Ecoregions due to its highlighted biodiversity (Olson and Dinerstein 1998; Ye et al. 2015). More than 30 lakes were located in this region, and most of them were tectonic lakes formed by faultage sinking with no connection with each other. The unique formation supports numerous endemic species. However, most of these lakes have been suffering from severe anthropogenic intervention in recent decades, resulting in extensive loss of endemic biodiversity (Ding et al. 2017; Liu et al. 2012a; Song et al. 2013; Wang et al. 2013; Yang et al. 2004; Ye et al. 2015).
Lake Dianchi is typical of these lakes. It has been experiencing strong ecosystem degeneration due to high nutrient level and grievous blue–green algal bloom (Gray and Wang 1999; Wang et al. 2012; Wu et al. 2016). However, the mechanism of how human-induced eutrophication drives the macroinvertebrate assemblages in this lake remains to be elucidated (Wang 1985; Wang et al. 2007). In the present study, this lake was chosen to carefully examine the spatiotemporal dynamics of macroinvertebrate assemblages under eutrophication stress for two years (from 2009 to 2010) and understand the response mechanism of macroinvertebrates to human activities (e.g., eutrophication). The specific objectives were (1) to portray the seasonal, yearly, and cross-region variations of macroinvertebrate community and (2) to detect how environmental variables drive these patterns, especially the eutrophication-related ones.
Materials and methods
Study area
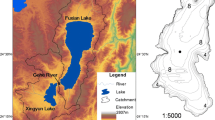
Lake Dianchi (24° 23′–26° 22′ N, 102° 10′–103° 40′ E), which is located in the southwestern outskirts of Kunming City, the capital of Yunnan Province, is the sixth largest freshwater lake in China and the most polluted plateau lake. This lake experiences a characteristic tropical plateau monsoon climate and hydrological condition and is separated into three distinct regions, namely, northern (NR), middle (MR), and southern (SR) regions, by their abiotic and biotic conditions (Liu et al. 2013). The lake mainly accommodates the surface runoffs of 22 river flows, with the rivers of the Panlong, Baoxiang, and Baiyu being the largest ones. The lake’s water discharges into the Tanglangchuan River via its only outlet in the southwest. The hydrologic residence time is approximately 2–4 years (Liu et al. 2013; Wang et al. 2004). The annual average water temperature is approximately 16.0 °C (ranging from 10 to 27 °C), and the mean annual precipitation is approximately 1070 mm (Wang et al. 2010).
Sampling sites and data collection
Benthic macroinvertebrate data were collected quarterly (April, July, October, and January) from April 2009 to January 2011. A total of 24 sampling sites were established in the whole lake, and NR, MR, and SR had 9, 8, and 7 sampling sites, respectively (Fig. 1). At each site, three quantitative bottom samples were collected using a modified Petersen grab (0.0625 m2 in area) and sieved using a 500-μm sieve in the field. Each site was positioned accurately by the Garmin GPS-76 system. Specimens were manually sorted from sediment on a white porcelain plate and preserved in 10% formalin. The animals were identified to the lowest feasible taxonomic taxa in accordance with related references (Brinkhurst 1986; Epler 2001; Liu et al. 1979; Morse et al. 1994), counted, and weighed (wet weight) using an electronic balance (accuracy of 0.0001 g).
Environmental data were also collected quarterly, but only for six seasons (from April 2009 to July 2010). On each sampling occasion, 18 environmental variables, namely, water temperature (WT), dissolved oxygen (DO), wind speed, water depth (WD), pH, Secchi depth (SD), total phosphorus (TP), total nitrogen (TN), ammonia nitrogen (NH4+–N), nitrate nitrogen (NO3−–N), nitrite nitrogen (NO2−–N), chlorophyll a (Chla), sediment pH (Sed pH), sediment oxidation–reduction potential (Sed Eh), total sediment nitrogen (Sed TN), total sediment phosphorus (Sed TP), sediment organic matter (Sed OM), and sediment oxidation–reduction capacity (Sed Ca), were measured. WT, DO, pH, Sed Eh, and Sed pH were measured in the field using the YSI Environmental Monitoring Systems 6600. SD, WS, and WD were measured at each site using the Secchi disc, anemoscope, and sounding lead, respectively. TN, NH4+–N, NO2−–N, NO3−–N, TP, Chla, Sed TN, Sed TP, Sed OM, and Sed Ca were determined in the laboratory in accordance with the Chinese Water Analysis Methods Standards (Huang et al. 1999).
Data analysis
The Kruskal–Wallis tests were used to detect the significance of region vs. season for all measured environmental variables. The differences in community parameters (total density, biomass, richness, Shannon–Wiener diversity, and abundance of dominant groups) among regions, seasons, and years were analyzed by one-way repeated measure analysis of variance (ANOVA). The repeated measure analysis was selected due to the temporally nonindependent data generated by the same sampling sites on eight subsequent occasions. When the assumption of data revealed a violation of the Mauchly’s test of sphericity, the Greenhouse–Geisser correction was used to correct the results of the within-subject analysis (Quinn and Keough 2002). When the between-subject effects of the ANOVA were significant, pairwise comparisons between regions were carried out using the Tukey’s honestly significant difference (HSD) multiple comparison analysis. In cases of nonhomogeneity (significant results of Levene’s test), the Games–Howell tests were applied for post hoc comparisons (Beckmann et al. 2005). When the within-subject effects of the ANOVA were significant, the Helmert contrasts were used to compare the two sampling seasons and the two years. Before executing the ANOVA, the macroinvertebrate assemblages’ data were tested for normality and transformed when needed. Nonmetric multidimensional scaling (NMDS) was performed using density data to examine the community compositional differences between regions, seasons, and years. Prior to NMDS analysis, the density data were subjected to log(x + 1) transformation. Then, the similarity matrix was calculated using the Bray–Curtis similarity coefficients. The similarity percentage analysis (SIMPER) was used to estimate the contribution of responsible species to the divergence of communities between groups identified from the NMDS and permutational analysis of variance (PERMANOVA). PERMANOVA was conducted to determine the spatial and temporal community variances based on the similarity matrix. The Kruskal–Wallis tests and ANOVA were run in the SPSS software (version 19.0), and NMDS, SIMPER, and PERMANOVA were performed in the PRIMER (version 6.1.16) and the PERMANOVA+ for PRIMER package (Anderson et al. 2008).
Constrained and partial canonical ordinations were used to assess the influence of environmental variables on macroinvertebrate variations. The detrended correspondence analysis (DCA) was used to classify the data and determine the appropriate type of model for direct gradient analysis (Ter Braak and Verdonschot 1995). DCA indicated that a linear model (gradient lengths < 3 standard units) would best fit the data. As such, redundancy analysis (RDA) was used. Prior to RDA, the fauna data were subjected to log(x + 1) transformation, and the down weighting option was used to reduce the influence of rare species. Environmental variables that did not fit the normality assumption (Shapiro–Wilk test, P < 0.05) were subjected to logarithm transformation. The environmental variables that had variance inflation factors > 20 were removed from the analysis to avoid high collinearity. The forward selection with Monte Carlo permutation tests (9999 permutations) was used to select a parsimonious set of explanatory variables under the cutoff point of 0.05. Finally, six RDAs were computed for six quarters to evaluate the key environmental variables affecting the macroinvertebrate assemblages. Variation partitioning analyses (Borcard et al. 1992; Legendre and Legendre 1998) were implemented to quantify the relative importance of aquatic and sediment factors in the community structure. The 12 aquatic variables were WT, wind speed, DO, WD, pH, SD, TP, TN, NH4+–N, NO2−–N, NO3−–N, and Chla. The sediment factors included Sed pH, Sed Eh, Sed TN, Sed TP, Sed OM, and Sed Ca. The “Varpart” function (R, vegan package) was used to partition the variations in the two sets of environmental variables (i.e., aquatic and sediment variables). The adjusted R2 values (“RsquareAdj” function) were calculated to determine the explanatory power of the final RDA models because these values were unbiased and had been recommended previously (Peres-Neto et al. 2006). DCA and RDA analyses were performed using the R version 3.3.1 (R Development Core Team 2016).
Results
Environmental parameters
Almost all water parameters (TN, TP, NH4+–N, NO2−–N, NO3−–N, and Chla) in the NR were significantly higher than those in the MR and SR, whereas the opposite was observed in terms of SD (Table 1, ESM Appendix A). However, most sediment parameters except pH and TP were not obviously different. All water factors except WD showed significant season differences (Table 1). Sed pH and Eh were high during summer and autumn, whereas Sed TN was low during spring (Table 1).
Spatial and temporal variation in macroinvertebrate assemblages
A total of 26 taxa (9 oligochaetes, 14 chironomids, 2 gastropods, and 1 leech), which belonged to 5 families of 4 classes of 3 phyla, were recorded in the 576 samples. Oligochaetes, which controlled the community structure, represented 96.36% of the total individuals due to its predominant members, namely, Limnodrilus hoffmeisteri (83.90%), Tubifex tubifex (8.44%), and Branchiura sowerbyi (2.07%). Chironomids (3.58%) were also common and mainly attributed to frequently occurring Chironomus plumosus (1.73%).
Repeated measure ANOVA indicated that the community parameters (total density, biomass, Oligochaeta density, and density and percentage of Chironomidae) in NR were significantly higher than those in MR and SR. In addition, the MR biomass was significantly higher than the SR biomass, and the richness and the percentage of Oligochaeta in NR were higher than those in SR (Table 2, Fig. 2). The richness and the abovementioned parameters were higher in 2009 than in 2010 (Table 2, Fig. 2). The high oligochaete density occurred during spring and/or autumn, and high chironomid density occurred during summer (Fig. 2). The interaction of season × region was significant for taxon richness, Shannon diversity, and biomass, whereas that of year × region was negligible (Table 2). Finally, the percentage of Oligochaeta had no significant difference among the three regions in different temporal scales (seasons or years) (Table 2).
PERMANOVA indicated that the region (F = 23.587, P = 0.0001), season (F = 6.4593, P = 0.0001), and year (F = 16.961, P = 0.0001) were the key explanatory variables for community variations. The interactions of region × year (F = 1.9391, P = 0.049) and season × year (F = 1.7774, P = 0.033) were also the sources of variation (Table 3). Detailed pairwise comparisons showed the significant interaction of region × year (P < 0.01 in all comparisons), and the assemblages in 2009 varied significantly across all three regions (Table 3, ESM Appendix B). Pairwise tests revealed that the assemblages during the seasons of 2009 were significantly different from those during the seasons of 2010 (P < 0.01 in all comparisons). Within the year, only the winter in 2009 evidently differed from spring and summer (P < 0.05), whereas all seasons in 2010 were distinct from each other (P < 0.05 in all comparisons) (Table 3, ESM Appendix B).
The variations in sampling sites among different regions, seasons, and years were distinct (Fig. 3). SIMPER analysis showed that the regional community variations were due to L. hoffmeisteri and T. tubifex, which accounted for 61.43% in NR, 77.40% in MR, and 87.96% in SR (Fig. 4a). Additional amounts were due to B. sowerbyi (13.01% in NR and 16.42% in MR), C. plumosus (12.92% in NR), and L. grandisetosus (4.40% in SR) (Fig. 4a). The seasonal differences were caused by T. tubifex (43.29%) during winter, L. hoffmeisteri (50.38 to 51.80%) and B. sowerbyi (10.67% to 15.52%) in other seasons, and C. plumosus (9.44%) during summer (Fig. 4b). Moreover, the annual variations were attributed to T. tubifex (34.59%) in 2009 and L. hoffmeisteri (51.80%) and B. sowerbyi (15.31%) in 2010 (Fig. 4c).
Relationships between macroinvertebrate and environmental variables
Among the 18 environmental variables detected, season-based RDAs retained 11 (Chla, pH, NO2−–N, NO3−–N, NH4+–N, wind speed, WD and TN, Sed TN, Sed TP, and Sed pH) for community variations, even though they were quite different across seasons (Table 4, Fig. 5). These retained variables were almost related to eutrophication. The final RDA models accounted for 22.0 to 46.0% of community variations in different seasons (Table 4).
The pure contributions of water and sediment variables to community variations, which ranged from 6.9 to 36.6% and from 5.3 to 14.7%, respectively, remarkably differed among seasons (Table 5). The conditions in the water environments, which were on the average three times higher (from 0.5 to 6.9) in value than those in the sedimentary environments, played important roles in regulating the community structures. However, their interactive effects were insignificant. Some variations (54.0 to 78.0%) remained unexplained by the RDA models.
Discussion
Characteristics of macroinvertebrate assemblages
Some pollution-tolerant species, i.e., oligochaetes and chironomids, dominated the benthic assemblages in Lake Dianchi, resulting in its low biodiversity and simple structure. Previous studies have shown that the lake’s benthic community has been experiencing a continuous diversity decline in recent decades (Wang 1985; Wang et al. 2007, 2011). For example, about 24 and 19 mollusk species, including a high endemic diversity (such as Cobicula fenouilliana, Radix yunnanensis, Margarya melanioides, M. mondi, M. elongata, and M. tropidophora) (Wang et al. 2011; Zhang et al. 2015), were recorded in the 1970s (Zhang and Wu 1983) and the 1980s (Wang 1985), respectively, but only two species, including the only endemic species (M. melanioides), which was rated as endangered (EN) by the IUCN in 2009 (http://www.iucnredlist.org), were occasionally collected during our investigation. Thus, the long-term eutrophication has destroyed the lake’s benthic diversity and structure through a series of processes, such as deterioration of water and sediment quality, the trophic shift, the disappearance of aquatic macrophytes, and extensive algal bloom (Cai et al. 2012a; Du et al. 2011; Erman and Erman 1984; Pan and Gao 2010; Tews et al. 2004). Therefore, the protection of these remnant benthic animals is a tremendous challenge for conservationists and stakeholders. Our results revealed that the dominant species of macroinvertebrates in Lake Dianchi (plateau lake) were similar to those of Lake Taihu (plain lake) (Cai et al. 2012a), suggesting that although the hydrological and climatic characteristics in the plateau lakes are different from the eastern plain lakes in China, human-induced eutrophication has promoted macroinvertebrate taxonomic and functional homogenization at broad spatial scales.
Spatial and seasonal patterns
Although extreme habitat homogenization occurred across the lake (e.g., the decline of aquatic macrophytes and fine silt–clay sediment), the remnant benthos showed a distinct spatial pattern (Du et al. 2011). This pattern can be attributed mainly to the predominant pollution-tolerant species. These species, which are good indicators of eutrophication, generally indicate positive correlation to environmental nutrient concentrations to some extent (Cai et al. 2015; Gong and Xie 2001; Lang 1978). Consequently, high water nutrients in NR produced high densities of oligochaetes and chironomids. Nevertheless, the low level of abundance and biodiversity in SR may be determined by the high level of Sed TP level in this region due to the undisposed wastewater discharge by nearby rich phosphorus mineral resources and phosphorus fertilizer factories (Gao et al. 2005; Liu et al. 2012b). The phosphorus level may exceed the tolerable thresholds of most benthic species in this region (Correll 1998). In the last two seasons of our investigation, the negative effects of high phosphorus on chironomids were further confirmed because of the disappearance of chironomids in the last two seasons. Cai et al. (2015) also found that chironomids had lower tolerance to phosphorus pollution than L. hoffmeisteri. Moreover, our RDA models indicated that Sed TP was significantly negatively correlated with macroinvertebrate variations. However, the extent of harm of these ultrahigh Sed TP levels to macroinvertebrate remains to be identified and needs further study.
Our study also demonstrated significant seasonal and interannual macroinvertebrate fluctuations. Natural community fluctuation is controlled by extrinsic (abiotic and biotic) and intrinsic (life history) factors (Cai et al. 2015, 2017; Hao et al. 1995; Pan et al. 2016; Talbot and Ward 1987). Since the environmental homogenization across the lake, the benthic community seasonal dynamics were mainly regulated by the community behavior. Such dynamics were determined by the fluctuations in four dominant pollution-tolerant species (L. hoffmeisteri, T. tubifex, B. sowerbyi, and C. plumosus), indicating high abundance during spring and/or autumn. These oligochaetes mainly complete their life cycles within 1 year, and their reproduction time is during spring and/or autumn (Wang et al. 2007). In addition, the high summer densities of chironomids should be related to the large-scale eclosion of chironomids larva during autumn. However, the cross-year abundance dynamics, which had a remarkable decline from 2009 to 2010 was also distinct. The abundance in 2009 was in the same level as that in 2002 (Wang et al. 2007, 2011), possibly indicating a turning point, in which the deteriorative benthic assemblage turned to a moderate condition. Our updated investigation (2014–2015) also indicated this trend (unpublished data). Thus, ongoing multiple restoration projects for Lake Dianchi (such as the control of point and nonpoint wastewater and the in-lake restoration act) may be effective in improving the environmental condition for benthic animals (Wang et al. 2012).
Community–environment relationships in different seasons
Eutrophication-mediated environmental factors drove the lake benthic assemblage variation across time despite their varying relative contributions in different seasons. These filters were mostly related to Chla, nutrients (nitrogen and phosphorus), pH, and transparency.
Chla played a strong role in regulating benthic assemblage during winter and spring. Cyanobacterial blooms persisted in Lake Dianchi all year round due to the high temperature during winter but with different dominant species through time (Zhang et al. 2012). Aphanizomenon flos-aquae dominated during the cold seasons (spring and winter), whereas Microcystis spp. were noted during the warm seasons (summer and autumn) (Wu et al. 2016). Massive blooming activity can consume the DO in water due to algal respiration and decomposition, leading to exasperated DO condition for benthos survival, especially during the cold season (Cai et al. 2016). Moreover, the cyanobacterial toxic metabolites (e.g., cyanotoxins) can be harmful to benthic animals (Nogueira et al. 2004; Zhang et al. 2012). For example, A. flos-aquae can synthesize one type of neurotoxin (paralytic shellfish poisons), which was regarded as one of the most hazardous toxins in cyanotoxins, and has been confirmed to lead to the malformation and mortality of aquatic organisms (Lefebvre et al. 2004; Liu et al. 2006; Mahmood and Carmichael 1986; Zhang et al. 2013). Nevertheless, Chla was not detected by the RDA model to affect significantly the benthos community in most serious cyanobacterial bloom seasons (summer and autumn). This situation can be attributed to other environmental factors (e.g., nitrogen and phosphorus) possibly outcompeting the other parameters in regulating the variations in these seasons (Qu et al. 2019). In addition, the lake’s algal growing time is during warm seasons, whereas moribund algae occur during the cold seasons, indicating that the high DO produced by algal photosynthesis should benefit animal survivals during summer and autumn (Wang et al. 2012).
Different forms of nitrogen had remarkable negative correlations with the assemblages across seasons. In recent decades, human-induced nitrogen inputs into this lake have sharply increased (Gao et al. 2015). These increased nitrogen concentrations caused by high sedimentation rates destroyed the benthic composition and structure through a battery of physical mechanisms of siltation, habitat modification, and oxygen depletion (Grall and Chauvaud 2002; Li et al. 2012; Pearson and Rosenberg 1978). The pollution-tolerant species, especially L. hoffmeisteri, have actively responded to the increase in the black layer and increase in the organic sedimentation in similar fashions (Lang and Hutter 1981; Zhong et al. 2008). This condition is beneficial for these species in terms of nutrient availability. The decomposition of organic matter led to oxygen depletion in the sediment and, in turn, to its enrichment with nutrients. This enrichment is known to generate an increase in the levels of NO3−–N and NH4+–N in the interstitial water through nitrification and denitrification (Hansen et al. 1998; Shang et al. 2014; Svensson et al. 2001). Their toxicities at high concentrations are considered key factors in the succession of benthic macroinvertebrates (Frazier et al. 1996; Sparks and Sandusky 1981; Wilson et al. 1995).
Sediment TP significantly affected the benthos assemblage dynamics. Phosphorus is a fundamental nutrient for invertebrate growth and metabolism (Elser et al. 2000), but a stoichiometric balance of carbon and phosphorus in the tissues is necessary (Elser 2005). Thus, a possible mechanism for the TP effect is that the very low carbon–phosphorus values in the lake’s animal tissue caused by highly phosphorus-enriched sediments can be harmful (Song et al. 2013).
Investigators have argued that pH, especially Sed pH, is an important factor regulating the benthos community (Shaw and Mackie 1989). Weakly acidic sediment is deleterious to molluscan fecundity, hatching survival, and formation of calcareous shells and can lead to high mortality of embryos and low recruitment (Echeverría et al. 2010). Thus, the weakly acidic sediment conditions in Lake Dianchi (ESM Appendix A) should play key roles in eradicating most of the molluscan biodiversity across the lake scale.
Naturally induced physical disturbance by wind and related factor (SD) had certain effects on the community dynamics during summer. From May onwards, the southwestern and southeastern monsoons can take shape in the Yunnan plateau (Yang et al. 2004). The south-directed long wind can historically cause severe water waves in the lake oriented in the north–south, accompanied by the low SD. These naturally caused activities, combined with the severe cyanobacterial bloom and high nutrient levels (Wang et al. 2010; Zhang et al. 2012), can aggravate the conditions for benthic survivals and other biological activities, especially in NR, where the moribund algae were heavily stacked due to the wind.
To our knowledge, few studies have assessed the effects of different environmental types (water and sediments) on macroinvertebrate community in the hypereutrophication plateau lakes. Inconsistent with previous studies (Cai et al. 2011, 2012b; Pan et al. 2015), the present study found that the water condition plays a fundamental role in the determination of macroinvertebrate structure compared with sediments in the lake studied. The wind-induced disturbance of the water/sediment interface in the large shallow lake is beneficial for nutrient exchange on the interface between the water and sediment, probably causing eutrophication-related aquatic factors affecting the benthic community in appearance (Cai et al. 2012a, 2016; Scheifhacken et al. 2007). Only a few sediment factors have been determined in our study. In general, only one factor in this phase became a significant driver in regulating the benthic assemblage. Nevertheless, a few sediment factors still showed quite important influences on the macroinvertebrate communities, implying that monitoring and protecting the sedimentary environment are essential for the restoration of eutrophic lakes and conservation of biodiversity.
Some unexplained variance of community variation across seasons was observed due to the failure to include key extrinsic and intrinsic variables. The extrinsic variables may include key environmental filters (e.g., human-induced habitat fragmentation and breakwater construction) and biotic reactions (e.g., alteration of algal resources available and exotic introduction). The intrinsic variables include community traits, such as the demographic characteristics (e.g., natality, fatality, and fecundity), and dispersal ability.
Conclusion and implications for conservation
The spatiotemporal dynamics of macroinvertebrate linking to ambient factors were carefully examined across two years in a once-fascinating plateau lake (Lake Dianchi). To date, this lake’s benthic members were severely destroyed, as demonstrated by the indications of the massive biodiversity loss (especially its endemism) and oversimple structure. The predominant members were all pollution-tolerant and widespread taxa (oligochaetes and chironomids), which governed the community composition and structure throughout the year. This homogenization had been driven strongly by human-induced eutrophication in recent decades. The eutrophication-related water and sediment factors independently played pivotal roles in the remnant benthos patterns across seasons, with the former being important. In addition, the information of a remarkable interannual fluctuation suggested that a long-term monitoring project be conducted to provide in-depth information for macroinvertebrates in lakes in this plateau. These findings can provide detailed information for the local government and environmental agency to restore and manage these eutrophic Yun-Gui Plateau lakes.
References
Anderson M, Gorley R, Clarke K (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth, UK
Bazzanti M, Mastrantuono L, Solimini AG (2012) Selecting macroinvertebrate taxa and metrics to assess eutrophication in different depth zones of Mediterranean lakes. Fundam Appl Limnol 180:133–143
Beckmann MC, Schöll F, Matthaei CD (2005) Effects of increased flow in the main stem of the River Rhine on the invertebrate communities of its tributaries. Freshw Biol 50:10–26
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Brinkhurst RO (1986) Guide to the freshwater aquatic microdrile oligochaetes of North America. Spec Publ Fish Aquat Sci Can 84:1–259
Burlakova LE, Karatayev AY, Pennuto C, Mayer C (2014) Changes in Lake Erie benthos over the last 50 years: historical perspectives, current status, and main drivers. J Great Lakes Res 40:560–573
Céréghino R, Ruggiero A, Angélibert PMS (2008) Influence of vegetation cover on the biological traits of pond invertebrate communities. Ann Limnol-Int J Limnol 44:267–274
Cai Y, Gong Z, Qin B (2011) Influences of habitat type and environmental variables on benthic macroinvertebrate communities in a large shallow subtropical lake (Lake Taihu, China). Ann Limnol-Int J Limnol 47:85–95
Cai Y, Gong Z, Qin B (2012a) Benthic macroinvertebrate community structure in Lake Taihu, China: effects of trophic status, wind-induced disturbance and habitat complexity. J Great Lakes Res 38:39–48
Cai Y, Gong Z, Xie P (2012b) Community structure and spatiotemporal patterns of macrozoobenthos in Lake Chaohu (China). Aquat Biol 17:35–46
Cai Y, Lu Y, Gong Z (2015) Changes in macrozoobenthic assemblages in a shallow subtropical lake (Lake Taihu, China): 1987–1988 vs. 2007. J Freshw Ecol 30:157–168
Cai Y, Lu Y, Liu J, Dai X, Xu H, Lu Y, Gong Z (2016) Macrozoobenthic community structure in a large shallow lake: disentangling the effect of eutrophication and wind-wave disturbance. Limnologica 59:1–9
Cai Y, Zhang Y, Wu Z, Chen Y, Xu J, Gong Z (2017) Composition, diversity, and environmental correlates of benthic macroinvertebrate communities in the five largest freshwater lakes of China. Hydrobiologia 788:85–98
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27:261–266
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. BioScience 49:119–127
Ding C, Jiang X, Xie Z, Brosse S (2017) Seventy-five years of biodiversity decline of fish assemblages in Chinese isolated plateau lakes: widespread introductions and extirpations of narrow endemics lead to regional loss of dissimilarity. Divers Distrib 23:171–184
Donohue I, Donohue LA, Ainín BN, Irvine K (2009) Assessment of eutrophication pressure on lakes using littoral invertebrates. Hydrobiologia 633:105–122
Du LN, Li Y, Chen XY, Yang JX (2011) Effect of eutrophication on molluscan community composition in the Lake Dianchi (China, Yunnan). Limnologica 41:213–219
Echeverría CA, Neves RAF, Pessoa LA, Paiva PC (2010) Spatial and temporal distribution of the gastropod Heleobia australis in an eutrophic estuarine system suggests a metapopulation dynamics. Nat Sci 2:860–867
Elser JJ (2005) Response of grazing snails to phosphorus enrichment of modern stromatolitic microbial communities. Freshw Biol 50:1826–1835
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Epler JH (2001) Identification manual for the larval Chironomidae (Diptera) of North and South Carolina. Crawford, South Carolina.
Erman DC, Erman NA (1984) The response of stream macroinvertebrates to substrate size and heterogeneity. Hydrobiologia 108:75–82
Fontanarrosa MS, Chaparro GN, O’Farrell I (2013) Temporal and spatial patterns of macroinvertebrates associated with small and medium-sized free-floating plants. Wetlands 33:47–63
Frazier B, Naimo T, Sandheinrich M (1996) Temporal and vertical distribution of total ammonia nitrogen and un-ionized ammonia nitrogen in sediment pore water from the upper Mississippi River. Environ Toxicol Chem 15:92–99
French JRP III, Schaeffer JS, Roseman EF, Kiley CS, Fouilleroux A (2009) Abundance and distribution of benthic macroinvertebrates in offshore soft sediments in Western Lake Huron, 2001–2007. J Great Lakes Res 35:120–127
Gao L, Zhou JM, Yang H, Chen J (2005) Phosphorus fractions in sediment profiles and their potential contributions to eutrophication in Dianchi Lake. Environ Geol 48:835–844
Gao W, Howarth RW, Swaney DP, Hong B, Guo HC (2015) Enhanced N input to Lake Dianchi Basin from 1980 to 2010: drivers and consequences. Sci Total Environ 505:376–384
Gong ZJ, Xie P (2001) Impact of eutrophication on biodiversity of the macrozoobenthos community in a Chinese shallow lake. J Freshw Ecol 16:171–178
Gong ZJ, Li YL, Shen J, Xie P (2009) Diatom community succession in the recent history of a eutrophic Yunnan plateau lake, Lake Dianchi, in subtropical China. Limnology 10:247–253
Grall J, Chauvaud L (2002) Marine eutrophication and benthos: the need for new approaches and concepts. Glob Chang Biol 8:813–830
Gray AV, Wang L (1999) Case study on water quality modelling of Dianchi Lake, Yunnan Province, South West China. Water Sci Technol 40:35–43
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32:5–12
Hao W, Wang S, Wang D (1995) The community structure of benthic macroinvertebrates and the assessment of water quality in Honghu lake. Acta Hydrobiol Sin 19:124–134 (in Chinese with English abstract)
Huang XF, Chen W, Cai QH (1999) Standard methods for observation and analysis in Chinese ecosystem research network survey, observation and analysis of lake ecology. Standards Press of China, Beijing (in Chinese)
Jackson JK, Füreder L (2006) Long-term studies of freshwater macroinvertebrates: a review of the frequency, duration and ecological significance. Freshw Biol 51:591–603
Jimenez A, Rennie MD, Sprules WG, La Rose J (2011) Temporal changes in the benthic invertebrate community of Lake Simcoe, 1983–2008. J Great Lakes Res 37:103–112
Kang S, Xu Y, You Q, Flügel WA, Pepin N, Yao T (2010) Review of climate and cryospheric change in the Tibetan Plateau. Environ Res Lett 5:75–82
Lang C (1978) Factorial correspondence analysis of Oligochaeta communities according to eutrophication level. Hydrobiologia 57:241–247
Lang C, Hutter P (1981) Structure, diversity and stability of two oligochaete communities according to sedimentary inputs in Lake Geneva (Switzerland). Aquat Sci 43:265–276
Lefebvre KA, Trainer VL, Scholz NL (2004) Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat Toxicol 66:159–170
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam, NL
Levin SA (1992) The problem of pattern and scale in ecology: the Robert H. MacArthur Award Lecture. Ecology 73:1943–1967
Li D, Erickson RA, Tang S, Zhang Y, Niu Z, Liu H, Yu H (2016) Structure and spatial patterns of macrobenthic community in Tai Lake, a large shallow lake, China. Ecol Indic 61:179–187
Li F, Cai Q, Jiang W, Qu X (2012) The response of benthic macroinvertebrate communities to climate change: evidence from subtropical mountain streams in central China. Int Rev Hydrobiol 97:200–214
Liu G, Liu Z, Chen F, Zhang Z, Gu B, Smoak JM (2013) Response of the cladoceran community to eutrophication, fish introductions and degradation of the macrophyte vegetation in Lake Dianchi, a large, shallow plateau lake in southwestern China. Limnology 14:159–166
Liu W, Li S, Bu H, Zhang Q, Liu G (2012a) Eutrophication in the Yunnan Plateau lakes: the influence of lake morphology, watershed land use, and socioeconomic factors. Environ Sci Pollut Res 19:858–870
Liu YM, Chen W, Li DH, Shen YW, Li GB, Liu YD (2006) First report of aphantoxins in China-waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicol Environ Saf 65:84–92
Liu Y, Li Y, Yang X, Zhang J, Guan J, Wang J, Zhang H, Xu C (2012b) Analysis of pollution in Dianchi Lake and consideration of its application in crop planting. Procedia Environ Sci 12:174–183
Liu YY, Zhang WZ, Wang YX, Wang EY (1979) Economic fauna of China: freshwater Mollusca. Science Press, Beijing (in Chinese)
Mahmood NA, Carmichael WW (1986) Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 24:175–186
Meng X, Jiang X, Xiong X, Wu C, Xie Z (2016) Mediated spatio-temporal patterns of macroinvertebrate assemblage associated with key environmental factors in the Qinghai lake area, China. Limnologica 56:14–22
Morse JC, Yang L, Tian L (1994) Aquatic insects of China useful for monitoring water quality. Hohai University Press, Nanjing
Nogueira ICG, Pereira P, Dias E, Pflugmacher S, Wiegand C, Franca S, Vasconcelos VM (2004) Accumulation of paralytic shellfish toxins (PST) from the cyanobacterium Aphanizomenon issatschenkoi by the cladoceran Daphnia magna. Toxicon 44:773–780
Olden JD, Poff NL (2004) Clarifying biotic homogenization. Trends Ecol Evol 19:283–284
Olson DM, Dinerstein E (1998) The Global 200: a representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv Biol 12:502–515
Pan BZ, Wang HZ, Pusch MT, Wang HJ (2015) Macroinvertebrate responses to regime shifts caused by eutrophication in subtropical shallow lakes. Freshw Sci 34:942–952
Pan B, Wang H, Li Z, Ban X, Liang X, Wang H (2016) Macroinvertebrate assemblages in relation to environments in the Dongting lake, with implications for ecological management of river-connected lakes affected by dam construction. Environ Prog Sustain Energy 36:914–920
Pan M, Gao L (2010) The influence of socio-economic development on water quality in the Lake Dianchi. Eng Sci 12:117–122 (in Chinese with English abstract)
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of marine environment. Oceanogr Mar Biol 16:229–311
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Qu X, Peng W, Liu Y, Zhang M, Ren Z, Wu N, Liu X (2019) Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol Indic 101:501–511
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge.
R Development Core Team (2016) R: a language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org
Rahel FJ (2000) Homogenization of fish faunas across the United States. Science 288:854–856
Scheifhacken NF, Fiek C, Rothhaupt KO (2007) Complex spatial and temporal patterns of littoral benthic communities interacting with water level fluctuations and wind exposure in the littoral zone of a large lake. Fundam Appl Limnol 169:115–129
Shang J, Liao Q, Zhang L, Fan C (2014) The influence of different benthic fauna on inorganic nitrogen flux and denitrification in a large shallow hyper-eutrophic lake. Fundam Appl Limnol 184:101–108
Shaw MA, Mackie GL (1989) Reproductive success of Amnicola limosa (Gastropoda) in low alkalinity lakes in south-central Ontario. Can J Fish Aquat Sci 46:863–869
Søndergaard M, Jeppesen E, Lauridsen TL, Skov C, Van Nes EH, Roijackers R, Lammens E, Portielje ROB (2007) Lake restoration: successes, failures and long-term effects. J Appl Ecol 44:1095–1105
Song Z, Zhang J, Jiang X, Wang C, Xie Z (2013) Population structure of an endemic gastropod in Chinese plateau lakes: evidence for population decline. Freshw Sci 32:450–461
Sparks RE, Sandusky MJ (1981) Identification of the factors responsible for decreased production of fish food organisms in the Illinois and Mississippi rivers. Illinois Natural History Survey River Research Laboratory, Havana II., USA Final Report Project 3-291-R
Strayer DL (2006) Challenges for freshwater invertebrate conservation. J N Am Benthol Soc 25:271–287
Svensson JM, Enrich-Prast A, Leonardson L (2001) Nitrification and denitrification in a eutrophic lake sediment bioturbated by oligochaetes. Aquat Microb Ecol 23:177–186
Takamura N, Ito T, Ueno R, Ohtaka A, Wakana I, Nakagawa M, Ueno Y, Nakajima H (2009) Environmental gradients determining the distribution of benthic macroinvertebrates in Lake Takkobu, Kushiro wetland, northern Japan. Ecol Res 24:371–381
Talbot JM, Ward JC (1987) Macroinvertebrates associated with aquatic macrophytes in Lake Alexandria, New Zealand. N Z J Mar Freshw Res 21:199–213
Ter Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Toussaint A, Beauchard O, Oberdorff T, Brosse S, Villéger S (2014) Historical assemblage distinctiveness and the introduction of widespread non-native species explain worldwide changes in freshwater fish taxonomic dissimilarity. Glob Ecol Biogeogr 23:574–584
Villéger S, Blanchet S, Beauchard O, Oberdorff T, Brosse S (2011) Homogenization patterns of the world's freshwater fish faunas. Proc Natl Acad Sci U S A 108:18003–18008
Wang CM, Xie ZC, Song LR, Xiao BD, Li GB, Li L (2011) Lake Dianchi macroinvertebrate community succession trends and retrogressive analysis. Zool Res 32:212–221 (in Chinese with English abstract)
Wang LZ (1985) A research of macroinvertebrates in Yunnan Dianchi Lake. J Yunnan Univ 7:73–84 (in Chinese with English abstract)
Wang LZ, Liu YD, Chen L, Xiao BD, Liu JT, Wu QL (2007) Benthic macroinvertebrate communities in Dianchi Lake Yunnan and assessment of its water. Acta Hydrobiol Sin 31:590–593 (in Chinese with English abstract)
Wang S, Wang J, Li M, Du F, Yang Y, Lassoie JP, Hassan MZ (2013) Six decades of changes in vascular hydrophyte and fish species in three plateau lakes in Yunnan, China. Biodivers Conserv 22:3197–3221
Wang YC, Peng YA, Li YM (2004) The characteristic of water pollution and engineering-oriented prevention on Dianchi. Areal Res Dev 23:88–92 (in Chinese with English abstract)
Wang Z, Xiao B, Wu X, Tu X, Wang Y, Sun X, Song L (2010) Linear alkylbenzene sulfonate (LAS) in water of Lake Dianchi—spatial and seasonal variation, and kinetics of biodegradation. Environ Monit Assess 171:501–512
Wang Z, Zhang Z, Zhang J, Zhang Y, Liu H, Yan S (2012) Large-scale utilization of water hyacinth for nutrient removal in Lake Dianchi in China: the effects on the water quality, macrozoobenthos and zooplankton. Chemosphere 89:1255–1261
Wilson DM, Naimo TJ, Wiener JG, Anderson RV, Sandheinrich MB, Sparks RE (1995) Declining populations of the fingernail clam Musculium transversum in the upper Mississippi River. Hydrobiologia 304:209–220
Wu Y, Li L, Zheng L, Dai G, Ma H, Shan K, Wu H, Zhou Q, Song L (2016) Patterns of succession between bloom-forming cyanobacteria Aphanizomenon flos-aquae and Microcystis and related environmental factors in large, shallow Dianchi Lake, China. Hydrobiologia 765:1–13
Yang Y, Tian K, Hao J, Pei S, Yang Y (2004) Biodiversity and biodiversity conservation in Yunnan, China. Biodivers Conserv 13:813–826
Ye S, Lin M, Li L, Liu J, Song L, Li Z (2015) Abundance and spatial variability of invasive fishes related to environmental factors in a eutrophic Yunnan Plateau lake, Lake Dianchi, southwestern China. Environ Biol Fish 98:209–224
Żbikowski J, Kobak J (2007) Factors influencing taxonomic composition and abundance of macrozoobenthos in extralittoral zone of shallow eutrophic lakes. Hydrobiologia 584:145–155
Żbikowski J, Kobak J, Żbikowska E (2010) Is Nuphar lutea (L.) Sm. a structuring factor for macrozoobenthos and selected abiotic parameters of water and bottom sediments throughout the year? Aquat Ecol 44:709–721
Zhang DL, Hu CX, Li DH, Liu YD (2013) Zebrafish locomoto capacity and brain acetylcholinesterase activity is altered by Aphanizomenon flos-aquae DC-1 aphantoxins. Aquat Toxicol 138-139:139–149
Zhang LJ, Chen SC, Yang LT, Jin L, Köhler F (2015) Systematic revision of the freshwater snail Margarya Nevill, 1877 (Mollusca: Viviparidae) endemic to the ancient lakes of Yunnan, China, with description of new taxa. Zool J Linn Soc 174:760–800
Zhang J, Wang Z, Song Z, Xie Z, Li L, Song L (2012) Bioaccumulation of microcystins in two freshwater gastropods from a cyanobacteria-bloom plateau lake, Lake Dianchi. Environ Pollut 164:227–234
Zhang QG, Wu TS (1983) Dianchi lake pollution and aquatic invertebrate investigation. Dianchi Lake pollution and hydrobios. Yunnan People’s Publishing House, Kunming, pp 31–37 (in Chinese)
Zhong F, Liu B, Cheng S, Wu Z (2008) Response of macrozoobenthos communities to ecological engineering remediation in a hypertrophic urban lake. Fresenius Environ Bull 17:829–836
Acknowledgments
The authors are greatly indebted to the Dianchi Workstation of the Institute of Hydrobiology, Chinese Academy of Sciences for the help in field sample collection. We also thank our colleagues Xiong Jing for field assistance and Luo Sheng, Xiong Jian, and Wu Yanlong for their help in providing the sediment characteristics and the aquatic physical chemistry and hydrochemistry.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31302191 and No. 41571495) and the Special S&T Project on Treatment and Control of Water Pollution (Grant No. 2013ZX07102-005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, C., Jiang, X. et al. Effects of human-induced eutrophication on macroinvertebrate spatiotemporal dynamics in Lake Dianchi, a large shallow plateau lake in China. Environ Sci Pollut Res 27, 13066–13080 (2020). https://doi.org/10.1007/s11356-020-07773-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07773-w