Abstract
In the context of new oil exploration/production areas, knowledge of the biological impact of dispersed oil in the deep-sea environment is essential. Hence, the aim of this study was to perform a comparison, at atmospheric pressure (0.1 MPa) and at a high hydrostatic pressure corresponding to 1000 m depth (10.1 MPa), of lethal concentrations (LC) on a model fish, Scophthalmus maximus, exposed to chemically dispersed oil. Fish were exposed concomitantly at 0.1 and 10.1 MPa using two exposure tanks connected to the same source tank thanks to a closed circuit. Acute toxicity was evaluated at 24 h through the determination of LC10 and LC50 (respectively, 10 and 50% of mortality) calculated from measured total petroleum hydrocarbon concentrations in the water. No statistical differences were observed between the LC10 at 0.1 MPa (46.1 mg L− 1) and the LC10 at 10.1 MPa (31.0 mg L− 1), whereas the LC50 of fish exposed to 0.1 MPa (90.8 mg L− 1) was significantly higher than the LC50 at 10.1 MPa (50.9 mg L− 1). These results clearly show an increase in oil toxicity under high hydrostatic pressure. This effect may be due to synergistic effects of pressure and oil contamination on fish energetic metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine waters with a depth greater than 1000 m represent 88% of oceanic areas (Jannasch and Taylor 1984). Until few decades, they were isolated from the direct footprint of anthropic activities. However, due to both technological improvements and an increase in resources demand (oil, gas, seafloor massive sulfide deposits), the exploitation of deep seas is more and more economically viable. Nowadays, offshore deep waters represent an important part of the new oil exploration/production areas (Maddahi and Mortazavi 2011). Discharge of chemicals, whether chronic or accidental, could upset the balance of these sensitive ecosystems. Consequently, the need for relevant data giving a better understanding of the potential environmental risks of anthropic activities in deep-sea areas is becoming increasingly crucial.
Ecological studies are difficult to carry out in these environments. However, they provide fundamental information after deep-sea contaminations, as in 2010 in the case of the Deepwater Horizon accident in the Gulf of Mexico: about 780,000 m3 of oil were released into the environment at 1500 m depth, and dispersant (7000 m3) was injected directly into the leaking Macondo well, reducing the rise of oil to the sea surface. This technical response led to the sedimentation of an estimated 3 to 14% of the 780,000 m3 released at sea (Chanton et al. 2014; Fingas 2017). Biological impacts of this sedimented oil were reported in many ecological studies (Beyer et al. 2016). Additionally, experimental approaches can provide relevant data to assess and understand the new field of deep-sea ecotoxicology. To date, few data are available, but several approaches could be used (Lemaire et al. 2012; Lemaire 2017; McConville et al. 2018). One of these is the study of the impact of chemicals on piezotolerant species at atmospheric pressure. This approach has been carried out on cold-water corals (DeLeo et al. 2016), on the benthic giant amphipod Eurythenes gryllus (Olsen et al. 2016), or on deep-sea fish species Coryphaenoides rupestris (Lemaire et al. 2012) and Anoplopoma fimbria (McConville et al. 2018). Nevertheless, in order to obtain a better understanding of the potential biological impact of a chemical in the deep-sea, it is necessary to develop experimental protocols to recreate contamination and assess its effects under high hydrostatic pressure (HP) (Lemaire et al. 2012). Ecobarotoxicology, i.e., the use of a hyperbaric chamber to assess the potential impact of chemicals at simulated depth, offers a relevant way to understand this new domain of ecotoxicology.
The ideal experiment would be the contamination of a deep species at depth or simulated depth (Lemaire et al. 2012). This type of experiment is difficult to achieve, but alternatives exist. It is possible to analyze the effects of HP on animals prior exposed to chemicals at atmospheric pressure. This kind of study was performed by exposing juvenile fishes contaminated with dispersed oil (Dussauze et al. 2017) or shallow shrimps exposed to different cadmium concentrations (Domingues 2015) to HP. Another way is to perform a comparison of toxicity at the surface and at simulated depth. In this case, the use of a reference species for scientific and legislative purposes is a good compromise. In our study, the turbot Scophthalmus maximus was selected as a model species. The originality of our experiment was to conduct contamination concomitantly at a simulated depth of 1000 m and at atmospheric pressure in order to compare the lethal concentrations (LC) of dispersed oil. The LCs were determined using the total hydrocarbon concentration (THC) during the exposure time. The LC results are presented for 10 and 50% of mortality (respectively, LC10 ad LC50) both at atmospheric pressure and at high hydrostatic pressure.
Materials and methods
Animals
Juvenile turbot (Scophthalmus maximus) (n = 792; weight: 2.89 ± 0.02 g mean ± SEM, France turbot -Noirmoutier, France) were acclimatized in a 300 L seawater tank for 2 weeks. The photoperiod was according to the season (June to August). Oxygen saturation (> 95%), pH (8.1 ± 0.2), and temperature (maintained at 15.2 ± 0.3 °C) were measured daily. The turbot were fed daily with dried commercial pellets provided by the hatchery.
Chemicals
The petroleum oil used in the study was a crude oil from the Lula oil field. This offshore oil field is located in ultra-deep water at approximately 2000 m depth. This oil contained 54% saturated hydrocarbons, 10% polar compounds, and 36% aromatic hydrocarbons. The dispersant was the Finasol OSR52, a third-generation formulation from the TOTAL Company.
Experimental systems
In this study, the oil concentration range (0 to 300 mg L− 1 of THC) and the oil exposure duration (24 h) were defined according to a pilot experimentation using an ecotoxicological bench of twelve replicates (see below). The complete procedure of LC50 determination, used for the pilot experimentation, was previously described in Dussauze et al. (2015).
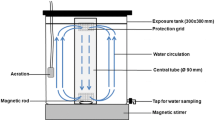
The experimental system (Fig. 1) is composed of one source tank, two exposure tanks and one hyperbaric chamber. The 100 L source tank was equipped with a water homogenizing system similar to the system described in detail in Milinkovitch et al. (2011). The two exposure tanks (15 L cylindrical plexiglass tanks) had continuous renewal (1.2 L min− 1) of their water from the source tank. One of these tanks was placed in the hyperbaric chamber and immediately next to it. The 130 L hyperbaric chamber (fully described in Theron et al. 2000) has hydraulic hull passageways allowing continuous water flow under pressure. This water circulation allows temperature and oxygen saturation to be controlled during the pressure exposure. The exposure tanks had a gas impermeable deformable membrane at one end, allowing the transmission of pressure to the water without gas dissolution. The configuration of the experimental device made it possible to directly compare contaminations carried out at atmospheric pressure and at a simulated depth of 1000 m.
Hyperbaric chamber and high-pressure water circulation system from Theron et al. (2000) coupled with one source tank and two exposure tanks
Experimental conditions
Thirty-three fish were randomly assigned to each of the two exposure tanks. Then, the tank used for pressure was placed in the hyperbaric chamber. The animals were acclimated for 24 h to the experimental system. Then, the pressure was gradually increased in the hyperbaric chamber at a speed of 0.2 MPa/h to reach 10.1 MPa. After 24 h of acclimatization, the oil-dispersant mixture was poured into the source tank every 6 h for 24 h. The exposure tanks were continuously supplied with contaminated water from the source tank. The total hydrocarbons concentrations (THC) were measured in water samples collected every 2 h. At the end of the 24 h exposure time, decompression of the hyperbaric chamber was performed at 0.1 MPa/min, and then survival of fish was assessed.
For this experimentation, ten oil exposures were performed concomitantly at 0.1 and 10.1 MPa, and four oil exposures were performed at 0.1 MPa. During the experimentation, 462 fish were assigned to the 0.1 MPa condition and 330 fish to the 10.1 MPa condition.
Measurement of seawater Total hydrocarbon concentrations (THC)
For the two exposure tanks, the THC was measured every 2 h during the contamination period in triplicate from 30 ml samples. Following the method described by Fusey and Oudot (1976), a separation of the aqueous and the organic phases was performed. Then, the absorbance of the organic phase was measured and quantified at a wavelength of 390 nm (UV-Vis spectrophometer, Unicam, France). The results are expressed in mg L− 1.
Statistics
Using the Excel™ macro: REGTOX© version 7.0.6, the LC10 and LC50 were calculated on the basis of the mean measured THC concentrations over the contamination period. LCs were expressed in mg.L−1 and given with their 95% confidence interval. The results were considered as statistically different when their confidence interval at 95% did not overlap.
Results and discussions
This study aims to compare the acute toxicity of dispersed oil on a model fish, at atmospheric pressure (0.1 MPa) and under high HP (10.1 MPa). As reported by Mestre et al. (2013), there is an important need to obtain information from ecotoxicological trials for environmental risk assessments in the deep-sea environment and particularly under high hydrostatic pressure. However, HP is a difficult parameter to control, and therefore, only few laboratories are able to produce data in this particular ecotoxicology context (Vevers et al. 2010; Dussauze et al. 2016, 2017; Lemaire et al. 2012; Lemaire 2017). Due to the difficulty in bringing and maintaining deep-sea species, the experimental protocol was carried out on a model fish, the turbot Scophthalmus maximus. This species was chosen for two main reasons: (1) this fish is one of the model species for ecotoxicological tests for the OSPAR Commission (OSPAR Protocols on Methods for the Testing of Chemicals Used in the Offshore Oil Industry),(2) this species is present from the surface to at least 70 m of depth (Muus and Dahlstrøm 1989) in European marine waters, from the Mediterranean Sea to the Arctic Circle (Imsland et al. 2001). This broad distribution area illustrates the variation of habitat that this species is able to cope with. These strong adaptive capacities of shallow species have previously been used in other ecobarotoxicological studies: on seabass Dicentrarchus labrax (Dussauze et al. 2017; Lemaire et al. 2016) or on shrimp Palaemon varians (Domingues 2015). In ecotoxicology, determination of the LC is the first step in the understanding of the toxicity of a chemical. It makes it possible to determine the sensitivity of the studied species to a given chemical, and it can be used to compare this sensitivity with other species or other chemicals. This approach was used in our study to compare the toxicity of a chemically dispersed oil both at simulated depth (1000 m) and at atmospheric pressure, and therefore to have information about the pressure dependence of toxicity responses.
In the pilot experiment, the LC50 at 24 h of exposure was 113.1 (104.1–126.2) mg L− 1. 0%, and 100% of mortality were reached respectively at 87 ± 22 and 209 ± 37 mg L− 1 of dispersed oil. A 48 h observation period showed that no mortality occurred after the first 24 h of contamination. This pilot experiment provided an exposure time (24 h) and a concentration range (0 to 300 mg L− 1 of THC) of interest to carry out the main experiment (i.e., comparison of the toxicity at the surface and at depth). In our experimental system, no mortality was observed in the control groups (without oil exposure) either at 0.1 and 10.1 MPa. Then, for an equivalent THC, a higher mortality was observed at 10.1 MPa when compared to fish kept at 0.1 MPa (Fig. 2). The LC10 were not statistically different at 0.1 and 10.1 MPa (Table 1). However, the LC50 for the surface experiment (90.8 mg L− 1) was statistically higher than the LC50 measured at 10.1 MPa (50.9 mg L− 1).
The differences in mortalities according to the experimental condition could be explained by the proteiform strong biological impacts of HP on:
(1) on molecular system with biological membranes and specially their fluidity (Cossins and Macdonald 1989; MacDonald 1997) or in the aryl hydrocarbon receptor pathway and enzymatic antioxidant systems involved in the defense against organic chemicals in marine fish (Lemaire 2017).
(2) the nervous system with high pressure nervous syndrome (HPNS) (Bennett and Towse 1971; Somero 1992). In our study, fish exposed to pressure was observed using the porthole of the hyperbaric chamber. From our observations, no erratic fish behaviors (swimming acceleration, convulsion or loss of balance), which are characteristic of HPNS (Dussauze et al. 2017), were observed. The only difference in behavior between fish exposed to atmospheric pressure and hydrostatic pressure was a trend to swim to the surface during the acclimatization of fish to pressure.
(3) energetic metabolism (Sébert et al. 1998; Sébert and Theron 2001). This last point is of particular interest since oil or hydrocarbons exposure is known to induce a reduction in the maximal O2 consumption of the respiratory chain (Stabenau et al. 2008; Dussauze et al. 2014). This effect is possibly mediated via reactive oxygen species (ROS) which have an impact on several complexes (I, III, and IV) of the respiratory chain (Musatov and Robinson 2012). Furthermore, oil is known to induce deleterious effects on the cardiovascular function of fish (Brette et al. 2014; Tissier et al. 2015). In our experiment, it is possible that oil exposure (through its impact on mitochondrial activity and cardiovascular function) induces a reduction of the fish metabolic scope and consequently limits energy availability at a time when HP induces an increase in the energetic demand. This assumption is in good coherence with a recent study showing that oil contamination has a significant deleterious impact on the adaptive capacities of juvenile Dicentrarchus labrax to an increase in HP (Dussauze et al. 2017).
Conclusion and outlook
Deep-sea ecotoxicology is an emerging phenomenon caused by the direct or indirect impact of human activities (oil and mining industries, red mud, microplastics, fisheries…) on deep-sea ecosystems. The scope is broad to understand these anthropic impacts, which were highlighted by the Deepwater Horizon oil spill. Future studies need to focus on true deep species. Several species could be good candidates: fish species such as sablefish (Anoplopoma fimbria) or Atlantic halibut (Hipoglossus hipoglossus) due to their presence at great depth and their controlled life cycle; Deep-sea corals such as Lophelia pertusa are also a possible and interesting species to study. Finally, species performing diel vertical migration, such as some calanoid zooplankton species can offer a wide range of possibilities to study the impact of oil on deep species.
References
Bennett PB, Towse EJ (1971) The high pressure nervous syndrome during a simulated oxygen-helium dive to 1500 ft. Electroencephalogr Clin Neurophysiol:383–393
Beyer J, Trannum HC, Bakke T, Hodson PV, Collier TK (2016) Environmental effects of the Deepwater horizon oil spill: a review. Mar Pollut Bull 110(1):28–51
Brette F, Machado B, Cros C, Incardona JP, Scholz NL, Block BA (2014) Crude oil impairs cardiac excitation-contraction coupling in fish. Science 14(343):772–776
Chanton J, Zhao T, Rosenheim BE, Joye S, Bosman S, Brunner C, Yeager KM, Diercks AR, Hollander D (2014) Using natural abundance radiocarbon to trace the flux of Petrocarbon to the seafloor following the Deepwater horizon oil spill. Environmental Science & Technology 49(2):847–854
Cossins AR, Macdonald AG (1989) The adaptation of biological membranes to temperature and pressure: fish from the deep and cold. J Bioenerg Biomembr 21(1):115–135
DeLeo DM, Ruiz-Ramos DV, Baums IB, Cordes EE (2016) Response of deep-water corals to oil and chemical dispersant exposure. Deep-Sea Res II Top Stud Oceanogr 129:137–147
Domingues GAJ (2015) Hydrostatic pressure on cadmium toxicity in Palaemon varians. Universidade de Aveiro, Dissertaçoes de mestrado em Ecologia Aplicada
Dussauze M, Camus L, Le Floch S, Karine Pichavant-Rafini K, Geraudie P, Coquillé N, Amerand A, Lemaire P, Theron M (2014) Impact of dispersed fuel oil on cardiac mitochondrial function on polar cod Boreogadus saida. Environ Sci Pollut Res 21(24):13779–13788
Dussauze M, Pichavant-Rafini K, Le Floch S, Lemaire P, Theron M (2015) Acute toxicity of chemically and mechanically dispersed crude oil on juvenile sea bass (Dicentrarchus labrax): absence of synergistic effects between oil and dispersants. Environ Toxicol Chem 34(7):1543–1551
Dussauze M, Pichavant-Rafini K, Le Floch S, Marziou A, Lemaire P, Fronteau L, Belhomme M, Theron M (2016) In vitro diving simulation: a new approach to assess biological impact of hydrocarbons at depth. Proceedings of the Thirty ninth AMOP Technical Seminar, Environment and Climate Change Canada, Ottawa, ON, pp 304–316
Dussauze M, Pichavant-Rafini K, Belhomme M, Buzzacott P, Privat K, Le Floch S, Lemaire P, Theron M (2017) Dispersed oil decreases the ability of a model fish (Dicentrarchus labrax) to cope with hydrostatic pressure. Environ Sci Pollut Res 24:3054–3062
Fingas M. 2017. A review of literature related to oil spill dispersants. Report to Prince William Sound Regional Citizens’ Advisory Council
Fusey P, Oudot J (1976) Comparaison de deux méthodes d’évaluation de la biodégradation des hydrocarbures in vitro. Material und Organismen (Berlin) 4:241–251
Imsland AK, Foss A, Nævdal G, Stefansson SO (2001) Selection or adaptation: differences in growth performance of juvenile turbot (Scophthalmus maximus Rafinesque) from two close-by localities off Norway. Sarsia 86:43–51
Jannasch HW, Taylor CE (1984) Deep-sea microbiology. Annu Rev Microbiol 38:487–514
Lemaire B (2017) Hydrostatic pressure and the experimental toxicology of marine fishes: the elephant in the room. Mar Pollut Bull 124(1):206–210
Lemaire B, Mignolet E, Debier C, Calderon PB, Thome JP, Rees JF (2016) High hydrostatic pressure influences the in vitro response to xenobiotics in Dicentrarchus labrax liver. Aquat Toxicol 173:43–52
Lemaire B, Debier C, Calderon PB, Thomé JP, Stegeman J, Mork J, Rees JF (2012) Precision-Cut Liver Slices To Investigate Responsiveness of Deep-Sea Fish to Contaminants at High Pressure. Environmental Science & Technology:120827130340000
MacDonald AG (1997) Hydrostatic pressure as an environmental factor in life processes. Comp Biochem Physiol 116A:291–297
Maddahi M, Mortazavi SJ (2011) A review on offshore concepts and feasibility study considerations. In SPE Asia Pacific Oil and Gas Conference and Exhibition, Society of Petroleum Engineers
Mestre NC, Calado R, Soares A (2013) Exploitation of deep-sea resources: the urgent need to understand the role of high pressure in the toxicity of chemical pollutants to deep-sea organisms. Environ Pollut:185. https://doi.org/10.1016/j.envpol.2013.10.021
Milinkovitch T, Kanan R, Thomas-Guyon H, Le Floch S (2011) Effects of dispersed oil exposure on the bioaccumulation of polycyclic aromatic hydrocarbons and mortality of juvenile Liza ramada. Sci Total Environ 409:1643–1650
Musatov A, Robinson NC (2012) Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res 46(11):1313–1326
Muus BJ, Dahlstrøm P (1989) Havfisk og Fiskeri i Nordvesteuropa. GEC Gads Forlag, København, 244 p
McConville MM, Roberts JP, Boulais M, Woodall B, Butler JD, Redman AD, Parkerton TF, Arnold WR, Guyomarch J, LeFloch S, Bytingsvik J, Camus L, Volety A, Brander SM (2018) The sensitivity of a deep-sea fish species () to oil-associated aromatic compounds, dispersant, and Alaskan North Slope crude oil. Environmental Toxicology and Chemistry 37 (8):2210-2221
Olsen GH, Coquillé N, Le Floch S, Geraudie P, Dussauze M, Lemaire P, Camus L (2016) Sensitivity of the deep-sea amphipod Eurythenes gryllus to chemically dispersed oil. Environ Sci Pol 23:6497–6505
Sébert P, Theron M (2001) Why can the eel, unlike the trout, migrate under pressure. Mitochondrion 1:79–85
Sébert P, Peragon J, Barroso JB, Simon B, Melendez HE (1998) High hydrostatic pressure (101 ATA) changes the metabolic design of yellow freshwater eel muscle. Comp Biochem Physiol 121B:195–200
Somero GN (1992) Adaptations to high hydrostatic pressure. Annu Rev Physiol 54:557–577
Stabenau EK, Sasser A, Schulte C (2008) The effects of pyrene exposure on exercise performance, muscle contraction, and mitochondrial O2 consumption in the leopard frog (Rana pipiens). J Environ Sci Health A Tox Hazard Subst Environ Eng 43(6):576–583
Theron M, Guerrero F, Sébert P (2000) Improvement in the efficiency of oxidative phosphorylation in the freshwater eel acclimated to 10.1 MPa hydrostatic pressure. J Exp Biol 203:3019–3023
Tissier F, Dussauze M, Lefloch N, Theron M, Lemaire P, Le Floch S, Pichavant-Rafini K (2015) Effect of dispersed crude oil on cardiac function in seabass Dicentrarchus labrax. Chemosphere 134:192–198
Vevers WF, Dixon DR, Dixon LRJ (2010) The role of hydrostatic pressure on developmental stages of Pomatoceros lamarcki (Polychaeta: Serpulidae) exposed to water accommodated fractions of crude oil and positive genotoxins at simulated depths of 1000–3000 m. Environ Pollut 158:1702–1709
Funding
This research project was completed with a funding from the Total Fluides company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dussauze, M., Pichavant-Rafini, K., Belhomme, M. et al. Deep-sea versus shallow conditions: a comparative ecobarotoxicological study. Environ Sci Pollut Res 27, 7736–7741 (2020). https://doi.org/10.1007/s11356-019-07590-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07590-w