Abstract
Fine particulate matter (PM2.5) concentrations at the Middle Adriatic coastal site of Croatia were affected by different air-mass inflows and/or local sources and meteorological conditions, and peaked in summer. More polluted continental air-mass inflows mostly affected the area in the winter period, while southern marine pathways had higher impact in spring and summer. Chemical characterization of the water-soluble inorganic and organic ionic constituents is discussed with respect to seasonal trends, possible sources, and air-mass inputs. The largest contributors to the PM2.5 mass were sea salts modified by the presence of secondary sulfate-rich aerosols indicated also by principal component analysis. SO42− was the prevailing anion, while the anthropogenic SO42− (anth-nssSO42−) dominantly constituted the major non-sea-salt SO42− (nssSO42−) fraction. Being influenced by the marine origin, its biogenic fraction (bio-nssSO42−) increased particularly in the spring. During the investigated period, aerosols were generally acidic. High Cl− deficit was observed at Middle Adriatic location for which the acid displacement is primarily responsible. With nssSO42− being dominant in Cl− depletion, sulfur-containing species from anthropogenic pollution emissions may have profound impact on atmospheric composition through altering chlorine chemistry in this region. However, when accounting for the neutralization of H2SO4 by NH3, the potential of HNO3 and organic acids to considerably influence Cl− depletion is shown to increase. Intensive open-fire events substantially increased the PM2.5 concentrations and changed the water-soluble ion composition and aerosol acidity in summer of 2015. To our knowledge, this work presents the first time-resolved data evaluating the seasonal composition of water-soluble ions and their possible sources in PM2.5 at the Middle Adriatic area. This study contributes towards a better understanding of atmospheric composition in the coastal Adriatic area and serves as a basis for the comparison with future studies related to the air quality at the coastal Adriatic and/or Mediterranean regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine aerosols play an important role in the direct and indirect effects on the global climate (O'Dowd and de Leeuw 2007). Chemical composition and size distribution of marine aerosols are important properties which affect their transport, transformation, removal, and the extent of global aerosol radiative forcing (Seinfeld and Pandis 2006). Water-soluble ionic constituents, particularly those within the fine-mode particles, could be important sources of cloud condensation nuclei (CCN), affecting cloud microphysics and consequently the climate (Ayers and Gras 1991; Liss and Lovelock 2007). In general, water-soluble ions account for about 60–70% of particulate mass (Wang and Shooter 2001). However, the water-soluble ion concentrations and distribution in atmospheric particles are characteristics of particular regions, because they considerably depend on local sources, weather conditions, and long-range transport (Abdalmogith and Harrison 2006). Thus, the ionic properties of atmospheric particles have been extensively studied in recent years.
In coastal areas, aerosols originate from natural and anthropogenic sources. Sodium chloride (NaCl) and other common seawater ions (such as SO42−, K+, Mg2+, and Ca2+) resulting from the primary emission by the action of the wind on sea surface are major constituents of sea-spray aerosols (e.g., Monahan et al. 1986), which significantly contribute to particulate mass in the coastal atmosphere (Adachi and Buseck 2015; Lewis and Schwartz 2004; Piazzola et al. 2009; Yoon et al. 2007). In addition, generation of smaller particles in the marine environment results from the secondary production from volatile biogenic precursors, such as dimethyl sulfide (DMS) of phytoplankton origin and isoprene from vegetative transpiration (e.g., Charlson et al. 1987; Meskhidze and Nenes 2006; Warneck 2003; Unger 2013). Moreover, coastal areas are significantly affected by continental and other anthropogenic sources (i.e., local transport and domestic heating), contrary to remote marine sites (Abbasse et al. 2003; Takami et al. 2005; Topping et al. 2004). In the Adriatic-Ionian port areas, the study of atmospheric impacts using inventories, experimental data, and modeling showed that ship traffic contributed 0.5–7.4% to particulate matter (PM2.5) mass (Merico et al. 2016). Although it is well-recognized that marine aerosols have a significant effect on the local air quality (Knipping and Dabdub 2003), their complex mixing with anthropogenic pollutants in coastal areas is still poorly understood.
The Mediterranean is recognized as a biodiversity hotspot, and one of the most susceptible areas to climate change, yet with large uncertainties regarding the fine-scale climate processes associated with the complex physiography of the region (Mermex Group 2011). The area continuously receives anthropogenic aerosols from industrial and domestic activities from the European region (Kanakidou et al. 2011). It is assumed that the impacts of human activities, including biomass burning (BB), are proportionally stronger in the Mediterranean than in any other sea-area of the world. Besides, the 85 million hectares of forests around the basin are also an occasional intense source of aerosols due to wildfire emissions (Turquety et al. 2014). Therefore, conducted field campaigns substantially contribute to the better process-level understanding of atmospheric physics and chemistry in the region. However, compared with the intensive research on the chemical characterization of airborne particles in other Mediterranean countries (e.g., Koçak et al. 2004, 2007; Luria et al. 1996; Mihalopoulos et al. 1997), in the Adriatic Sea sub-basin, such investigations are scarce. With the exception of the northern part and the area in the vicinity of the Venice lagoon (e.g., Gambaro et al. 2009; Ivošević et al. 2016; Morabito et al. 2014; Piazzola et al. 2016; Rossini et al. 2005; Stortini et al. 2009; Turšič et al. 2006), no extensive investigations on chemical composition of aerosols and their potential sources have been conducted at the Adriatic region thus far. As typical for coastal areas, the Adriatic is under the combined influence of local, regional, and long-range natural and anthropogenic pollution sources (Richon et al. 2017). Additionally, during summer, the risk of fire is assessed as high to very high, especially in the Middle Adriatic.
Our current study includes an intensive sampling program, which was conducted seasonally from April 2015 to July 2016 at the eastern coast of Middle Adriatic, Croatia. The primary objective was to get an insight into the variability of the chemical composition of fine aerosol particles in respect to water-soluble inorganic and organic ions in relation to different seasons, air-mass impacts, and special events at the local level, such as open-fire emissions typical for the Middle Adriatic area. Relative contributions of local and long-range transported particles as well as relative contributions of natural and anthropogenic sources to fine atmospheric aerosol loadings are important issues for the coastal Adriatic due to the limited data available in this region thus far. To our knowledge, our study presents the first data evaluating the composition of water-soluble ions and their possible sources in PM2.5 at the Middle Adriatic area. Every new quantitative data set on aerosol components and its sources in still poorly explored or completely unexplored regions will help in future improvements of atmospheric models, which are necessary to resolve and clarify the aerosol impact on the global climate.
Materials and methods
Sampling
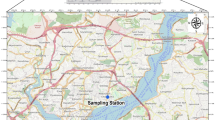
Aerosol sampling was conducted at the peninsula Gradina in the vicinity of the small touristic settlement Rogoznica (43°31′52“N 15°57′34”E) at the Middle Adriatic coast, Croatia (Fig. 1). The topography of this suburban region is characterized by a wide belt of hinterland with fields in the karst landscape and Mediterranean vegetation as well as mountainous area in the north and northeast. The sampling site is located 2 km away from the most eastern part of the eastern Adriatic coast (Cape Planka) where the north and south winds collide strongly. The eastern Middle Adriatic is generally characterized by the extensive tourism and mariculture, and the low impact of local industrial activities. The region is affected by the arid summer conditions of the Mediterranean and thus under high to very high fire risks.
The PM2.5 samples were collected using the low volume sequential sampler SEQ 47/50 (SEQ47/50) (Sven Leckel, Ingenieuburo GmbH, Germany) on a pre-combusted (450 °C for 4 h) glass fiber filters (Whatman, Grade GF/F, d = 47 mm) at a flow rate of 2.3 m3/h for 48 h from April 2015 to July 2016, capturing 6 consecutive seasons: spring 2015 (8 April–6 May 2015), summer 2015 (15 July–15 September 2015), autumn 2015 (23 September–29 October 2015), winter 2016 (20 January–24 March 2016), spring 2016 (29 March–22 June 2016), summer 2016 (22 June–13 July 2016). Three subsequent winter samples as well as two subsequent spring samples from 2016 were removed from the data set because of a suspected direct contamination. The concentrations of sea salt in those samples were more than ten times higher than in the sample with the next highest concentration. A total of 143 samples were stored in Petri Slides (Millipore Inc.) in a freezer (− 50 °C) until the analysis.
Aerosol chemical analyses
The PM2.5 mass was obtained using Mettler Toledo XP205DR microbalance (reading precision of 10 μg) by weighing the filters before and after the sampling under constant conditions of 20 ± 1 °C and the relative humidity (RH) of 50 ± 5%. For ion analysis, approximately 25% of exposed filter was extracted with 10 mL Milli-Q water (18.2 MΩ; 20 min ultrasonic bath; stored at 4 °C overnight). The solutions were filtrated through 0.22 μm PTFE disk filters and analyzed through ion chromatography. The anions were measured on a Dionex ICS 3000 with a conductivity detector. Anion separation was achieved on an analytical column (Dionex IonPac AS11-HC, 4 × 250 mm) with a precolumn (Dionex IonPac AG11-HC, 4 × 50 mm) at 1 mL min−1 flow rate applying the following elution program: 0–15 min isocratic elution with 1 mM KOH, 15–29 min gradient to 15 mM KOH, 29–42 min gradient to 30 mM KOH, 42–50 min gradient to 60 mM KOH, and 50–55 min isocratic elution to 60 mM KOH. Injection volume was 50 μL. Under these conditions, 12 anions were quantified: fluoride (F−), lactate (C3H5O3−), acetate (C2H3O2−), formate (CHO2−), methanesulfonate (CH3SO3−, MS−), chloride (Cl−), nitrate (NO3−), malate (C4H4O52−), maleate (C4H2O42−), sulfate (SO42−), oxalate (C2O42−), and phosphate (PO43−). The cations, sodium (Na+), ammonium (NH4+), and potassium (K+), were measured on a Thermo Separation Products ion chromatograph equipped with a Shodex CD-5 conductivity detector, using a Dionex IonPac CG/CS12A precolumn (4 × 50 mm) and column (4 × 250 mm). The isocratic elution with 20 mM methanesulfonic acid (MSA) at the flow rate of 1.5 mL min−1 was set up for 23 min and the injection volume was 50 μL. The limits of quantification (LOQ) for cations and anions in PM2.5 samples were < 0.006 μg m−3 and < 0.002 μg m−3, respectively.
Chloride depletion calculations
Chloride depletion refers to the percentage loss in Cl− from sea salt leading to higher Na+ to Cl− ratios due to the Cl− reaction with inorganic and organic acids and is estimated as mass percentage:
where (Na+) and (Cl−) are mass concentrations (μg m−3) and 1.81 is the typical mass ratio of Cl− to Na+ in seawater.
The theoretical maximum amount (%) of observed Cl− depletion that could be attributed to a specific acidic species was estimated by the following equation:
where A is the mass concentration (μg m−3) of the acidic species in ambient air, y is the charge of the fully deprotonated conjugate base of A, and MWchloride and MWA represent molecular weights of chloride and acid species, respectively.
Accounting that nssSO42− reacts with available NH4+, concentration (in μg m−3) of the nssSO42− not associated with ammonium, (ex-nssSO42−) was obtained following the equation by Brown et al. (2017):
Meteorological data
Meteorological parameters recorded at the ground meteorological station located 400 m away from the sampling site are presented in Fig. 2 which include 2-day average atmospheric temperature, wind speed, RH, and precipitation level measured during the campaign. Local ambient temperature affected by solar radiation varied throughout the year from 6 to 30 °C (average 19 ± 6 °C). Air temperature started to increase in March and reached its maximum in July–August (approximately 27 °C) after which it decreased below 10 °C in January. From October to March, the wind circulation was northerly (from NNE to ENE), while from April to September, the wind direction changed southerly with a domination of SW wind during the summer (coming from the sea). The sampling site was relatively wet during the autumn, winter, and early spring and was mostly dry during the summer months. In summer, the whole Mediterranean region is typically dry; therefore, the removal of aerosol particles by wet deposition is minimized.
Air-mass backward trajectories
The air-mass backward trajectory analysis was performed by using the NOAA HYSPLIT model (https://www.ready.noaa.gov/HYSPLIT.php) and GDAS 1 (Global Data Assimilation System) meteorological datasets at 10 m a. g. l. to differentiate major air-mass origins in order to assist in the data interpretation. The plots represent a trajectory ensemble of 3 single backward trajectories, ending at the sampling site. All trajectories were calculated for 72 h time intervals and are, within an ensemble, shifted for 24 h, which corresponds to the three representative time points during the 48 h sampling (at the beginning, middle, and end of the sampling time for each sample).
In order to identify the potential importance of different source regions on the aerosol composition, the 143 samples collected were generally classified into airflow sectors according to the dominant air-mass trajectory direction: (a) marine (MAR)—the air mass spent most of the time over the open Adriatic Sea, Mediterranean, and/or coastal region (Fig. 3a) and (b) continental (CONT)-the air mass spent most of the time over the continental Europe (Fig. 3b). On average, only 35% of the air masses arriving at the Middle Adriatic site originated from the continental north sector (online supplementary information, SI Tables 1–5). MAR inflow significantly impacted the area with a lower contribution during the autumn and winter (on average 50 and 55%, respectively) in comparison to the spring (spring 2015, 72%; spring 2016, 64%) and summer (summer 2015, 67%; summer 2016, 64%) periods. This is in line with the mean wind vectors over the sampling site (“Meteorological data” section).
Open-fire events
A fire-influenced sample set was identified in summer 2015 (6 samples; from 04 August–16 August 2015) according to the air-mass backward trajectory analysis and the Šibenik county fire department archive data (http://www.vatrogastvo-sibenik-knin.hr/), where compiled information on the intervention type (e.g., air-force intervention), fire duration, attacked surface area, and type of vegetation affected are registered. During the selected period, intensive simultaneous and/or continuous open fires (overall 42 registered events) of grass, low plants, and pine and olive tree forests were reported in the N/NW Šibenik county area in the radius of around 30 km from the sampling location (Fig. 1). The overall affected area was over 2 km2. On 15 August 2015, an open-fire event happened in Rogoznica, 50 m away from the sampling site.
Statistical data treatment
To evaluate the sources of major ions, a multivariate statistical method principal component analysis (PCA) by Statistica Release 7.0 was performed. PCA was performed after data set homogenization (half-range and central value transformation), cross-validation, and normalization (varimax rotation).
Results and discussion
Temporal variability of PM2.5 concentration and ion composition
Figure 4 presents the PM2.5 mass concentrations for the Middle Adriatic site, including the period with intensive regional open fires marked with yellow. Results of average individual water-soluble ion concentrations along with the average contributions of the main ion constituents to PM2.5 at the coastal Middle Adriatic site during the investigated period are presented in Table 1. Note that only samples without the identified influence of open-fire emissions were further considered while fire-event samples will be discussed separately in the “Impact of open fires on water-soluble ion composition” section. In addition, data from 18 autumn samples corresponding to 12% of all data are not included in the further discussion related to ion composition because of high analytical uncertainties due to the low PM2.5 mass concentrations (caused by intensive rainfalls in autumn 2015). Complete data set presenting concentrations of PM2.5 mass and ion species in different seasons are shown in SI Tables 1–5. In the following, the temporal and seasonal variations of the PM2.5 constituents are discussed with respect to the meteorological conditions, air-mass origin, and interrelationship between ionic species.
PM2.5 mass concentration
Excluding the fire period, the average PM2.5 mass showed seasonal variability and decreased in the order: summer 2016 (13.4 ± 5.4 μg m−3) > summer 2015 (12.7 ± 4.8 μg m−3) > spring 2015 (10.8 ± 3.2 μg m−3) > winter 2016 (9.0 ± 4.9 μg m−3) > spring 2016 (7.4 ± 2.5 μg m−3) > autumn 2015 (5.0 ± 2.5 μg m−3). High summer PM concentrations are also typical for the whole Mediterranean (e.g., Erduran and Tuncel 2001; Koçak et al. 2004, 2007; Luria et al. 1996). The average PM2.5 mass concentration (9.2 ± 4.7 μg m−3) obtained for the investigated period at the Middle Adriatic site is enclosed with the ranges reported for several rural sites in Portugal (between 5.6 and 17.8 μg m−3) (Freitas et al. 2005) and suburban Lisbon area (2.4–30.0 μg m−3) (Almeida et al. 2006). The comparable average PM2.5 mass of 12.6 μg m−3 was also observed at the regional background site in the western Mediterranean in the period from 2002 to 2010 (Cusack et al. 2012). In contrast, higher PM2.5 concentrations were reported for several European regions, such as suburban (19 ± 9 μg m−3) (Perrone et al. 2011) and urban sites (34.5 ± 19.4 μg m−3) of Italy (Daher et al. 2012) and rural sites of Crete (23.5 ± 5.8 μg m−3) (Kopanakis et al. 2012). Higher annual mean value of 25 ± 8 μg m−3 was reported also at a coastal site in the south of Italy, located away from large sources of local pollution, but exposed to periodic dust events (Perrone et al. 2013).
Meteorological conditions largely affected temporal PM2.5 variabilities in the area. A positive correlation between PM2.5 mass and air temperature (r = 0.478, p < 0.01, N = 143; SI Fig. 1a) points to the possible influence of photochemical processing and the related secondary aerosol formation during summer contributing to the highest measured PM concentrations in this period. High PM2.5 concentrations in summer can be also attributed to the prolonged lifetime of aerosol particles in the atmosphere due to the absence of rain shower, since weak but still significant negative correlation between the PM2.5 mass and precipitation level was observed (r = 0.324, p < 0.05, N = 51; SI Fig. 1b). Accordingly, during abundant rain events in autumn 2015 (Fig. 2), PM2.5 concentrations drastically decreased, possibly because rain droplets efficiently scavenged particles from the atmosphere. More frequent and intensive rainfalls during spring 2016 (Fig. 2) most probably caused also lower average PM concentrations in comparison to spring 2015, despite higher temperatures measured. A negative correlation between wind speed and PM mass concentrations (r = 0.291, p < 0.01, N = 143; SI Fig. 1c) additionally suggests some PM dispersal with the increasing wind speed at the coastal Middle Adriatic site, as typically observed over urban locations in comparison to the open ocean (Vardoulakis and Kassomenos 2008; O’Dowd and Hoffmann 2005).
As far as the long-range transport of atmospheric PM is concerned, PM samples mostly affected by the continental north sector were generally characterized by higher average concentrations (except in autumn with comparable average masses of CONT and MAR samples; see Fig. 5). This indicates more polluted continental impact in comparison to the air masses arriving from the southern marine regions. Considering also the frequency of samples influenced by the different air-mass origins in particular season (“Air-mass backward trajectories” section), more polluted continental air-mass inflows mostly affected the area in the winter period. The highest PM2.5 concentrations measured in summer could be also expected due to the pronounced photochemistry as well as higher impact of southern marine pathways during the stagnant summer meteorological conditions.
Total ion composition
The average total water-soluble ion concentrations (Table 1) showed a similar pattern to the PM2.5 mass concentrations (Fig. 4) with the minimum in winter and the maximum in summer. The sum of ions contributed an average of 76% to PM2.5. No significant difference in the total ion concentrations with regard to the air mass origin was observed. (SI Table 7). The most abundant ion was SO42− followed by Na+, NO3−, Cl−, NH4+, and the anions of carboxylic acids (hereafter organic acids) indicating that secondary inorganic aerosol (SIA) and sea salts presented the major portion of PM2.5 at the Middle Adriatic site. The percentages of SIA (i.e., the summation of SO42−, NO3−, and NH4+) ranged from 20 to 85% (average 49 ± 13%) and from 11 to 64% (average 36 ± 9%) in total measured ions and PM2.5 mass, respectively, having the minimum in winter and the maximum in summer.
Sea salt
Sea salt concentration estimated as [ss] = [Cl−] + [Na+] × 1.47 (Quinn et al. 2002), contributed on average 52 and 40% to the total measured ions and PM2.5 mass, respectively (Fig. 6, Table 1). Despite no significant correlation between sea salt concentrations and average wind speeds, the highest contribution of sea salts was observed during winter and spring 2016, when the highest average wind speeds were detected as well (Fig. 2). In marine environment, more turbulent weather conditions during transitional winter and spring periods are expected to enhance the primary emission of sea salt aerosols in contrast to summer, characterized by weaker winds and a calmer sea surface. Thus, since higher sea salt contributions to total ions were observed when both MAR (spring 2015) and CONT (winter 2016) inflows preferentially influenced the area (SI Table 7), we may assume that the regional/local meteorological conditions become more important factor for the sea salt enhancement in PM than the southern marine air inflows.
Sulfate and methanesulfonate
SO42− as the prevailing anion constitutes on average 28% of the PM2.5 mass (Table 1) and 37% of the total measured ion fraction (Fig. 6) with the minimum in winter and the maximum in the summer time. The average SO42− concentration of 2.79 ± 1.66 μg m−3 obtained for this study agrees with high SO42− concentrations observed over the Mediterranean (Bougiatioti et al. 2013; Koçak et al. 2004).
By using Na+ as a sea-spray marker to determine the concentration of SO42− associated with sea salt as [ssSO42−] = [Na+] × 0.252, where 0.252 is the (SO42−/Na+) mass ratio of bulk seawater (Millero 2006), it was found that on average ssSO42− contributed only up to 19% of the total SO42− measured. Thus, the variations observed in the SO42− time series are attributed to the variations in the prevailing non-sea-salt fraction (nssSO42−). Besides gas-phase oxidation and gas-to-particle conversion of anthropogenic SO2, as well as in-cloud oxidation of dissolved SO2 (Harris et al. 2013), a substantial amount of secondary nssSO42− (as well as MS−) could originate from biogenic phytoplankton emissions of DMS and its subsequent oxidation in the marine troposphere (Andreae et al. 1985; Charlson et al. 1987; Stefels et al. 2007).
Methane sulfonic acid is a well-known oxidation product of DMS and, hence, an important constituent of marine aerosols, especially in spring and summer (Sciare et al. 2009). Seasonal trend was indeed observed for MS− concentrations in fine aerosols at the coastal Adriatic site, with much higher concentrations measured in spring–summer than in winter (Table 1). Significantly, higher MS− contribution to MAR aerosols was observed in spring, being attributed to the increased phytoplankton activity (SI Table 7).
The observed average nssSO42− concentration (2.4 ± 1.6 μg m−3) is in a good agreement with the annual nssSO42− average of several Mediterranean cities (2.2–3.9 μg m−3) (Salameh et al. 2015) but considerably higher than those for remote marine regions (Savoie and Prospero 1989), implying that the studied aerosols comprised both anthropogenically (anth-nssSO42−) and biogenically (bio-nssSO42−) derived nssSO42−.
The bio-nssSO42− content is often estimated as the product of MS− concentration and the asymptotic value of nssSO42−/MS− ratio during enhanced MS− levels which is assumed to be a characteristic of investigated region and exclusively related to DMS (Park et al. 2017; Udisti et al. 2012; Udisti et al. 2016). Seasonal variabilities of average bio-nssSO42− and anth-nssSO42− following the asymptotic approach by Park et al. (2017) are shown in SI Fig. 2a. On average, the bio-nssSO42− contribution to total water-soluble ions and PM2.5 was 9 and 7%, respectively. Since anth-nssSO42− background levels at the sampling site presumably affected the asymptotic value assumed to relate exclusively to DMS oxidation, the obtained bio-nssSO42− concentrations (average 0.61 ± 0.41 μg m−3) are considered to be overestimated. Up to 50% overestimation of bio-nssSO42− by the asymptotic approach was also indicated by its correlation with that using the stable S-isotope ratio (Park et al. 2017). If accounting for the recent modeling simulation findings implying that the multiphase DMS oxidation produces equal amounts of MS− and SO42− (Hoffmann et al. 2016), bio-nssSO42− concentrations (average 0.06 ± 0.04 μg m−3) are an order of magnitude lower than those obtained by the asymptotic approach (Table 1). We may assume that the realistic bio-nssSO42− content is between the both calculated limit values.
Independent of the applied approach, bio-nssSO42− contributions to the total nssSO42− were particularly high in spring, followed by the summer periods (SI Fig. 2a and b) when air-mass inputs from the southern marine pathways were enhanced (“Air-mass backward trajectories” section). This is in agreement with the well-known increased phytoplankton primary production in the Adriatic Sea in spring due to favorable conditions, i.e., the availability of sunlight and nutrients within the sea surface layers (Marić et al. 2012). Significant correlation between the air temperature and the nssSO42− concentrations was detected (r = 0.512, p < 0.01, N = 119; SI Fig. 3). Thus, in addition to the lower rate of wet removal during summer, photochemical processing could also enhance the formation of secondary nssSO42− via oxidation by OH radicals. Note however, anth-nssSO42− dominantly constituted the nssSO42− fraction (SI Fig. 2a and b) being generally enhanced in the samples affected by the CONT inflow (SI Table 7) and having the highest impact on the Middle Adriatic area in winter.
Nitrogen-containing species
The determined NO3− concentrations were approximately 5–10 times lower than those of SO42−, indicating that secondary inorganic aerosols at the Middle Adriatic originated dominantly from the sulfur-containing sources rather than being nitrogen-controlled. Opposite to nssSO42−, the lowest NO3− contribution to the total measured ions was determined in summer, while the maximum was detected in winter (Fig. 6). A good correlation between the NO3− and nssSO42− observed only in winter (Table 2) implies that nssSO42−and NO3− had a common anthropogenic origin during that period. During spring and summer, an additional source of nssSO42−, such as the production through photochemical processes from both anthropogenic and biogenic precursors occurred.
In winter, higher NO3− levels are expected due to the larger availability of NOx precursors resulting from additional emissions not operating in summer (e.g., domestic heating). Moreover, since the equilibrium dissociation constant for NH4NO3 is sensitive to temperature and humidity, more NH4NO3 (from the same quantity of ammonia and nitric acid vapor) is expected to form at low temperature and at high relative humidity as observed in winter 2016. NO3− contribution did not differ significantly within MAR and CONT inflows (SI Table 7), implying comparable impacts of both CONT and MAR air-mass inflows in the area. In addition, local emission sources (e.g., domestic heating, land and ship traffic, and agricultural activities) could be important factors for the NO3− levels at the Middle Adriatic coastal site.
The average NH4+ concentrations at the Middle Adriatic site (Table 1) were lower than the average values determined at the eastern Mediterranean coastal sites (Bougiatioti et al. 2013; Koçak et al. 2007). Being the principal neutralizing agent for atmospheric acids, ammonia plays an important role in the secondary aerosol formation, while its conversion depends on the content of acidic species, temperature, and humidity in the atmosphere. Significant correlation with nssSO42− (Table 2) points to their related secondary origin. No seasonal trends or significant difference between CONT and MAR samples were observed at the Adriatic site, the same as for NO3−, indicating that both long-range transport and/or local sources could influence NH4+ levels in fine aerosols (SI Table 7). Local/regional sources of NH3 in the coastal and suburban Adriatic region are likely to come from agricultural practices, such as fertilizers use and agricultural waste burning.
Oxalate and potassium
Among the organic acid ions, oxalate was the most abundant species (Table 1). The oxalate levels, especially those measured during summer, are in very good agreement with the values reported by Bardouki et al. (2003) for Finokalia, Crete, in summer. These levels are among the highest reported in the literature for rural and even urban areas (Kawamura and Ikushima 1993). High insolation and the high levels of O3 prevailing in summer in the Mediterranean (Kouvarakis et al. 2002) could account for the extreme concentrations of oxalate in this region, indicating the influence of photochemistry on the ambient aerosol composition. It is believed that marine emissions of DMS, ethane, isoprene, and other biogenic precursors in combination with subsequent photochemical aqueous-phase reactions lead to the formation of oxalate in the marine atmosphere (Kawamura and Bikkina 2016; Kawamura and Sakaguchi 1999). On the other hand, a significant correlation of airborne oxalate with SO42− and NO3− has been reported, suggesting also the pollution source (Norton et al. 1983). Significant correlation observed in this work between oxalate and nssSO42− (Table 2) suggests its common secondary origin, likely related to in-cloud formation in the atmosphere.
High correlation between the oxalate and K+ was also obtained during the investigated period (Table 2). K+ emitted from sea salt (determined as [ssK+] = [Na+] × 0.038, where 0.038 is the (K+/Na+) mass ratio in bulk seawater (Millero 2006)) was a minor contributor to the total K+ (on average 2%). The anthropogenic origin of K+ indicates the relation to the combustion of vegetation and wood and waste incineration (Pachon et al. 2013 and reference herein). The oxalate/K+ mass ratio in this work averaged at 1.1, which is apparently larger than the reported values for the oxalate directly measured in BB plumes (0.03–0.1) (Yamasoe et al. 2000). Hence, the secondary formation of oxalate from vegetation and wood burning emitted VOCs could not be excluded.
Correlation of acidic species with NH4+ and implications for aerosol acidity
The most acidic constituents of atmospheric aerosol particles are H2SO4 and HNO3, both of secondary origin and predominantly related to anthropogenic pollution sources. Gas-to-particle partitioning of oxidized nitrogen species to form particulate NO3− is largely affected by acidic sulfate and neutralizing ammonium content (Seinfeld and Pandis 2006). The sum of measured nssSO42− and NO3− concentrations during the campaign at the coastal Middle Adriatic was strongly correlated with NH4+ (Table 2), with a slope of linear regression larger than 1, indicating an incompletely neutralized system. Such a strong correlation was due to the dominance of nssSO42− over NO3− governing the acidity of the atmosphere. There were several samples from April to May 2015 (spring 2015) and July 2015 (summer 2015) as well as from March 2016 (winter 2016) that were characterized by significantly higher NH4+ content, possibly due to the increased emissions of NH4+ from local agricultural activities and more intensive BB in the region. Specifically, those samples were characterized with NH4+/nssSO42− molar ratios > 1.2 as well as NO3− concentrations being on average up to 30% higher in comparison to seasonal averages. The excess in NH4+ during April, May, and August was also observed in the coastal Aveiro area, Portugal (Alves et al. 2007). In general, the average molar NH4+/nssSO42− ratio of 0.8 obtained for the overall period suggests that aerosols were acidic and predominantly in the form of NH4HSO4. Due to NH4+-limited conditions, HNO3 could not be fully neutralized by NH4+ to NH4NO3. Therefore, a statistically significant correlation between NH4+ and NO3− could only be observed in winter 2016 (Table 2). This is in line with higher NO3− content observed in winter as discussed previously.
A positive correlation was found between NH4+ + nssK+ and nssSO42− + NO3− + organic acids in equivalent concentrations (on average r = 0.812, p < 0.01, slope = 0.70, N = 96) and the ratio of the main cations to anions decreased in the order: summer 2015 > spring 2015 > summer 2016 > spring 2016 > winter 2016 (Table 2). Thus, except for several samples in spring and summer 2015, acidic components generally dominated over alkaline ones, rendering an acidic nature of aerosols throughout the sampling period. According to these calculations, winter samples were the most acidic, which is related to enhanced anthropogenic emissions and less pronounced Cl− depletion due to shorter residence time of aerosol particle in the atmosphere in this season (refer here to the following section). This in fact also agrees with the higher contribution of polluted CONT air masses in comparison to spring and summer aerosols.
Processes controlling Cl− depletion
In the atmospheric aerosols along the coastline, Cl− can be partially depleted due to chemical reactions of sea salts with acidic aerosol components, forming volatile HCl. Cl− deficit in marine aerosols has been observed at many coastal sites in the Mediterranean (e.g., Athanasopoulou et al. 2015; Malaguti et al. 2015; Mihalopoulos et al. 1997). In the polluted areas, where precursors of strong acids (H2SO4 and HNO3) constituting aerosols are abundant, simple acid-displacement reactions are likely responsible for the majority of Cl− depletion, leading to large Cl− deficits (and concomitantly pH increase) in respect to sea salt composition if only enough time is available.
The relationship between Na+ and Cl− was examined in the Middle Adriatic samples for the first time. Cl−/Na+ mass ratios (on average 0.50) were lower than that of bulk seawater (0.81), revealing a substantial Cl− depletion in the investigated fine aerosols. Moreover, no significant correlation could be found between Na+ and Cl− in the Middle Adriatic samples (r = 0.138, N = 143), pointing to complex processes leading to Cl− depletion in the investigated samples.
The calculated Cl− depletion (Eq. 1) at the Middle Adriatic ranged from 27 to 93% (on average 72 ± 12%) (Fig. 7a). Cl− deficits were higher in summer than in winter, which could be explained by the highest content of main acidic components, such as nssSO42−, NO3−, and organic acids in summer aerosols. Note, however, that despite the lower content of acidic species, winter aerosols were the most acidic. The lowest calculated Cl− depletion (and the highest aerosol acidity) of winter samples are attributable to shorter aerosol lifetimes in the atmosphere during winter, not giving enough time for efficient Cl− depletion. In addition, other mechanisms than acid-displacement reactions, such as photochemical reactions with O3 or NOx (Behnke and Zetzsch 1990) could be responsible for the observed enhanced Cl− depletion during hot summer period. Due to limited data set regarding the role of meteorological conditions or irradiation, our data suggest, but do not prove that photochemical reactions could play a role in the observed Cl− deficit.
Total Cl− depletion and average concentrations of acidic compounds: non-sea-salt sulfate (nssSO42−), NO3−, and the sum of organic acid anions, (a); theoretical Cl− depletion for acidic species: nssSO42−, NO3−, and the sum of organic acid anions, (b); theoretical Cl− depletion for acidic species: ex-nssSO42− (obtained after nssSO42− neutralization by NH4+), NO3−, and the sum of organic acid anions, (c); and theoretical Cl− depletion for organic acid species capped at 100%, (d); obtained for different seasons, and intensive open-fire period in the summer 2015 at the Middle Adriatic site
Theoretically, nssSO42− contributed the most to the observed Cl− depletion (Eq. 2) in the fine aerosols of all seasons (on average 72 ± 36%), with the maximum of 96% in summer and the minimum of 54% in winter (Fig. 7b). Assuming the upper limit of bio-nssSO42− concentrations obtained by the asymptotic approach following Park et al. (2017) (Table 1), bio-nssSO42− alone could account for up to 17% of Cl− displacement, with the maximum of 27% during the spring period. However, after accounting for the neutralization of H2SO4 by NH3 (Eq. 3), the average contribution of the obtained ex-nssSO42− drops to 37% (Fig. 7c), which should also be considered in the case of bio-nssSO42− contribution. In general, in the atmosphere over the Middle Adriatic, the reaction of sea salt with H2SO4 is the important pathway for the reduction of aerosol acidity in the atmosphere.
The chemical reaction between sea salt particles and HNO3 leading to Cl− depletion has been also well-documented (Hsu et al. 2007; Quinn and Bates 2005; Wu and Okada 1994). However, owing to lower NO3− concentrations in aerosols over the Middle Adriatic, the other acid-displacement reaction between H2SO4 and NaCl to Na2SO4 and HCl was favored. The calculated average ((Cl− + NO3−)/Na+) ratio was around 0.4, confirming that the contribution of HNO3 in the chemical displacement in the sea salt aerosols was minor. However, considering the theoretical scenario of lower nssSO42− availability after accounting for the neutralization by NH4+, the role of HNO3 becomes more significant for the Cl− depletion, especially in winter and spring (Fig. 7c).
Considerable theoretical contribution of total organic acids to the observed Cl− depletion was detected, clearly exceeding that of NO3− in summer (Fig. 7b). Among the organic acid anions, oxalate, MS−, and malate exhibited the highest theoretical contributions to the observed Cl− depletion (Fig. 7d). The maximal contribution of MS− to Cl− depletion (in respect to the contribution of the total fraction of organic acids) was up to 19% in spring, which is in relation to the enhanced phytoplankton productivity and DMS emissions. On the other hand, oxalate potentially contributed the most to the observed Cl− loss among all organic acids (on average 65 ± 10%), with the maximum in summer (Fig. 7d). Note that the formation and precipitation of organic salts in fine marine aerosols can modify the internal composition of atmospheric particles and, in turn, inhibit acid-catalyzed reactions to secondary organic aerosol (SOA) aging (Laskin et al. 2012). Moreover, the ability of particles to serve as CCN can be modified. Due to the high abundance of sea salt and SOA, acid-displacement reactions may have a considerable impact on the climate in coastal areas, where marine aerosols are mixed with anthropogenic and/or biogenic emissions.
Overall, assuming that all available NO3−, nssSO42−, and organic acids were involved in Cl− depletion (even without considering their neutralization with other nss-cations), acid contribution could explain the total Cl− loss only in summer, and up to 80% in spring and winter times. At this point, the unexplained Cl− loss can be attributed to the replacement by organic acids not considered within this study. In addition, this could also imply that at the coastal Middle Adriatic, being influenced by combined air-mass inputs and variable meteorology throughout the year, alternative pathways may also play a partial role in triggering the Cl− liberation from sea salt. The additional particulate Cl− loss can be thus partially attributed to heterogeneous and interface chemistry with a variety of atmospheric trace species, including OH, HO2, O3, NO2, N2O5, and ClONO2 (Finlayson-Pitts 2003 and references therein).
Sources of major ions of PM2.5
A principal component analysis (PCA) was applied to identify the factors influencing the variance of the major ionic constituents in different seasons to get more insight into the possible sources of aerosol components at the Middle Adriatic site. Thus, the MS−, Cl−, NO3−, oxalate, Na+, NH4+, K+, and nssSO42− concentrations obtained in different seasons were the discriminating variables used in the analysis. No restrictions to principal components (PCs) were made prior to the analysis and the decision of how many were selected relied on the Kaiser criterion (factors having an eigenvalue greater than 1.0) (Kaiser 1960). The rotated factors loading matrix from PCA is shown in Table 3. Results show that 74.9, 84.1, and 72.1% of the total variance in spring 2015, summer 2015, and winter 2016, respectively, can be explained by two PCs. Three PCs were able to explain the main variances in spring and summer 2016 (they accounted for 80.9 and 93.1% of the variabilities, respectively) (Table 3).
For all seasons, the main principal component (PC1) was loaded with Na+, nssSO42−, NH4+, oxalates, and K+ and occasionally NO3−, Cl−, and MS−. This suggests that marine aerosols (sea salts and biogenic emissions) modified by the presence of secondary components (sulfur and nitrogen compounds and oxalates) were the largest contributors to the analyzed PM2.5 samples. nssSO42−, oxalates, and K+ were always included only in PC1, indicating their constant inflow. Moreover, Na+ and NH4+ were underrepresented in 2016 in comparison to 2015 samples if only accounting for PC1. Therefore, their enrichment in 2016 was corrected by the addition of PC2. The observed correlation between Na+ and NH4+ could also point to the connection between NH4+ and natural marine sources in the Middle Adriatic, as has previously been observed in other marine regions (Clarke and Porter 1993; Fomba et al. 2014; Jickells et al. 2003; Quinn et al. 1988). Cl− tends to be a more variable contributor to the PM mass as Na+ (less significant correlation in PC1 or minor PC2 was observed), which indicates that acid catalyzed Cl− depletion is dependent on the weather conditions. In general, the second and third source factors (PC2 and PC3) can be considered as correction factors of PC1. PC2 is mainly related to natural marine sources and describes the enrichment of sea-spray aerosols in 2016 and Cl− depletion, as well as the seasonal biogenic marine sources of secondary MSA aerosols (i.e., MS− together with PC3). In addition, PC2 also accounts for the variations in NO3− concentrations, again together with PC3.
Impact of open fires on water-soluble ion composition
Numerous studies have investigated BB impacts on ambient aerosol mass and composition (e.g., Chuang et al. 2013; Engling et al. 2009; Jaffe et al. 2008). Distinct regional characteristics of BB smoke have been observed, suggesting that local BB emissions dominate aerosol chemistry. However, the transport and mixing of BB aerosols from upwind sources are also common (e.g., Zhang et al. 2012). Our results showed that fire events in summer 2015 substantially increased PM2.5 mass concentrations (Fig. 4) and changed the abundance of the main ions in comparison to the no-fire samples in the same season (Table 1, Fig. 6). PM2.5 mass concentrations reached on average 24.2 μg m−3 (summer average was 12.7 μg m−3), and the significant increase of average of SO42−, NH4+, and K+ concentrations was also observed (Table 1, SI Table 6). While no significant changes were observed for ssSO42−, the average concentration of nssSO42− increased by more than twice (from 3.6 to 8.0 μg m−3). On the other hand, there was also no significant change in the concentrations of NO3−. BB particles were thus more likely exposed to high ambient levels of H2SO4 than HNO3. The fresh BB particles which are thought to be initially composed of KCl, have most likely chemically evolved by H2SO4 + KCl reactions, due to the abundance of H2SO4 (Yamasoe et al. 2000; Li et al. 2003).
According to the NH4+/nssSO42− molar ratio being on average 1.5, all NH4+ was consumed in the neutralization with nssSO42− and (NH4)HSO4 was predominantly formed in the aerosol phase. Overall, Cl− depletion increased slightly from 82 to 88% in relation to the no-fire period (Fig. 7a). Other studies have also shown that Cl− is more efficiently depleted in wildfire plumes (Brown et al. 2017; Maudlin et al. 2015; Zauscher et al. 2013). The theoretical maximum percentages of observed Cl− loss attributable to the specific acidic species during the fire period are shown in Fig. 7b. Assuming that all available nssSO42− was involved in Cl− depletion, its contribution could explain the total Cl− loss during the open-fire events in summer 2015. However, after accounting for the neutralization of nssSO42− by NH4+, the average contribution from ex-nssSO42− again dropped to 38% (Fig. 7c).
Conclusions
Seasonal PM2.5 concentrations, water-soluble ion composition, and possible sources were investigated for the first time at the Middle Adriatic coast, Croatia, in the period from April 2015 to July 2016. PM2.5 is shown to be generally more affected by the southern marine inflow, especially in spring and summer, while more polluted continental air masses had more pronounced influence in winter. In addition, meteorological conditions largely affected temporal PM2.5 levels in the area. Hot arid stagnant conditions typical of the region in summer are pertinent for prolonged lifetime of airborne particles and their photochemical aging in the atmosphere. As a result, more long-range transport and secondary aerosols were observed in summer, in comparison to rainy autumn and windy winter and spring times.
The most abundant ion was SO42− followed by Na+, NO3−, Cl−, NH4+, and anions of carboxylic acids. Chemical analysis of PM2.5 supported by PCA indicated that both secondary sulfate aerosols and sea salts from primary sea-spray emissions were the major contributors to fine aerosols at the coastal Adriatic area. Anthropogenic sulfates (anth-nssSO42−) dominantly constituted the nssSO42− fraction throughout the year and are shown to be enhanced by the continental air transport to the Middle Adriatic site. In spring and summer periods, when air-mass inputs from the southern marine pathways prevailed, the estimated contribution of biogenic sulfates (bio-nssSO42−) to the total nssSO42− presumably increased due to the enhancement of primary DMS production by phytoplankton and subsequent photochemical processing.
The investigated aerosols were mostly acidic. A substantial Cl− depletion was observed, greatly corresponding to the gas-phase loss in a form of HCl. The calculated average Cl− depletion was 72 ± 12%, with the highest contribution of nssSO42− in all seasons. When accounting for the neutralization of H2SO4 by NH3, the potential of HNO3 and organic acids to considerably influence Cl− depletion is shown to increase. Due to the fraction of unexplained Cl− loss by nssSO42−, NO3−, and organic acid ions, additional atmospheric chemistry processes potentially play a role in Cl− loss at the coastal Middle Adriatic area, but it merits further investigation.
We show that in addition to long-range transport, regional/local emission sources, such as domestic heating, land and ship traffic, and agricultural activities could influence air quality of the area. In particular, intensive open-fire events in summer 2015 substantially increased PM2.5 mass concentrations, changed water-soluble ion composition in favor of nssSO42−, NH4+, and K+, and slightly increased aerosol acidity, being also in line with the observed slight increase in Cl− depletion.
As there is no available data on the chemical composition of ambient aerosols at the Middle Adriatic coast, the present study serves as a basis for the comparison with future studies related to their various sources as well as the air quality conditions in the Adriatic and/or Mediterranean regions. Moreover, the presented data provides valuable background knowledge to better understand the type and variabilities of atmospheric deposition to this highly oligotrophic area of the Adriatic Sea and the other air–sea exchange processes.
References
Abbasse G, Ouddane B, Fischer JC (2003) Determination of trace metal complexes by natural organic and inorganic ligands in coastal seawater. Anal Sci 19:529–535. https://doi.org/10.2116/analsci.19.529
Abdalmogith SS, Harrison RM (2006) An analysis of spatial and temporal properties of daily sulfate, nitrate and chloride concentrations at UK urban and rural sites. J Environ Mon 8:691–699. https://doi.org/10.1039/b601562j
Adachi K, Buseck PR (2015) Changes in shape and composition of sea-salt particles upon aging in an urban atmosphere. Atmos Environ 100:1–9. https://doi.org/10.1016/j.atmosenv.2014.10.036
Almeida SM, Pio CA, Freitas MC, Reis MA, Trancoso MA (2006) Approaching PM2.5 and PM2.5−10 source apportionment by mass balance analysis, principal component analysis and particle size distribution. Sci Total Environ 368:663–674. https://doi.org/10.1016/j.scitotenv.2006.03.031
Alves A, Pio C, Campos E, Barbedo P (2007) Size distribution of atmospheric particulate ionic species at a coastal site in Portugal. Quim Nova 30(8):1938–1944. https://doi.org/10.1590/S0100-40422007000800027
Andreae MO, Ferek RJ, Bermond F, Byrd KP, Engstrom RT, Hardin S, Houmere PD, Lemarrec F, Raemdonck H, Chatfield RB (1985) Dimethyl sulfide in the marine atmosphere. J Geophys Res-Atmos 90(D7):2891–2900. https://doi.org/10.1029/JD090iD07p12891
Athanasopoulou E, Protonotariou AP, Bossioli E, Dandou A, Tombrou M et al (2015) Aerosol chemistry above an extended archipelago of the eastern Mediterranean basin during strong northern winds. Atmos Chem Phys 15:8401–8421. https://doi.org/10.5194/acp-15-8401-2015
Ayers GP, Gras JL (1991) Seasonal relationship between cloud condensation nuclei and aerosol methanesulphonate in marine air. Nature 35(6347):834–835. https://doi.org/10.1038/353834a0
Bardouki H, Liakakou H, Economou C, Sciare J, Smolik J et al (2003) Chemical composition of size resolved atmospheric aerosols in the eastern Mediterranean during summer and winter. Atmos Environ 37:195–208. https://doi.org/10.1016/S1352-2310(02)00859-2
Behnke W, Zetzsch C (1990) Heterogeneous photochemical formation of Cl atoms from NaCl aerosol, NOx and ozone. J Aerosol Sci 21:S229–S232. https://doi.org/10.1016/0021-8502(90)90226-N
Bougiatioti A, Zarmpas P, Koulouri E, Antonou M, Theodosi C, Kouvarakis G, Saarikoski S, Mäkelä T, Hillamo R, Mihalopoulos N (2013) Organic, elemental and water-soluble organic carbon in size segregated aerosols, in the marine boundary layer of the eastern Mediterranean. Atmos Environ 64:251–252. https://doi.org/10.1016/j.atmosenv.2012.09.071
Brown RA, Dadashazar H, MacDonald AB, Aldhaif AM, Maudlin LC et al (2017) Impact of wildfire emissions on chloride and bromide depletion in marine aerosol particles. Environ Sci Technol 51(16):9013–9021. https://doi.org/10.1021/acs.est.7b02039
Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326(6114):655–661. https://doi.org/10.1038/326655a0
Chuang MT, Chou CCK, Sopajaree K, Lin NH, Wang JL et al (2013) Characterization of aerosol chemical properties from near-source biomass burning in the northern Indochina during 7-SEAS/Dongsha experiment. Atmos Environ 78:72–81. https://doi.org/10.1016/j.atmosenv.2012.06.056
Clarke AD, Porter JN (1993) Pacific marine aerosol: 2. Equatorial gradients in chlorophyll, ammonium, and excess sulfate during SAGA 3. J Geophys Res-Atmos 98:16997–17010. https://doi.org/10.1029/92JD02481
Cusack M, Alastuey A, Perez N, Pey J, Querol X (2012) Trends of particulate matter (PM2.5) and chemical composition at a regional background site in the Western Mediterranean over the last nine years (2002–2010). Atmos Chem Phys 12:8341–8357. https://doi.org/10.5194/acp-12-8341-2012
Daher N, Ruprecht A, Invernizzi G, De Marco C, MillerSchulze J, Heo JB, Shafer MM, Shelton BR, Schauer JJ, Sioutas C (2012) Characterization, sources and redox activity of fine and coarse particulate matter in Milan, Italy. Atmos Environ 49:130–141. https://doi.org/10.1016/j.atmosenv.2011.12.011
Engling G, Lee JJ, Tsai YW, Lung SCC, Chou CCK, Chan CY (2009) Size-resolved anhydrosugar composition in smoke aerosol from controlled field burning of rice straw. Aerosol Sci Technol 43:662–672. https://doi.org/10.1080/02786820902825113
Erduran MS, Tuncel SG (2001) Gaseous and particulate air pollutants in the Northeastern Mediterranean Coast. Sci Total Environ 281:205–215. https://doi.org/10.1016/S0048-9697(01)00847-6
Finlayson-Pitts BJ (2003) The tropospheric chemistry of sea salt: a molecular-level view of the chemistry of NaCl and NaBr. Chem Rev 103(12):4801–4822. https://doi.org/10.1021/cr020653t
Fomba KW, Muller K, van Pinxteren D, Poulain L, van Pinxteren M, Herrmann H (2014) Long-term chemical characterization of tropical and marine aerosols at the Cape Verde Atmospheric Observatory (CVAO) from 2007 to 2011. Atmos Chem Phys 14:8883–8904. https://doi.org/10.5194/acp-14-8883-2014
Freitas MC, Farinha MM, Ventura MG, Almeida SM, Reis MA, Pacheco AMG (2005) Gravimetric and chemical features of airborne PM10 and PM2.5 in mainland Portugal. Environ Monit Assess 109:81–95. https://doi.org/10.1007/s10661-005-5841-9
Gambaro M, Radaelli R, Piazza R, Stortini AM, Contini D et al (2009) Organic micropollutants in wet and dry depositions in the Venice lagoon. Chemosphere 76(8):1017–1022. https://doi.org/10.1016/j.chemosphere.2009.04.063
Harris E, Sinha B, Hoppe P, Ono S (2013) High-precision measurements of 33S and 34S fractionation during SO2 oxidation reveal causes of seasonality in SO2 and sulfate isotopic composition. Environ Sci Technol 47:12174–12183. https://doi.org/10.1021/es402824c
Hoffmann EH, Tilgner A, Schrodner R, Brauer P, Wolke R, Herrmann H (2016) An advanced modeling study on the impacts and atmospheric implications of multiphase dimethyl sulfide chemistry. PNAS 113(42):11776–11781. https://doi.org/10.1021/es402824c
Hsu SC, Liu SC, Kao SJ, Jeng WL, Huang YT et al (2007) Water-soluble species in the marine aerosol from the northern South China Sea: high chloride depletion related to air pollution. J Geophysl Res 112:D19304. https://doi.org/10.1029/2007JD008844
Ivošević T, Stelcer E, Orlić I, Bogdanović Radović I, Cohen D (2016) Characterization and source apportionment of fine particulate sources at Rijeka, Croatia from 2013 to 2015. Nucl Instrum Meth B 371:376–380. https://doi.org/10.1016/j.nimb.2015.10.023
Jaffe D, Hafner W, Chand D, Westerling A, Spracklen D (2008) Interannual variations in PM2.5 due to wildfires in the Western United States. Environ Sci Technol 42:2812–2818. https://doi.org/10.1021/es702755v
Jickells TD, Kelly SD, Baker AR, Biswas K, Dennis PF et al (2003) Isotopic evidence for a marine ammonia source. Geophys Res Lett 30:1374. https://doi.org/10.1029/2002GL016728
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Meas 20:141–151. https://doi.org/10.1177/001316446002000116
Kanakidou M, Mihalopoulos N, Kindap T, Im U, Vrekoussis M et al (2011) Megacities as hot spots of air pollution in the East Mediterranean. Atmos Environ 45:1223–1235. https://doi.org/10.1016/j.atmosenv.2010.11.048
Kawamura K, Bikkina S (2016) A review of dicarboxylic acids and related compounds in atmospheric aerosols: molecular distributions, sources and transformation. Atmos Res 170:140–160. https://doi.org/10.1016/j.atmosres.2015.11.018
Kawamura K, Ikushima K (1993) Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ Sci Technol 27:2227–2235. https://doi.org/10.1021/es00047a033
Kawamura K, Sakaguchi F (1999) Molecular distributions of water-soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics. J Geophys Res 104(D3):3501–3509. https://doi.org/10.1029/1998JD100041
Knipping EM, Dabdub D (2003) Impact of chlorine emissions from sea-salt aerosol on coastal urban ozone. Environ Sci Technol 37(2):275–284. https://doi.org/10.1021/es025793z
Koçak M, Kubilay N, Mihalopoulos N (2004) Ionic composition of lower tropospheric aerosols at a northeastern Mediterranean site: implications regarding sources and long-range transport. Atmos Environ 38:2067–2077. https://doi.org/10.1016/j.atmosenv.2004.01.030
Koçak M, Mihalopoulos N, Kubilay N (2007) Chemical composition of the fine and coarse fraction of aerosols in the northeastern Mediterranean. Atmos Environ 41(34):7351–7368. https://doi.org/10.1016/j.atmosenv.2007.05.011
Kopanakis I, Eleftheriadis K, Mihalopoulos N, Lydakis Simantiris N, Katsivela E et al (2012) Physico-chemical characteristics of particulate matter in the Eastern Mediterranean. Atmos Res 106:93–107. https://doi.org/10.1016/j.atmosres.2011.11.011
Kouvarakis G, Vrekoussis M, Mihalopoulos N, Kourtidis K, Rappenglueck B et al (2002) Spatial and temporal variability of tropospheric ozone in the boundary layer above the Aegean Sea (eastern Mediterranean). J Geophys Res 107:8137. https://doi.org/10.1029/2000JD000081
Laskin A, Moffet RC, Gilles MK, Fast JD, Zaveri RA, Wang B, Nigge P, Shutthanandan J (2012) Tropospheric chemistry of internally mixed sea salt and organic particles: surprising reactivity of NaCl with weak organic acids. J Geophys Res 117:D15302. https://doi.org/10.1029/2012JD017743
Lewis ER, Schwartz SE (2004) Sea salt aerosol production: mechanisms, methods, measurements and models–a critical review. Geophysical monograph series 152. AGU, Washington DC, p 413
Li J, Pósfai M, Hobbs PV, Buseck PR (2003) Individual aerosol particles from biomass burning in southern Africa: 2, compositions and aging of inorganic particles. J Geophys Res 108:347–362. https://doi.org/10.1029/2002JD002310
Liss PS, Lovelock JE (2007) Climate change: the effect of DMS emissions. Environ Chem 4(6):377–378. https://doi.org/10.1071/EN07072
Luria M, Peleg M, Sharf G, Siman Tov-Alper D, Spitz N et al (1996) Atmospheric sulfur over the Eastern Mediterranean region. J Geophys Res 101:25917–25930. https://doi.org/10.1029/96JD01579
Malaguti A, Mircea M, La Torretta TMG, Telloli C, Petralia E et al (2015) Chemical composition of fine and coarse aerosol particles in the central Mediterranean area during dust and non-dust conditions. Aerosol Air Qual Res 15:410–425. https://doi.org/10.4209/aaqr.2014.08.0172
Marić D, Kraus R, Godrijan J, Supić N, Djakovac T, Precali R (2012) Phytoplankton response to climatic and anthropogenic influences in the north-eastern Adriatic during the last four decades. Estuar Coast Shelf Sci 115:98–112. https://doi.org/10.1016/j.ecss.2012.02.003
Maudlin LC, Wang Z, Jonsson HH, Sorooshian A (2015) Impact of wildfires on size-resolved aerosol composition at a coastal California site. Atmos Environ 119:59–68. https://doi.org/10.1016/j.atmosenv.2015.08.039
Merico E, Donateo A, Gambaro A, Cesari D, Gregoris E et al (2016) Influence of in-port ships emissions to gaseous atmospheric pollutants and to particulate matter of different sizes in a Mediterranean harbour in Italy. Atmos Environ 139:1–10. https://doi.org/10.1016/j.atmosenv.2016.05.024
Mermex Group (2011) Marine ecosystems responses to climatic and anthropogenic forcings in the Mediterranean. Prog Ocean 91(2):97–166
Meskhidze N, Nenes A (2006) Phytoplankton and cloudiness in the Southern Ocean. Science 314(5804):1419–1423. https://doi.org/10.1126/science.1131779
Mihalopoulos N, Stephanou E, Kanakidou M, Pilitsidis S, Bousquet P (1997) Tropospheric aerosol ionic composition in the eastern Mediterranean region. Tellus B 49:1–13. https://doi.org/10.1034/j.1600-0889.49.issue3.7.x
Millero FJ (2006) Chemical oceanography, 3rd edn. CRC Press, 530 pp
Monahan EC, Spiel DE, Davidson KL (1986) A model of marine aerosol generation via whitecaps and wave disruption. In: Monahan EC, MacNiocaill G (eds) Oceanic whitecaps and their role in air–sea exchange processes. D. Reidel, Dordrecht, Netherlands, pp 167–174
Morabito E, Contini D, Belosi F, Stortini AM, Manodori L et al (2014) Atmospheric deposition of inorganic elements and organic compounds at the inlets of the Venice lagoon. Adv Meteorol 158902:1–10. https://doi.org/10.1155/2014/158902
Norton RB, Roberts JM, Huebert BJ (1983) Tropospheric oxalate. Geophys Res Lett 10:517–520. https://doi.org/10.1029/GL010i007p00517
O’Dowd CD, Hoffmann T (2005) Coastal new particle formation: a review of the current state of the art. Environ Chem 2(4):245–255. https://doi.org/10.1071/EN05077
O'Dowd CD, de Leeuw G (2007) Marine aerosol production: a review of the current knowledge. Philos T Roy Soc A 65(1856):1753–1774. https://doi.org/10.1098/rsta.2007.2043
Pachon JE, Weber RJ, Zhang X, Mulholland JA, Russell AG (2013) Revising the use of potassium (K) in the source apportionment of PM2.5. Atmos Pollut Res 4:14–21. https://doi.org/10.5094/APR.2013.002
Park KT, Jang S, Lee K, Jun Yoon Y, Kim MS et al (2017) Observational evidence for the formation of DMS-derived aerosols during Arctic phytoplankton blooms. Atmos Chem Phys 17:9665–9675. https://doi.org/10.5194/acp-17-9665-2017
Perrone MR, Piazzalunga A, Prato M, Carofalo I (2011) Composition of fine and coarse particles in a coastal site of the central Mediterranean: carbonaceous species contributions. Atmos Environ 45:7470–7477. https://doi.org/10.1016/j.atmosenv.2011.04.030
Perrone MR, Becagli S, Orza JAG, Vecchi R, Dinoi A, Udisti R, Cabello M (2013) The impact of long-range transport on PM1 and PM2.5 at a central Mediterranean site. Atmos Environ 71:176–186. https://doi.org/10.1016/j.atmosenv.2013.02.006
Piazzola J, Forget P, Lafon C, Despiau S (2009) Spatial variation of sea-spray fluxes over a Mediterranean coastal zone using a sea state model. Bound Lay Meteorol 132(1):167–183. https://doi.org/10.1007/s10546-009-9386-2
Piazzola J, Mihalopoulos N, Canepa E, Tedeschi G, Prati P et al (2016) Characterization of aerosols above the northern Adriatic Sea: case studies of offshore and onshore wind conditions. Atmos Environ 132:153–162. https://doi.org/10.1007/s10546-009-9386-2
Quinn PK, Bates TS (2005) Regional aerosol properties: comparisons of boundary layer measurements from ACE1, ACE2, aerosols99, INDOEX, ACE asia, TARFOX, and NEAQS. J Geophys Res 110:D14202. https://doi.org/10.1029/2004JD004755
Quinn PK, Charlson RJ, Bates TS (1988) Simultaneous observations of ammonia in the atmosphere and ocean. Nature 335:336–338. https://doi.org/10.1038/335336a0
Quinn PK, Coffman DJ, Bates TS, Miller TL, Johnson JE, Welton EJ, Neususs C, Miller MP, Sheridan J (2002) Aerosol optical properties during INDOEX 1999: means, variability, and controlling factors. J Geophys Res 107(D19):1–19. https://doi.org/10.1029/2000JD000037
Richon C, Dutay JC, Dulac F, Wang R, Balkanski Y et al (2017) Modeling the impacts of atmospheric deposition of nitrogen and desert dust–derived phosphorus on nutrients and biological budgets of the Mediterranean Sea. Prog Oceanogr 163:21–39. https://doi.org/10.1016/j.pocean.2017.04.009
Rossini P, Guerzoni S, Molinaroli E, Rampazzo G, de Lazzari A, Zancanaro A (2005) Atmospheric bulk deposition to the lagoon of Venice: part I. Fluxes of metals, nutrients and organic contaminants. Environ Int 31(7):959–974. https://doi.org/10.1016/j.envint.2005.05.006
Salameh D, Detournay A, Pey J, Pérez N, Liguori F et al (2015) PM2.5 chemical composition in five European Mediterranean cities: a 1-year study. Atmos Res 155:102–117. https://doi.org/10.1016/j.atmosres.2014.12.001
Savoie DL, Prospero JM (1989) Comparison of oceanic and continental sources of non-sea-salt sulphate over the Pacific Ocean. Nature 339:685–689. https://doi.org/10.1038/339685a0
Sciare J, Favez O, Sarda-Estève R, Oikonomou K, Cachier H, Kazan V (2009) Long-term observations of carbonaceous aerosols in the Austral Ocean atmosphere: evidence of a biogenic marine organic source. J Geophys Res 114:D15302. https://doi.org/10.1029/2009JD011998
Seinfeld JH, Pandis SN (2006) Atmospheric chemistry and physics: from air pollution to climate change, 2nd edn. John Wiley, New York
Stefels J, Steinke M, Turner S, Malin G, Belviso S (2007) Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 83(1–3):245–275. https://doi.org/10.1007/s10533-007-9091-5
Stortini M, Freda A, Cesari D, Cairns WRL, Contini D et al (2009) An evaluation of the PM2.5 trace elemental composition in the Venice lagoon area and an analysis of the of the possible sources. Atmos Environ 43(44):6296–6304. https://doi.org/10.1016/j.atmosenv.2009.09.033
Takami A, Miyoshia T, Shimonob A, Hatakeyamaet S (2005) Chemical composition of fine aerosol measured by AMS at Fukue Island, Japan during APEX period. Atmos Environ 39:4913–4924. https://doi.org/10.1016/j.atmosenv.2005.04.038
Topping D, Coe H, McFiggans G, Burgess R, Allan J et al (2004) Aerosol chemical characteristics from sampling conducted on the island of Jeju, Korea during ace Asia. Atmos Environ 38:2111–2123. https://doi.org/10.1016/j.atmosenv.2004.01.022
Turquety S, Menut L, Bessagnet B, Anav A, Viovy N et al (2014) APIFLAMEv1.0: high-resolution fire emission model and application to the Euro-Mediterranean region. Geosci Model Dev 7:587–612. https://doi.org/10.5194/gmd-7-587-2014
Turšič J, Podkrajšek B, Grgić I, Ctyroky P, Berner et al (2006) Chemical composition and hygroscopic properties of size-segregated aerosol particles collected at the Adriatic coast of Slovenia. Chemosphere 63(7):1193–1202. https://doi.org/10.1016/j.chemosphere.2005.08.040
Udisti R, Dayan U, Becagli S, Busetto M, Frosini et al (2012) Sea spray aerosol in Central Antarctica. Present atmospheric behaviour and implications for paleoclimatic reconstructions. Atmos Environ 52:109–120. https://doi.org/10.1016/j.atmosenv.2011.10.018
Udisti R, Bazzano A, Becagli S, Bolzacchini E, Caiazzo L, Cappelletti D, Ferrero L, Frosini D, Giardi F, Grotti M, Lupi A, Malandrino M, Mazzola M, Moroni B, Severi M, Traversi R, Viola A, Vitale V (2016) Sulfate source apportionment in the Ny-Ålesund (Svalbard Islands) Arctic aerosol. Rend Lincei Sci Fis 27:85–94. https://doi.org/10.1007/s12210-016-0517-7
Unger N (2013) Isoprene emission variability through the twentieth century. J Geophys Res Atmos 118:13606–13613. https://doi.org/10.1002/2013JD020978
Vardoulakis S, Kassomenos P (2008) Sources and factors affecting PM10 levels in two European cities: implications for local air quality management. Atmos Environ 42:3949–3963. https://doi.org/10.1016/j.atmosenv.2006.12.021
Wang HB, Shooter D (2001) Water soluble ions of atmospheric aerosols in three New Zealand cities: seasonal changes and sources. Atmos Environ 35(34):6031–6040. https://doi.org/10.1016/S1352-2310(01)00437-X
Warneck P (2003) In-cloud chemistry opens pathway to the formation of oxalic acid in the marine atmosphere. Atmos Environ 37:2423–2427. https://doi.org/10.1016/S0045-6535(00)00171-5
Wu PM, Okada K (1994) Nature of coarse nitrate particles in the atmosphere–a single particle approach. Atmos Environ 28:2053–2060. https://doi.org/10.1016/1352-2310(94)90473-1
Yamasoe MA, Artaxo P, Miguel AH, Allen AG (2000) Chemical composition of aerosol particles from direct emissions of vegetation fires in the Amazon Basin: water soluble species and trace elements. Atmos Env 34:1641–1653. https://doi.org/10.1016/S1352-2310(99)00329-5
Yoon YJ, Ceburnis D, Cavalli F, Jourdan O, Putaud JP, Facchini MC et al (2007) Seasonal characteristics of the physicochemical properties of North Atlantic marine atmospheric aerosols. J Geophys Res J 112:D04206. https://doi.org/10.1029/2005JD007044
Zauscher MD, Wang Y, Moore MJK, Gaston CJ, Prather KA (2013) Air quality impact and physicochemical aging of biomass burning aerosols during the 2007 San Diego wildfires. Environ Sci Technol 47(14):7633–7643. https://doi.org/10.1021/es4004137
Zhang YN, Zhang ZS, Chan CY, Engling G, Sang XF, Shi S, Wang XM (2012) Levoglucosan and carbonaceous species in the background aerosol of coastal Southeast China: case study on transport of biomass burning smoke from the Philippines. Environ Sci Poll Res 19:244–255. https://doi.org/10.1007/s11356-011-0548-7
Acknowledgments
The authors also acknowledge Jadranka Škevin Sović, Ana Šutić and Ivona Igrec from Croatian Meteorological and Hydrological Service for gravimetric measurements.
Funding
The authors acknowledge the financial support from the projects “The Sulphur and Carbon Dynamics in the Sea and Fresh-Water Environment” (IP-11-2013-1205 SPHERE), Slovenian Research Agency (Contract No. P1-0034), STSM COST Action European Network on New Sensing Technologies for Air-Pollution Control and Environmental Sustainability (EuNetAir), and Croatian-Slovenian bilateral project “Estimating the role of marine biogenic organosulfur compounds in the formation and properties of atmospheric organic aerosols.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 502 kb)
Rights and permissions
About this article
Cite this article
Cvitešić Kušan, A., Kroflič, A., Grgić, I. et al. Chemical characterization of fine aerosols in respect to water-soluble ions at the eastern Middle Adriatic coast. Environ Sci Pollut Res 27, 10249–10264 (2020). https://doi.org/10.1007/s11356-020-07617-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07617-7