Abstract
Sediments core within the Yangtze River Estuary was collected for metal and grain size analysis. The vertical distribution characteristics of eight metals along the core were investigated based on 137Cs and 210Pb radionuclide dating. The sediment was mainly composed of sand and silt. The metals concentrations were Al, 4.67–6.83; Fe, 2.3–3.94; Mn, 0.046–0.07; Cr, 69.5–103; Cu, 14.3–32.1; Zn, 47.3–96.7; Cd, 0.037–0.212; Pb, 13.7–23; Ni, 18.8–38.9 (mg·kg−1, except Al, Fe, and Mn as %), respectively. Geoaccumulation indexes (Igeo) indicated that Cu, Zn, and Pb were of pollution-free level; Cd, Cr, and Ni were in a slight polluted level. Based on potential ecological risk factors (EI), Cd posed a moderate risk to the local environment. Correlation analysis showed that Fe, Al, and Mn had a close association with Cu, Zn, Pb, and Ni at p < 0.01. Clay was significantly correlated with other metals except Cr and Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuary is a region where land runoff interacts most with the ocean and a region most strongly affected by anthropogenic activities. A large amount of heavy metals substances produced by human activities enter the Estuary through rivers and are deposited in the Estuary (Sheng et al. 2008). Sediments are important components of the estuarine ecosystems and the major sources and sinks of the toxic substances including heavy metals, which are originated from anthropogenic activities, such as industrial, agricultural, and domestic wastewater (Monikh et al. 2013). Therefore, it is of necessity to assess the sediment contamination in the estuarine areas (Liu et al. 2016).
The Yangtze River is the fourth largest river in the world in terms of sediment flux (4.86 × 108 t·a−1) (Milliman and Meade 1983). It originates from the Goradandon Mountains in the Qinghai-Tibet Plateau and flows from the west to the east into the East China Sea in Shanghai. Located at the junction of the East China Sea and the Yellow Sea, the Yangtze River Estuary, which resembles a trumpet, is divided into two main branches by Chongming Island and one of the largest estuaries in the world (Chen et al. 2001; Liu et al. 2016). As a mesotidal and partially mixed Estuary characterized by semidiurnal tides, with a mean tidal amplitude of 2.8 m (Shi 2004) and tidal currents of 1.0–2.0 m/s, under fair weather conditions, the Yangtze River Estuary coast is less influenced by waves (Zhang et al. 2009). Shanghai, as the main city along the Yangtze River Estuary and one of the developed cities in China, accounting for 3.71% of the Chinese Gross Domestic Product (GDP), covers 6340 km2 and has a population of 24.18 million (Hu et al. 2017; Shanghai Statistical Yearbook 2018). More than 5 million tons of sewage is discharged into the Yangtze Estuary every year (Zhang et al. 2009). 15 thousand tons of heavy metals were discharged into the East China Sea from the Yangtze River in 2002, and this value reached 22.6 thousand tons in 2008 (Chen et al. 2014; Lin et al. 2002). Consequently, this leads to a large amount of pollutant deposition and poses a threat to the Estuary ecological environment (Zhang et al. 2009).
Qiantang River is located in the northwest of Zhejiang Province, which flows into the Hangzhou Bay. Hangzhou Bay is loaded with 786 × 104 tons sediment from Qiantang River and 486 × 106 tons from Yangtze River every year, respectively. Consequently, both sedimentation and geochemical characteristics of the Hangzhou Bay sediments were dominated by the Yangtze River (Pang et al. 2015; Wright and Nittrouer 1995) and areas surrounding of Qiantang River and Hangzhou Bay with developed industry and huge population. Yang et al. (2017) found that Cd and Cu concentrations in the Qiantang River are worse than Marine Sediments Quality Grade II in 2014, which may pose a threat to the environment.
In recent years, investigations of the distribution, pollution status, and toxicity of heavy metals (such as Cr, Cu, Zn, Cd, Pb, and Ni) in the coastal or estuarine surface sediments in the Yangtze River Estuary Qiantang River and Hangzhou Bay have been carried out (Cao et al. 2015; Chen et al. 2014; Li et al. 2018; Liu et al. 2016; Yang et al. 2017; Yu et al. 2013). Some studies have used isotopes to trace the sources of heavy metals in the Yangtze Estuary. Other scholars have assessed the toxicity of metals in surface sediments from the Yangtze River Estuary based on acid-volatile sulfide (AVS)/simultaneously extracted metals (SEM) methods (Wang et al. 2015; Wang et al. 2013; Li et al. 2018).
However, research reflecting the vertical distribution, toxicity assessment, and sources of heavy metals combined with a chronology in the sediments of the Yangtze River Estuary is still lacking. The aims of this study are to (1) examine the vertical variation of metals in the sediments of the Yangtze River Estuary, (2) study the grain size characteristics of sediments, (3) assess the level of sediments contamination and toxicity, and (4) identify the possible sources of heavy metals.
Materials and methods
Sampling and analysis
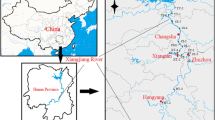
A sediment core (86 cm) was collected from Jinshan Petrochemical Base in the northeast of Jinshan District, Shanghai in 2011 (Fig. 1) for the metals and physical-chemical properties analysis of the sediment cores. The sediment core was cut into immediately at 2 cm intervals respectively, with a plastic knife and placed into plastic bags. All sediment samples were stored at − 20 °C in the refrigerator until analysis (Liu et al. 2011).
Sediment samples were digested through following steps: (1) dried sediment samples (0.5 g) were put into Teflon vessel and added 6 mL HCl (guaranteed reagent) and 4 mL HNO3 (guaranteed reagent) placed on an electronic hot plate and then heat to 110 °C for 2 h; (2) added 10 mL HF (guaranteed reagent) and 3 mL HClO4 (guaranteed reagent), samples were stayed overnight with residual temperature; (3) heating to 150 °C till there was no smoke, after cooling and added 3 mL HCl (1:1); and (4) the mixture was poured into reagent tubes and diluted to 50 mL.
The analysis of major and trace elements was carried out in the Beijing Research Institute of Uranium Geology (BRIUG). The metals, i.e., Al, Fe, and Mn concentrations, in sediments were analyzed by X-ray fluorescence spectrometer (AB-104 L, PW2404, Ltd., the Netherlands). Trace metals of Cu, Pb, Zn, Ni, and Cd in sediments were analyzed by ICP-MS (ELEMENT I, Ltd., USA). Standard reference materials (Chinese National Standard, GBW07309) were used in the analysis for quality assurance and quality control. The errors of the analyses as relative standard deviation were less than 10%, and the recovery was in the range of 88.7–97.6%.
Sediments grain size was determined after H2O2 digestion using a laser size analyzer (Mastersizer 2000, Ltd., UK) at Laboratory of Ocean School, China University of Geosciences (Beijing). 210Pb and 137Cs dating analysis were finished at the Nanjing Institute of Geography and Limnology, Chinese Academy of Science.
Grain size parameter
The average particle size (\( \overline{x} \)), sorting coefficient (σ), skewness (SK) and kurtosis (K) were calculated by Folk and Ward’s (1957):
where Ф = −log2 D and D denotes diameter and Фi represents the cumulative content on i percentage of the particle size.
Contamination and risk assessment methods
Geo-accumulation index (Igeo)
The geo-accumulation index (Igeo) is commonly used to evaluate the pollution in sediments (Hui et al. 2015; Hussain et al. 2015); the formula of Igeo is defined as (Müller 1969):
where Ci is the measured concentration of the examined metal (n) in the sediment and Bi is the geochemical background concentration of the metal (i). 1.5 acts as a constant to correct factors due to lithogeny effects.
Igeo not only can effectively determine the degree of heavy metal pollution of human activities but also considerate the impact of the geological process to the natural background values (Zheng et al. 2015), The Igeo for each metal was classified in seven classes by Müller (1969) as follows: Igeo ≤ 0, uncontaminated; 0 < Igeo ≤ 1, slightly contaminated; 1 < Igeo ≤ 2, moderately contaminated; 2 < Igeo ≤ 3, moderately to heavily contaminated; 3 < Igeo ≤ 4, heavily contaminated; 4 < Igeo ≤ 5, severely contaminated; and Igeo > 5, extremely contaminated.
Potential ecological risk index
Potential ecological risk index (RI) was widely introduced to assess the heavy metals contamination of sediments, according to the toxicity of heavy metals and the response of the environment (Wu et al. 2011; Xiao et al. 2015). Potential carcinogenic risk can be evaluated through the following formula (Hakanson 1980):
where\( {C}_k^i \), \( {C}_n^i \), \( {T}_r^i \), and \( {E}_r^i \)represent the measured concentration, background concentration, toxicity coefficient, and potential ecological risk factors of heavy metal i respectively. RI is the potential ecological risk caused by the overall contamination. The toxicity coefficients of Hg, Cr, Cu, Zn, Cd, Pb, and Ni are 40, 10, 30, 5, 2, 5, and 1, respectively.
According to\( {E}_r^i \), the ecological risk can be classified as follows (Hakanson et al. 1980): \( {E}_r^i \)≤ 40, low risk; 40 <\( \kern0.50em {E}_r^i \)≤ 80, moderate risk; 80 <\( {E}_{r\kern0.5em }^i \)≤ 160, relatively high risk; 160 <\( {E}_r^i \)≤ 320, high risk; and \( {E}_r^i \)> 320, extremely high risk. RI is calculated as follows: RI ≤ 150, low risk; 150 < RI ≤ 300, moderate risk; 300 < RI ≤ 600, relatively high risk; and 600 < RI, extremely high risk.
Result and discussion
Sediments core dating
210Pb is a natural radionuclide with a half-life of 22.3 a, which was first used in glacial dating and then widely used in 100-year-scale dating of lake sediments and marine sediments (Begy et al. 2011; Edgington et al. 1991; Lin 2009; Ren et al. 2007; Robbins and Edgington 1975; Saravana-Kumar et al. 1999; Wan 1997; Zheng et al. 2016). The specific activity of 210Pbtotal and 226Ra in core sediments at the same depth was measured (Fig. 2). The 210Pbex activity decreased approximately exponentially as a function with depth, making it possible to use the CIC (constant initial concentration) model to calculate the sedimentation rates (Zhang et al. 2008). Therefore, we have concluded an average deposition rate is 1.82 cm·a−1 of the core and estimated the age of the bottom of sedimentary rock core was approximately 1965.
Grain size analysis
Figure 3 shows the vertical profiles of physical-chemical parameters in the core. The sediment is mainly composed of sand and silt. The percentages of sand ranged from 10.94% to 73.01%, with an average of 39.55%. The percentages of silt were 25.40% to 78.03%, with an average of 56.38%. Sand and silt percentages generally stayed stable in the upper part of the core except for a large fluctuation at the bottom of the core. The minimum and maximum of the sand percentages were observed at 86 cm and 66 cm depth of the core, respectively. The maximum of silt percentages occurred at 18 cm depth of the core. The percentages of clay ranged from 1.60% to 19.39%, with an average of 4.06%, staying nearly stable throughout the core, with a sharp increase between 78 cm and 86 cm at the bottom of the core. TOC content ranged from 0.22% to 0.80%, and then generally kept stable from 4 cm to the bottom.
The average particle size reflects the distribution of sediments particles and the sediment environment in the sampling area. The particle size of the sample ranged from − 6.71Ф to − 3.78Ф (mean − 5.71Ф), which was relatively stable throughout the core, except for two peak values at the bottom of the core. The Md of the core had the similar trend with that of the average particle size of the core. The sorting coefficient ranged from − 1.95 to − 0.62, showing a good sorting. Skewness ranged from − 0.49 to 0.18 (mean − 0.17). Kurtosis is from 0.95 to 1.76 (mean 1.35), which is ranked as medium to sharp level.
Concentrations and distribution of metals in the sediment core
The concentration of heavy metals in the sediment core from Yangtze River Estuary and some references are shown in Table 1. The concentration ranges of metals in the sediment core were as follows: Al, 4.67–6.83%; Fe, 2.3–3.94%; Mn, 0.046–0.07%; Cr, 69.5–103 mg·kg−1; Cu, 14.3–32.1 mg·kg−1; Zn, 47.3–96.7 mg·kg−1; Cd, 0.037–0.212 mg·kg−1; Pb, 13.7–23 mg·kg−1; Ni, 18.8–38.9 mg·kg−1, respectively. The average concentrations of Cu, Zn, Cd, Pb, and Ni were generally lower than those in the Liaodong Bay. Cr concentration was higher than Liaodong Bay, the Yellow River Estuary, and Shallow Sea Sediments in China and background values. The concentrations of Cu and Cd were higher than those in the Shallow Sea Sediments in China, while the concentrations of Zn, Pb, and Ni were lower than. The concentrations of Cr, Cd, and Ni were higher than those in the background values, and, especially, Cr was 2.35 times higher than those in the background values. Cu, Zn, Cd, and Pb average concentrations except for Cr were lower than in Hangzhou Bay and Qiantang River. The concentrations of Cu, Zn, Cd, and Pb were lower than those in the Pearl River Estuary and Marine Sediments Quality Grade I. Cr and Ni concentrations in the sediment from the Yangtze River Estuary were higher than Kavala Gulf and lower than Strymonikos Gulf and Ierissos Gulf, and Cu, Zn, and Pb concentrations were lower than Kavala gulf, Strymonikos Gulf, and Ierissos Gulf in Greece.
Vertical profiles of physical-chemical parameters and total metals concentrations in the solid phase are shown in Fig. 3. The variations of concentrations of Al, Fe, Mn, Cu, Zn, Pb, and Ni are very similar, keeping generally stable since 1972. The concentration of Cr stays stable in the core except for a maximum value at the depth of 66 cm depth of the core, approximately in 1976. The concentration of Cd reaches two peak values at 34 cm and 6 cm depth, about in 1993 and 2006.
Assessment of the heavy metals contamination
Igeo of the metals is shown in Fig. 4. The average Igeo values of the metals followed the order: Cr > Ni > Cd > Zn > Pb > Cu. The Igeo values for Cr ranged from 0.4 to 0.97 (mean 0.64), indicating that the pollution level of the metals was slight on the whole. Cr could be affected by both industrial activities and natural processes. The maximum Igeo values for Cu, Zn, and Pb throughout core were all less than 0, suggesting that the environment has not been polluted overall by these metals. The Igeo values of the metals were − 2.02 to 0.5 (mean − 0.4) for Cd and − 0.67 to 0.37 (mean − 0.37) for Ni, showing that the metals on the whole were in the pollution-free level and only in the individual layer there were some slight pollution for the metals.
The potential ecological risk diagrams for heavy metals in the sediments core is shown in Fig. 5. Potential ecological risk index (RI) of heavy metals in sediments core samples from the Yangtze Estuary decreased as follows: Cd > Ni > Cr > Pb > Cu > Zn. The potential ecological risk index of Cd in the samples ranged from 11.1 to 63.6 (mean 37.42), of which 12 samples (54.5%) were ranked as low risk and 10 (45.5%) were ranked as moderate risk. The potential ecological risk index of Cd was relatively high in the Yangtze River Estuary, and as a heavy metal, its pollution has been widely reported (Liu et al. 2016; Zhang et al. 2007). The potential ecological risk index of Cr, Cu, Zn, Pb, and Ni in all samples was less than 40, indicating a low risk to the ecological environment. RI ranged from 30.08 to 81.98 with an average of 56.05, indicating low risk of heavy metals to ecosystem in the Yangtze River Estuary.
Source identification of metals
To further identify heavy metals sources, correlation analysis was carried out. As shown in Table 2, Fe, Al, and Mn have high correlations with Cu, Zn, Pb, and Ni at p < 0.01. Since Al and Fe mainly exist in fine-grained sediments and clastic minerals and are not significantly affected by antigenic and biological processes (Ren et al. 2003), the high correlation coefficients among Al, Fe, and the heavy metals suggest that the metals are little affected by human activities and the metals might be originated from same sources or influenced by similar geochemical processes. There are significant correlations among the four elements of Cu, Zn, Pb, and Ni (p < 0.01), indicating that they have similar sources. In addition, the low correlation coefficient between Cr, Cd, and Al and Fe suggest that there might be other sources such as anthropogenic activities for Cd and Cr. In addition, clay was high correlated with other metals except Cr and Cd, possibly due to that clay has a large specific surface area and, therefore, has a strong adsorption on metals (Mayer and Rossi 1982; Shen and Ma 2004), which is consistent with previous studies (Wang et al. 2018; Yao et al. 2016).
Shanghai, the most important industrial city, witnessed a great development of Chinese industry from 1949 to 1965, which results in Al, Fe, Mn, Cu, Zn, Pb, and Ni reached peaks in 1965. Then the Chinese economy was seriously damaged by the Cultural Revolution between 1966 and 1976, possibly resulting in a drastic decrease in industrial (Chen et al. 2014). From the reform and opening up to 2011, Chinese economy has developed steadily. With the emphasis on environmental protection, the content of heavy metals (Al, Fe, Mn, Cu, Zn, Pb, and Ni) has remained stable as a whole.
As the largest estuarine delta in China, the cities surrounding of the Yangtze River Estuary, Hangzhou Bay, and Qiantang River such as Shanghai, Jiaxing, Hangzhou, Shaoxing, and Ningbo cities, etc. have the most developed economy and the highest degree of industrialization in China. A large amount of toxic and harmful substances were discharged into the Yangtze River Estuary through rivers and the atmosphere annually surrounding cities. Shanghai, as a petrochemical city, discharged large quantities of Cd into the Yangtze River Estuary. Human activities (including mining development and urbanization) are one of the main ways to cause Cd pollution (Barcellos and Lacerda 1994; Luo 2009). Cr and Cd may come from discharge sewage from pharmaceutical factories in Shaoxing and Hangzhou city. Jinhua, located in the upstream of Qiantang River, is mainly made of metal fittings hardware, which can also have a certain impact on the sampling point. Meanwhile, the agricultural non-point source runoff and industrial sewage is also an important source for Pb (Cao et al. 2015). Cu concentration was lower than background and other research in Yangtze River Estuary, indicating that it was less affected by anthropogenic activities.
Moreover, atmospheric deposition is also an important source for Pb, Cr, Zn, and Ni. Pb content had remained stable after leaded gasoline was banned by Chinese government in 2001, suggesting that the consumption of coal for combustion and for nonferrous smelting has become the principal anthropogenic source (Sun et al. 2019). Cr concentration was 2.35 times higher than background value, suggesting an influence by anthropogenic activities. With the rapid development of industry, we could presume that Cr and Ni may be derived from such as fossil fuel consumption and metallurgical industry (Tian et al. 2012).
Conclusion
The sediment in this study area was composed of silt with an average particle size of − 5.71Ф. The sorting coefficient was very good, the skewness was negative, and the kurtosis was medium.
The average concentration of Al, Fe, Mn, Cr, Cu, Zn, Cd, Pb, and Ni were as follows: 4.98, 2.6, 0.051, 82.3, 17.16, 58, 0.125, 15.24, and 23.48 (mg·kg−1, except Al, Fe and Mn as %). Except for Cr, Cd, and Ni, the concentrations of other four heavy metals were lower than background values.
Fe, Al, and Mn had high correlation with Cu, Zn, Pb, and Ni at p < 0.01. Cu, Zn, Pb, and Ni were significant correlations among each other (p < 0.01). Clay was significantly correlated with other metals except Cr and Cd.
Results of Igeo suggested that metals Cu, Zn, and Pb are of pollution-free level. Cd and Ni were in a level from unpolluted to slight polluted throughout the core. Cr was in slightly polluted level throughout the core. Based on EI, Cd posed a moderate risk to the local environment. The average of RI was 56.05, indicating that the Yangtze River Estuary was at a low risk level.
References
Barcellos C, Lacerda LD (1994) Cadmium and zinc source assessment in the Sepetiba Bay and basin region. Environ Monit Assess 29(2):183–199
Begy RC, Alida TG, Janos S, Constantin C (2011) A sedimentation study of St. Ana Lake (Romania) applying the210Pb and137Cs dating methods. Geochronometria 38(2):93–100
Cao L, Hong GH, Liu SM (2015) Metal elements in the bottom sediments of the Changjiang estuary and its adjacent continental shelf of the East China Sea. Mar Pollut Bull 95(1):458–468
Chen B, Fan DJ, Li WR, Li WR, Wang L, Zhang XL, Liu M, Guo ZG (2014) Enrichment of heavy metals in the inner shelf mud of the East China Sea and its indication to human activity. Cont Shelf Res 90:163–169
Chen ZY et al (2001) Yangtze River of China: historical analysis of discharge variability and sediment flux. Geomorphology 41(2):77–91
Edgington DN, Klump JV, Robbins JA, Kusner YS, Pampura VD, Sandimirov IV (1991) Sedimentation rates, residence times and radionuclide inventories in Lake Baikal from 137Cs and 210Pb in sediment cores. Nature 350(6319):601–604
Folk RL, Ward WC (1957) Brazos River Bar: a study in the significance of grain size parameters. J Sediment Res 27(1):3–26
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sediment ecological approach. Water Res 14(8):975–1001
He ZF (2018) Geochemical background values of sediments in the Yangtze estuary and offshore marine areas. Shanghai Land Resour 39(1):81–85 (in Chinese)
Hu BF, Jia XL, Hu J, Xu DY, Xia F, Li Y (2017) Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int J Environ Res Public Health 14(9):1–18
Hui Y, Yuan SY, Li MY, Xiao J (2015) Evaluation of heavy metal contamination in agricultural top soils in suburban Xuchang city, China. Environ Earth Sci 74(3):475–2480
Hussain R, Khattak SA, Shah MT, Ali L (2015) Multistatistical approaches for environmental geochemical assessment of pollutants in soils of Gadoon Amazai industrial estate, Pakistan. J Soils Sediments 15(5):1119–1129
Ke S, Zhang JB, Sun SL, Xie Q (2017) The source characteristics and the relationship between enrichment and biological toxicity of heavy metals in surface sediment of pearl river estuary. J Guangdong Ocean Univ 37(4):69–77 (in Chinese)
Li FP, Mao LC, Jia YB, Gu ZJ, Shi WL, Chen L, Ye H (2018) Distribution and risk assessment of trace metals in sediments from Yangtze River estuary and Hangzhou Bay, China. Environ Sci Pollut Res 25:855–866
Lin BQ (2009) Prediction of environmental Kuznets curve of carbon dioxide in China and analysis of its influencing factors. Manage World 187(4):27–36 (in Chinese)
Lin S, Hsieh IJ, Huang KM, Wang CH (2002) Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem Geol 182:377–394
Liu BL, Hu K, Jiang ZL, Qu FG, Su X (2011) A 50-year sedimentary record of heavy metals and their chemical speciations in the Shuangtaizi River estuary (China): implications for pollution and biodegradation. Front Environ Sci Eng China 5(3):435–444
Liu RM, Men C, Liu YY, Yu WW, Xu F, Shen ZY (2016) Spatial distribution and pollution evaluation of heavy metals in Yangtze estuary sediment. Mar Pollut Bull 110(1):564–571
Luo YM (2009) Trends in soil environmental pollution and the prevention-controlling-remediation strategies in China. Environ Pollut Control 31(12):27–31 (in Chinese)
Marine sediment quality standard (2002) GB18668. 4–2002. (in Chinese)
Mayer LM, Rossi PM (1982) Specific surface areas in coastal sediments: relationships with other textural factors. Mar Geol 45:241–252
Milliman JD, Meade RH (1983) World-wide delivery of river sediment to the oceans. J Geol 91(1):1–21
Monikh FA, Safahieh A, Savari A, Doraghi A (2013) Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa estuary (Persian gulf). Environ Monit Assess 185(1):215–222
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geol J 2:108–118
Pang HJ, Lou ZH, Jin AM, Yan, KK, Jiang Y, Yang XH, Chen CTA, Chen XG (2015) Contamination, distribution, and sources of heavy metals in the sediments of Andong tidal flat, Hangzhou Bay, China. Cont Shelf Res 110:72–84
Ren JL, Liu SM, Zhang J, Xie L, Li DD, Cheng Y, Zhu DD (2003) Effects of terrestrial input on the harmful algal bloom area-with aluminum as an example. Chin J Appl Ecol 14(7):1117–1121
Ren Y, Wei CH, Wu CF, Li BG (2007) Environmental and biological characteristics of coking wastewater. Acta Sci Circumst 27(7):1094–1100 (in Chinese)
Robbins JA, Edgington DN (1975) Determination of recent sedimentation rates in Lake Michigan using Pb-210 and Cs-137. Geochim Cosmochim Acta 39(3):285–304
Saravana-Kumar U, Navada SV, Rao SM, Nachiappan RP, Kumar B, Krishnamoorthy TM, Jha SK, Shukla VK (1999) Determination of recent sedimentation rates and pattern in Lake Naini, India by 210Pb and 137Cs dating techniques. Appl Radiat Isot 51(1):97–105
Shanghai Statistical Bureau (2018) Shanghai Statistical Yearbook 2018. (in Chinese)
Shen PY, Ma YJ (2004) Progress in research on contaminated environment remediation of clay minerals. China Min Mag 13:47–50 (in Chinese)
Sheng JJ, Fan DJ, Yang DF, Qi HY, Xu L (2008) Distribution patterns of heavy metals in surface sediments of the Yangtze estuary and its adjacent areas and environmental quality assessment. Environ Sci 29(9):2405–2412 (in Chinese)
Shi QX, Sheng PC, Fang WP, Sun BY, Zhou DR, Wang J, Xu L, Wu QF (2018) Ecological risk and bioaccumulation of heavy metals in the surface sediments in Qiantang River. J Shanghai Ocean Univ 27(5):710–717 (in Chinese)
Shi Z (2004) Behaviour of fine suspended sediment at the north passage of the Changjiang estuary, China. J Hydrol 293(1–4):180–190
Song YG, Yu CF, Zhang YF, Wang K, Du J, Jiang B, Song L (2016) Geochemical characteristics of trace metals in sediments of Liaodong Bay based on multivariate statistical analysis. Res Environ Sci 29(5):692–699 (in Chinese)
Stamatis N, Kamidis N, Pigada P, Sylaios G, Koutrakis E (2019) Quality indicators and possible ecological risks of heavy metals in the sediments of three semi-closed East Mediterranean Gulfs. Toxics 7(2):1–16
Sun XS, Fan DJ, Liu M, Liao HJ, Tian Y (2019) Persistent impact of human activities on trace metals in the Yangtze River estuary and the East China Sea: evidence from sedimentary records of the last 60 years. Sci Total Environ 654:878–889
Tian HZ, Lu L, Cheng K, Hao JM, Zhao D, Wang Y, Jia WX, Qiu PP (2012) Anthropogenic atmospheric nickel emissions and its distribution characteristics in China. Sci Total Environ 417–418:148–157
Wan GJ (1997) 210Pb dating for recent sedimentation. Quat Sci 17:230–239
Wang C, Lin DJ, Wang PF, Ao YH, Hou J, Zhu HG (2015) Seasonal and spatial variations of acid-volatile sulphide and simultaneously extracted metals in the Yangtze River estuary. Chem Ecol 31(5):466–477
Wang CL, Zou XQ, Feng ZY, Hao Z, Gao JH (2018) Distribution and transport of heavy metals in estuarine-inner shelf regions of the East China Sea. Sci Total Environ 644:298–305
Wang YJ, Zhou LM, Zheng XM, Qian P, Wu YH (2013) Influence of Spartina alterniflora on the mobility of heavy metals in;salt marsh sediments of the Yangtze River estuary, China. Environ Sci Pollut Res 20(3):1675–1685
Wright LD, Nittrouer CA (1995) Dispersal of river sediments in coastal seas-6 contrasting cases. Estuary 18(3):494–508
Wu B, Song JM, Li XG (2013) Environmental characteristics of heavy metals in surface sediments from the Huanghe estuary. Environ Sci 34(4):1324–1332 (in Chinese)
Wu ZH, He MC, Lin CY, Fan YH (2011) Distribution and speciation of four heavy metals (cd, Cr, Mn and Ni) in the surficial sediments from estuary in Daliao river and Yingkou Bay. Environ Earth Sci 63(1):163–175
Xiao Q, Zong YT, Lu SG (2015) Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotox Environ Safe 120:377–385
Yang Q, Liu ML, Han YC, Yu ZM, Shen X (2017) Source identification and ecological risk assessment of heavy metals in sediments of Qiantang River. Environ Sci Technol 40(12):278–283 (in Chinese)
Yang YF, Cao W, Zhu ZQ, Yu HB, Mao WH (2013) Accumulation of heavy metals in surface sediments of Hangzhou Bay and its potential ecological risk assessment. Ocean Dev Manage (1):51–58 (in Chinese)
Yao QZ, Wang XJ, Jian HM, Chen HT, Yu ZG (2016) Behavior of suspended particles in the Changjiang estuary: size distribution and trace metal contamination. Mar Pollut Bull 103(1–2):159–167
Yu Y, Song JM, Li XG, Yuan, HM, Li N (2013) Fractionation, sources and budgets of potential harmful elements in surface sediments of the East China Sea. Mar Pollut Bull 68(1–2):157–167
Zhang J, Mou DH, Du JZ, Zhang J (2008) Study on comparison of excess 210Pb chronology of several models. Mar Environ Sci 27(4):370–374, 382 (in Chinese)
Zhang LX, Jiang XS, Zhao M, Li ZE (2007) Pollution of surface sediments and its assessment of potential ecological risk in the Yangtze estuary. Ecol Environ 16:89–393 (in Chinese)
Zhang WG, Feng H, Chang JN, Qu JG, Xie HX, Yu LZ (2009) Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environ Pollut 157(5):1533–1543
Zheng H, Yang D, Xing XL, Zhang ZZ, Shu QL (2016) Historical records, distribution characteristics and sources of heavy metals from sediment core in Honghu Lake, China. China Environ Sci 36:2139–2145 (in Chinese)
Zheng XX, Zhao WJ, Yan X, Shu TT, Xiong QL, Chen FT (2015) Pollution characteristics and health risk assessment of airborne heavy metals collected from Beijing bus stations. Int J Environ Res Public Health 12:9658–9671
Funding
This work was supported by the National Natural Science Foundation of China (41106108) and Special Fund for Public Welfare Industrial Research of the Ministry of Land and Resources of China (201011019-05).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The vertical distribution characteristics of eight metals along the core were investigated based on 137Cs and 210Pb radionuclide dating.
• Study the grain size characteristics of sediments.
• Assess the level of sediments contamination and toxicity and identify the possible sources of heavy metals.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, B. & Zhang, W. Distribution and risk analysis of heavy metals in sediments from the Yangtze River Estuary, China. Environ Sci Pollut Res 27, 10802–10810 (2020). https://doi.org/10.1007/s11356-019-07581-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07581-x