Abstract
Diatomite is an economical and environmentally friendly adsorbent, and its use has been applied widely for the treatment of water contaminated by heavy metals. Despite this, the mechanism for the removal of the heavy metal Cd(II) remains unclear. In this work, we explored the adsorption mechanism of Cd(II) by diatomite using batch experiment, and characterized the diatomite using scanning electron microscopy, energy-dispersive spectrometry, specific surface area, and pore size distribution analysis. Our results showed that, under the experimental conditions, the kinetic adsorption approached equilibrium within 5 min, and the Sips isotherm model was most suitable for data fitting. EDS characterization of the Cd-loaded diatomite indicated that Cd(II) was adsorbed onto the diatomite. Furthermore, desorption experiments showed that Ca2+ and Mg2+ in the diatomite caused an ion exchange interaction, and this was primarily responsible for Cd(II) adsorption. Moreover, we found that its contribution to the whole adsorption reaction could reach 80%, while the remainder of Cd(II) was probably trapped in the microporous structure of the diatomite. Additionally, our data indicated that the adsorption mechanism did not change significantly after regeneration. These results have provided special insight into the deep understanding of the mechanism of Cd(II) adsorption by diatomite, and could provide theoretical support and guidance for further development and application of diatomite in the treatment of Cd(II)-contaminated water.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, water pollution caused by heavy metals has received increasing attention (Salman et al. 2019). Cadmium (Cd) is a heavy metal with highly acute toxicity, and has been identified as a carcinogen, teratogen, and mutagen (Lodeiro et al. 2006). The main sources of cadmium pollution are metal ore mining and industrial wastewater discharge (Kumar et al. 2015; Ulmanu et al. 2003), such as electroplating (Qu et al. 2019), battery manufacturing (Zhang et al. 2010), printing and dyeing (Patil and Sawant 2009), and metallurgy (Sun et al. 2016; Tang and Gschneidner 1996). Due to the non-degradable characteristic, it can easily accumulate in the soil and food chain, eventually leading to various diseases of the human body, including rickets and renal dysfunction (Li et al. 2019; Zhang et al. 2019). As a result, the World Health Organization has set the maximum concentration of cadmium in drinking water at 0.003 mg/L. Therefore, treating water contaminated with Cd(II) is extremely important to ensure the safety of human life.
As we all know, adsorption is the most widely used method for water treatment. Some adsorbents have been studied for water treatment (Dhir 2014; Khairy et al. 2014; Rao et al. 2010). Common adsorbents, activated carbon, silica gel, activated alumina, etc., have the advantage of a large specific surface area, leading to high removal efficiency for contaminants (Bhatnagar and Sillanpää 2010; Wang et al. 2018). However, the costs of these adsorbents for application are generally high, due to the complicated preparation process of the adsorbents (Dhir 2014; Gupta et al. 2009). Therefore, it is essential to develop new low-cost and environmentally friendly adsorbents.
In comparison, diatomite resource is abundant in nature, leading to easy access and low cost to obtain (Wang and Chen 2009). Among many emerging natural adsorbents, diatomite attracts researchers’ attention by its unique properties such as high porosity, high permeability, small particle size, large specific surface area, low thermal conductivity, chemical inertness, and low economic cost (Bailey et al. 1999; Danil de Namor et al. 2012). Due to these properties, diatomite has exhibited high removal efficiency for heavy metals (Danil de Namor et al. 2012; ElSayed 2018). Although some studies have focused on diatomite as a potential adsorbent (Al-Ghouti et al. 2009; Miretzky et al. 2011), studies on the adsorption of Cd(II) by diatomite for contaminated water treatment are not comprehensive up to now, mainly due to insufficient research on the adsorption mechanism. Therefore, in order to make full use of diatomite as an adsorbent for water treatment, it is necessary to study the adsorption mechanism of Cd(II).
At present, there have been some studies on the adsorption of heavy metal ions by diatomite, including Pb2+ (Beheshti and Irani 2016; Irani et al. 2011), Cu2+ (Danil de Namor et al. 2012), Zn2+ (Valente Flores-Cano et al. 2013), Cr3+ (ElSayed 2018), and Cd2+ (Miretzky et al. 2011). Most of these studies focused on adsorption behaviors (Beheshti and Irani 2016; ElSayed 2018; Vassileva et al. 2011), while research on adsorption mechanism was limited. Most studies stated that ion exchange played a significant role in the reaction (Safa et al. 2012; Sheng et al. 2009; Ulmanu et al. 2003), while some others believed electrostatic attraction (Miretzky et al. 2011) determined adsorption capacity. The mechanism of Cd(II) adsorption by diatomite has not been clearly pointed out. Therefore, it is still unclear about the contribution of ion exchange and electrostatic attraction in the interaction between diatomite and Cd(II). In order to evaluate and enhance the value of diatomite as a promising adsorbent for Cd-contaminated water, it is crucial to carry out experimental research on the adsorption mechanism of Cd(II) by diatomite.
In this manuscript, we carried out an experiment to reveal the contribution of ion exchange and electrostatic attraction in the adsorption of Cd(II) by diatomite through batch experiment and a combination of various characterization methods. This work could provide theoretical support and guidance for further development of diatomite and its application as a useful adsorbent.
Experimental
Materials
We obtained the adsorbent raw diatomite, whose main chemical composition is SiO2, from Qingzhong Bishui (Beijing) Technology Co., Ltd. The chemicals used in the experiment, cadmium chloride (CdCl2), hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium chloride (NaCl), etc., were all of analytical purity and purchased from Beijing Chemical Corp. (China). All solutions were prepared with deionized water.
Characterization
The microscopic morphology and elemental composition of the diatomite were obtained with a scanning electron microscope (JEOL JSM-6701F, Japan) and an energy dispersive spectrometer (20.0 kV, Takeoff Angle 35.0°, Elapsed Livetime 30.0). Nitrogen adsorption and desorption isotherms were obtained by measurement of the Brunauer-Emmett-Teller surface area and the pore size distribution (MicroActive for ASAP 2460 2.01). The point of zero charge of the diatomite was measured according to the salt titration method (Mustafa et al. 2002).
Adsorption and desorption experiments
Diatomite (0.5 g) was added to a centrifuge tube containing 25 mL of a solution of the desired concentration of Cd(II) with 0.01 M NaCl as the background electrolyte. The pH of the solution was adjusted to 5.0 using 0.1 M NaOH or HCl as needed. The tube was shaken at 175 rpm (25 °C) for 4 h, and then centrifuged at 2000 rpm for 3 min. The supernatant was passed through a 0.45-μm filter and the concentration of Cd(II) in the decanted solution was determined by ICP-AES (Spectro, Germany).
In the isothermal adsorption experiment, the initial concentration of Cd(II) was changed and the tube was shaken for 2 h at 25 °C before sampling. After the centrifugation, the supernatant was poured out, and the Cd-loaded diatomite was desorbed with 25 mL of 0.1 M MgCl2 solution, and then shaken again for another 2 h. The initial pH of the solution was changed from 3.0 to 8.0, and these samples were used to investigate the effect of pH.
After desorption, the diatomite was used as the second adsorption samples, and the adsorption experiments were carried out as the first one. The mass loss ratio after the first adsorption was around 3% according to preliminary experiment, so the effect of mass loss was negligible.
All samples in the above experiments were conducted in triplicate. This study is based on the treatment of acidic water containing Cd(II).
Results and discussion
Characterization of the diatomite
The specific surface area of the diatomite was determined to be 27 m2/g, which was within the range (18.8–58 m2/g) found in previously reported studies (Sheng et al. 2009; Zhu et al. 2013). The SEM images of diatomite are shown in Fig. 1a and b, which showed the disc shape of the diatomite used in this experiment, an observation that was consistent with previous literature (Ye et al. 2015). There were many porous channels distributed on the surface of the disc with a small amount of floc inside. The porous channels were irregularly distributed and centered on the center of the disc, forming a circular area. A large number of nanoscale pores were radially distributed at the curved edge, forming a dense distribution like the arrangement of plant petals. The pore size distribution is shown in Fig. 1c; it can be seen that around 90% of the pore radii were less than 16 nm, and the cumulative proportion of micropores smaller than 8 nm was greater than 50%. It can also be seen that the porous channel structure of diatomite was well developed and tended to provide potential adsorption sites. The elemental analysis result is shown in Fig. 1 d. It can be concluded that the diatomite was composed of many elements such as O, Si, C, Al, Mg, Fe, Ca, and K.
The point of zero charge (pHPZC) of the diatomite was determined to be 4.5 by the salt titration method. This indicated that when the pH was greater than or less than this value, the surface of the diatomite was negatively charged and positively charged, respectively.
Adsorption experiment
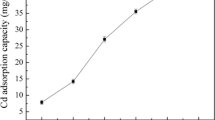
The kinetic adsorption at an initial concentration of 100 mg/L for Cd(II) is shown in Fig. 2a. After 5 min, the Cd(II) concentration decreased to 53 mg/L, and then equilibrated at a final concentration of 50 mg/L after 2 h. This indicated that the adsorption rate of Cd(II) by diatomite was fast. The removal percentage of Cd(II) was 50%, indicating that the content of Cd(II) in the solution was excessive. The adsorption data were fitted with pseudo-first-order kinetics (Lagergren 1898), pseudo-second-order kinetics (Ho 1995), intra-particle diffusion (Weber and Morris 1963), and Elovich equation (Cheung et al. 2000), respectively (Supplementary Material 1). The results showed that the experimental data of Cd(II) adsorption by diatomite was well fitted to pseudo-second-order kinetics model (R2 = 0.999), and the equation is shown below. This implied that the adsorption of Cd(II) by diatomite was controlled by chemisorption.
Qe and Qt (mg g−1) are the amounts of Cd(II) adsorbed per unit mass of the adsorbent at equilibrium and at time t (min), respectively; k2 (g(mg min)−1) is the pseudo-second-order adsorption rate constant.
In order to explore the thermodynamic adsorption mechanism more comprehensively, we adopted Langmuir, Freundlich, and Sips isotherm models to fit the adsorption data. The results showed that the Sips isotherm model was the most suitable for describing the adsorption of Cd(II) by diatomite with a highest correlation coefficient (R2 = 0.9965, Table 1). It can also be seen that this model was more suitable than the Langmuir isotherm or Freundlich isotherm (Fig. 2b).
The Sips isotherm is a combination of the Langmuir and Freundlich isotherms by introducing a parameter (mS) related to heterogeneity, and it is more suitable for describing the adsorption process on heterogeneous surfaces (Ahmed and Theydan 2012; Gökırmak Söğüt and Caliskan 2017). Therefore, the adsorption of Cd(II) by diatomite was more likely to be heterogeneous interface adsorption.
The Sips and the two basic adsorption isotherm equations are shown below.
Langmuir isotherm (Langmuir 1916):
Freundlich isotherm (Freundlich 1906):
Sips isotherm (Kumar and Porkodi 2006):
Where Ce is the equilibrium concentration of Cd(II) in solution (mg/L), Qe is the adsorption capacity at adsorption equilibrium (mg/g); KL is the Langmuir adsorption constant (L/mg), and Qm is the maximum adsorption capacity of the adsorbent (mg/g); 1/n is the heterogeneous coefficient, and KF is the Freundlich adsorption constant (L/mg); KS is the Sips constant, mS represents the heterogeneity of the adsorbent.
Desorption experiment
The binding mode of adsorbed Cd(II) on diatomite was investigated through desorption experiments. Under the same experimental conditions, the diatomite after isothermal adsorption was desorbed with deionized water and 0.1 M MgCl2, respectively, and the results are shown in Fig. 3a. Although the amount of desorbed Cd(II) by deionized water gradually increased with the increase of Ce, the values of the amount were much smaller than that by MgCl2. As known to all, the deionized water system cannot perform ion exchange according to the law of charge conservation. The small amount of desorbed Cd(II) by deionized water indicated the electrostatic attraction was very weak in the system, which was consistent with the result of pHPZC determination of the diatomite (pHPZC = 4.5).
The Mg2+ in the desorption solution could displace the ion-exchanged Cd(II), which had the same charge. Since the amount of Mg2+ in the high concentration solution was much higher than that of Cd(II) adsorbed on the diatomite, it can be inferred that the Cd(II) adsorbed on the diatomite via ion exchange could be completely desorbed into the solution. The desorption results are shown in Fig. 3b. We found that both the amounts of adsorbed Cd(II) and desorbed Cd(II) increased with increasing initial Cd(II) concentration. The desorption percentage of adsorbed Cd(II) was about 80%.
Detection of cations was carried out on the solution following isothermal adsorption. The results are shown in Fig. 4a. The cations in the solution were mainly Ca2+ and Mg2+, with a small amount of K+. NaCl served as the background electrolyte for the system, and the Na+ concentration remained unchanged before and after adsorption. The content of each cation in the solution increased with the amount of Cd(II) adsorbed, and the total cationic milliequivalent concentration was consistent with the equivalent concentration of desorbed Cd(II). The result demonstrated that all of the Cd(II) desorbed by MgCl2 was adsorbed onto the diatomite by ion exchange interaction. Therefore, we could conclude that ion exchange interaction dominated the removal of Cd(II) by diatomite due to a high contribution to the whole adsorption reaction (around 80%). More importantly, Ca2+ and Mg2+ in the diatomite were responsible for ion exchange.
Furthermore, we found that Mg2+ could not replace all of the Cd(II) adsorbed on the diatomite. The result suggested that ion exchange was not the only interaction during the adsorption process. In order to investigate the adsorption mechanism of Cd(II) adsorption on diatomite, we performed characterization of the diatomite after Cd(II) adsorption and desorption using EDS mapping. We found that Cd(II) was adsorbed onto the diatomite after the adsorption experiment; however, there was no Cd signal after the desorption experiment (Fig. 5, Fig. S2). It indicated that the retained Cd(II) was trapped inside the diatomite by the porous channel. The pore volume values of the original and Cd-loaded (C0 = 100 mg/L) diatomite were determined to be 0.057 and 0.055 cm3/g, respectively.
This proved that partially adsorbed Cd(II) was retained inside the diatomite, causing a decrease in the pore volume. It can be seen from Fig. 1 that there were enough micropore structures on the surface and inside of the diatomite, and the ratio of pores with diameters less than 2 nm was as high as 7%. The hydration radius of Cd(II) is 0.428 nm according to a previous study (Nightingale 1958). Due to the small diameter of the pores, Cd(II) in the solution could easily enter the pores inside the diatomite and be trapped by the microporous structure, and thus cannot be desorbed by deionized water or MgCl2 solution.
In summary, we found that ion exchange was the main driving force of Cd(II) adsorption, while the rest was probably trapped into the pores. The desorption data (T = 298 K) was nonlinear fitted, as can be seen in Fig. 4b. It can also be seen that the extension of the ion exchange fitting line had an increasing tendency, while the remaining ones had a smaller line increment and slowly approach the equilibrium. The curve shows the relatively large number of ion exchange sites on diatomite, which is consistent with the result that ion exchange was the main effect in Cd(II) adsorption.
The pH effect
The point of zero charge (pHPZC) of the diatomite was determined to be 4.5. Theoretically, the surface of diatomite was negatively charged at pH > 4.5 (pHPZC), which showed electrostatic attraction for Cd(II).
As shown in Fig. 6, the amounts of adsorption and desorption of Cd(II) on diatomite slightly increased when the solution pH ranged from 4 to 7, whereas that were obviously lower and higher at pH 3 and 8, respectively. It can be seen that pH has an obvious effect on the adsorption amount only under strongly acidic or basic condition. This indicated that electrostatic attraction had less contribution to the adsorption compared with ion exchange, which was consistent with the desorption results by deionized water.
It can also be seen that the percentage of desorption was comparable over the pH range of 3 to 7, while the desorption percentage was a little lower at pH 8. This implied that hydroxide precipitation played a role in the adsorption (Hahne and Kroontje 1973), due to the small solubility product of Cd(OH)2 (Ksp = 2.2 × 10−14).
The second adsorption
The regeneration performance of diatomite is an important index for the evaluation of adsorbents used in water treatment. Therefore, in the effort to better understand adsorption performance, the significance of investigating the change in the adsorption mechanism before and after regeneration cannot be overstated. As shown in Fig. 7, in the second adsorption process, the capacity for adsorption was smaller than that in the first adsorption, accounting for 85% of the first one.
Although there was a decrease in the amount of Cd(II) adsorption retained in the second adsorption experiment, the contribution rate for total adsorption when compared with the first adsorption was equivalent, indicating that trapped Cd(II) did not reach saturation. Additionally, in the second adsorption experiment, the high concentration of Cd(II) in the solution led to a relatively strong concentration gradient force, allowing more Cd(II) to diffuse into the micropores of the diatomite. Therefore, the amount of trapped Cd(II) was slightly higher than that in the low Cd(II) concentration samples.
The results of pore volume determination (Fig. S3 and Fig. S4 in Supplementary Material 3) showed that the pore volume of pores with diameters less than 2 nm decreased after Cd(II) adsorption on diatomite, and it decreased again after the second adsorption conducted. The decreasing trend of pore volume indicated Cd(II) was trapped into diatomite again.
Mechanism
Based on the analysis above, the mechanism of interaction that occurred between diatomite and Cd(II) can be concluded in the following statements. Under the conditions of this study, electrostatic attraction plays a minor role in the adsorption of Cd(II) by diatomite. Driven by the concentration gradient, the Cd(II) in the solution diffused freely onto diatomite, and was then exchanged with the ions on the solid phase exchange site of the diatomite, mainly Ca2+ and Mg2+. The ion exchange equations are listed as follows:
In summary, the equations can be written as:
The exchanged ions diffused to the surface of the solid phase, then diffused into the solution. Simultaneously, a small amount of Cd(II) in the solution diffused from the large pores on the surface of the diatomite to the inner pores and micropores, thereby becoming trapped inside the diatomite. It was thus difficult for this portion of Cd(II) to be exchanged or desorbed in the desorption experiment. The ion exchange interaction caused by Ca2+ and Mg2+ in the diatomite was the main force driving Cd(II) adsorption, and its contribution to the whole adsorption reaction reached 80%. The remainder was most likely trapped in the micropores of the diatomite.
The retained Cd(II) inside the diatomite decreased the adsorption site in the second adsorption experiment. However, the adsorption trend and mechanism remained unchanged after the first-time regeneration of diatomite.
Conclusion
Under the experimental conditions examined in this study, we found that the adsorption of Cd(II) by diatomite was very fast because the reaction was close to equilibrium within 5 min. In order to explore the adsorption mechanism, we carried out kinetic, isothermal, and desorption experiments. The results showed that the main interaction force driving the adsorption was ion exchange, accounting for more than 80% of the total adsorption efficiency, while the rest of the Cd(II) was probably trapped in the micropore structure of the diatomite. Moreover, the adsorption mechanism did not change significantly after regeneration. To our knowledge, this is the first time to clearly reveal the specific types of interactions involved in Cd(II) adsorption by diatomite. We believe that these findings have provided new insight into the mechanism of Cd(II) adsorption by diatomite, which will have great significance for the application of diatomite in the treatment of Cd(II) contaminated water.
References
Ahmed MJ, Theydan SK (2012) Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib 317:9–14
Al-Ghouti MA, Khraisheh MAM, Ahmad MNM, Allen S (2009) Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study. J Hazard Mater 165:589–598
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Beheshti H, Irani M (2016) Removal of lead(II) ions from aqueous solutions using diatomite nanoparticles. Desalin Water Treat 57:18799–18805
Bhatnagar A, Sillanpää M (2010) Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—a review. Chem Eng J 157:277–296
Cheung CW, Porter JF, McKay G (2000) Elovich equation and modified second-order equation for sorption of cadmium ions onto bone char. J Chem Technol Biotechnol 75:963–970
Danil de Namor AF, El Gamouz A, Frangie S, Martinez V, Valiente L, Webb OA (2012) Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J Hazard Mater 241:14–31
Dhir B (2014) Potential of biological materials for removing heavy metals from wastewater. Environ Sci Pollut Res 21:1614–1627
ElSayed EE (2018) Natural diatomite as an effective adsorbent for heavy metals in water and wastewater treatment (a batch study). Water Sci 32:32–43
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Gökırmak Söğüt E, Caliskan N (2017) Isotherm and kinetic studies of Pb (II) adsorption on raw and modified diatomite by using non-linear regression method. Fresenius Environ Bull 26:2721
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment a review. Crit Rev Environ Sci Technol 39:783–842
Hahne HCH, Kroontje W (1973) Significance of pH and chloride concentration on behavior of heavy metal pollutants : mercury (II), cadmium (Il), zinc (ll), and lead (ll). J Environ Qual 2:444
Ho YS (1995) Adsorption of heavy metals from waste streams by peat. Dissertation, University of Birmingham
Irani M, Amjadi M, Mousavian MA (2011) Comparative study of lead sorption onto natural perlite, dolomite and diatomite. Chem Eng J 178:317–323
Khairy M, El-Safty SA, Shenashen MA (2014) Environmental remediation and monitoring of cadmium. TrAC Trends Anal Chem 62:56–68
Kumar KV, Porkodi K (2006) Relation between some two- and three-parameter isotherm models for the sorption of methylene blue onto lemon peel. J Hazard Mater 138:633–635
Kumar R, Chawla J, Kaur I (2015) Removal of cadmium ion from wastewater by carbon-based nanosorbents: a review. J Water Health 13:18–33
Lagergren S (1898) About the theory of so-called adsorption of solution substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Li Y, Yang Z, Chen Y, Huang L (2019) Adsorption, recovery, and regeneration of Cd by magnetic phosphate nanoparticles. Environ Sci Pollut Res 26:17321–17332
Lodeiro P, Herrero R, Sastre de Vicente ME (2006) Thermodynamic and kinetic aspects on the biosorption of cadmium by low cost materials: a review. Environ Chem 3:400–418
Miretzky P, Munoz C, Cantoral-Uriza E (2011) Cd2+ adsorption on alkaline-pretreated diatomaceous earth: equilibrium and thermodynamic studies. Environ Chem Lett 9:55–63
Mustafa S, Dilara B, Nargis K, Naeem A, Shahida P (2002) Surface properties of the mixed oxides of iron and silica. Colloid Surf A 205:273–282
Nightingale ERJ (1958) Phenomenological theory of ion solvation. Effective radii of hydrated ions. J Phys Chem 63:1381–1387
Patil KL, Sawant CP (2009) An evaluation of heavy metals content in dyeing and printing industrial effluents in GIDC Surat: a statistical approach. Asian J Chem 21:7097–7100
Qu J, Tian X, Jiang Z, Cao B, Akindolie MS, Hu Q, Feng C, Feng Y, Meng X, Zhang Y (2019) Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121718
Rao KS, Mohapatra M, Anand S, Venkateswarlu P (2010) Review on cadmium removal from aqueous solutions. Int J Eng Sci Technol 2:81–103
Safa M, Larouci M, Meddah B, Valemens P (2012) The sorption of lead, cadmium, copper and zinc ions from aqueous solutions on a raw diatomite from Algeria. Water Sci Technol 65:1729–1737
Salman M, Jahan S, Kanwal S, Mansoor F (2019) Recent advances in the application of silica nanostructures for highly improved water treatment: a review. Environ Sci Pollut Res 26:21065–21084
Sheng G, Wang S, Hu J, Lu Y, Li J, Dong Y, Wang X (2009) Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloid Surf 339:159–166
Sun Z, Chen J, Wang X, Lv C (2016) Heavy metal accumulation in native plants at a metallurgy waste site in rural areas of northern China. Ecol Eng 86:60–68
Tang J, Gschneidner KA (1996) Physical metallurgy and magnetic behavior of Cd-stabilized bcc beta-Gd alloys. J Alloys Compd 234:26–33
Ulmanu M, Maranon E, Fernandez Y, Castrillon L, Anger I, Dumitriu D (2003) Removal of copper and cadmium ions from diluted aqueous solutions by low cost and waste material adsorbents. Water Air Soil Pollut 142:357–373
Valente Flores-Cano J, Leyva-Ramos R, Padilla-Ortega E, Mendoza-Barron J (2013) Adsorption of heavy metals on diatomite: mechanism and effect of operating variables. Adsorpt Sci Technol 31:275–291
Vassileva P, Gentscheva G, Ivanova E, Tzvetkova P, Voykova D, Apostolova M (2011) Characterization of natural diatomites from Bulgaria. C R Acad Bulg Sci 64:823–830
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang W, Liu Y, Liu X, Deng B, Lu S, Zhang Y, Bi B, Ren Z (2018) Equilibrium adsorption study of the adsorptive removal of Cd2+ and Cr6+ using activated carbon. Environ Sci Pollut Res 25:25538–25550
Weber WJ, Morris JC (1963) Intraparticle diffusion during the sorption of surfactants onto activated carbon. J Sanit Eng Div Am Soc Civ Eng 89:53–61
Ye X, Kang S, Wang H, Li H, Zhang Y, Wang G, Zhao H (2015) Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J Hazard Mater 289:210–218
Zhang J, Xu Y, Wu Y, Hu S, Zhang Y (2019) Dynamic characteristics of heavy metal accumulation in the farmland soil over Xiaoqinling gold-mining region, Shaanxi, China. Environ Earth Sci 78:25
Zhang Z, Li M, Chen W, Zhu S, Liu N, Zhu L (2010) Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ Pollut 158:514–519
Zhu J, Wang P, Lei M, Zhang W, Wang T, Wu X (2013) Analysis of the adsorption behaviour of cadmium on aluminium-pillared diatomite in a solid/liquid system using classical adsorption theory. Adsorpt Sci Technol 31:659–670
Funding
This work was financially supported by the Research Fund of China Geological Survey (DD20190703), the Beijing Natural Science Foundation (No. 8182049), Guangxi key research project (GuikeAB18050026), and the Natural Science Foundation of China (Grant 41672239).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 239 kb)
Rights and permissions
About this article
Cite this article
Chang, J., Zhang, J., Tan, B. et al. New insight into the removal of Cd(II) from aqueous solution by diatomite. Environ Sci Pollut Res 27, 9882–9890 (2020). https://doi.org/10.1007/s11356-020-07620-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07620-y