Abstract
Non-alcoholic fatty liver disease (NAFLD), the most common form of liver disease, affects over 30% of the US population. Our group and others have previously demonstrated that low-level environmental pollutant exposures were associated with increased odds ratios for unexplained alanine aminotransferase (ALT) elevation, a surrogate biomarker for NAFLD, in the adult National Health and Nutrition Examination Survey (NHANES). However, recently, more sensitive and lower ALT cutoffs have been proposed. The objective of this observational study is to utilize these ALT cutoffs to determine new associations between environmental chemicals and the surrogate NAFLD biomarker. Adult NHANES 2003–2004 participants without viral hepatitis, hemochromatosis, or alcoholic liver disease were analyzed in this cross-sectional study. ALT elevation was defined as > 30 IU/L in men and > 19 IU/L in women. Odds ratios adjusted for potential confounders for ALT elevation were determined across exposure quartiles for 17 pollutant subclasses comprised of 111 individual pollutants. The overall prevalence of ALT elevation was 37.6%. Heavy metal and organochlorine insecticide subclasses were associated with dose-dependent increased adjusted odds ratios for ALT elevation of 1.6 (95% CI 1.2–2.3) and 3.5 (95% CI 2.3–5.5) respectively, for the highest vs. lowest exposure quartiles (ptrend < 0.01). Within these subclasses, increasing whole blood levels of lead and mercury, and lipid-adjusted serum levels of dieldrin, and the chlordane metabolites, heptachlor epoxide, and trans-nonachlor, were associated with increased odds ratios for ALT elevation. In conclusion, organochlorine insecticide, lead, and mercury exposures were associated with ALT elevation and suspected NAFLD in adult NHANES 2003–2004.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD), and its more advanced form, non-alcoholic steatohepatitis (NASH), are currently the most common forms of liver disease in the United States with a prevalence of approximately 30% in the general population (Le et al. 2017). NAFLD is a spectrum of disorders in the liver ranging from lipid accumulation (steatosis) to steatohepatitis, which is often accompanied by inflammation and hepatocyte death (Aguilera-Mendez 2019). NASH may progress to cirrhosis with severe clinical sequela including hepatocellular carcinoma, death, and liver transplantation. Non-invasive biomarkers of NAFLD and NASH, among others, include elevated activity levels of liver enzymes namely alanine aminotransferase (ALT) and aspartate transaminase (AST), as well as investigational biomarkers such as cytokeratin 18 (Cave et al. 2011; Zhou et al. 2019).
In general, in randomly selected individuals without a clinical diagnosis of NAFLD, ALT activity level greater than two standard deviations of the mean value of 100 is considered the upper limit of normal laboratory references. Previously, numerous epidemiologic studies on the National Health and Nutrition Examination Survey (NHANES) data adopted the following criteria for this range: > 40 IU/L for men and > 31 IU/L for women, to estimate the prevalence of liver disease in the general US population (Cave et al. 2010a; Clark 2006; Clark et al. 2003; Lazo et al. 2008; Liangpunsakul and Chalasani 2004, 2005). Unexplained ALT elevation which is liver enzyme elevation that is not due to either viral hepatitis, excessive ethanol consumption, or iron overload has become an accepted surrogate biomarker for NAFLD in NHANES studies (Clark et al. 2003). Prati et al. performed a 4-year, comprehensive study to define ALT cutoffs for NAFLD diagnosis by eliminating participants who had ultrasound readings suggestive of fatty liver, and then re-evaluated ALT ranges in that population (Prati et al. 2002). These ALT reference ranges, namely > 30 IU/L for men and > 19 IU/L for women, were much lower than previously used ranges and have been increasingly recognized by many research groups (Dunn et al. 2008; Kwo et al. 2017; Martin-Rodriguez et al. 2017; Takyar et al. 2017). Therefore, it becomes important to consider these revised ALT cutoffs in environmental liver disease population studies.
Historically, most cases of NAFLD have been attributed to obesity, insulin resistance, and the metabolic syndrome. With time, an increasing number of studies have reported that exposure to several industrial chemicals and environmental toxicants is associated with NAFLD and NASH, and termed as toxicant-associated steatohepatitis (TASH) (Al-Eryani et al. 2015; Bassler et al. 2019; Cave et al. 2010b; Guardiola et al. 2016; Wahlang et al. 2013). Additionally, utilizing previous ALT reference ranges ≥ 48 IU/L for men and ≥ 31 IU/L for women (≥ 21 years of age); Cave et al. demonstrated that chronic low-level exposures to environmental toxicants were dose-dependently associated with increased odds ratios for ALT elevation and suspected NAFLD in the general US adult population (NHANES 2003–2004) (Cave et al. 2010a). Specifically, polychlorinated biphenyl (PCB) exposures were implicated, as well as lead and mercury. In addition, Serdar et al. confirmed that liver enzyme levels were significantly higher in the highest exposure groups of PCBs and organochlorine pesticides in this population (NHANES 2003–2004). Moreover, additional epidemiological and toxicological studies have confirmed the reported association between environmental chemical exposures, primarily PCBs, and NAFLD (Clair et al. 2018; Wahlang et al. 2016; Wahlang et al. 2014). However, to date, there is still limited epidemiologic information on associations between organochlorine insecticide/pesticide exposures and NAFLD.
Although ALT is a commonly used biomarker for liver disease in epidemiologic and clinical studies, its reported sensitivity for fatty liver diseases is somewhat ambiguous, especially at higher cutoff values (Hadizadeh et al. 2017). In fact, in some patients with biopsy-proven NAFLD, ALT may be normal, and ALT levels do not always correlate with disease severity (Wong et al. 2009). Furthermore, in fatty liver related to industrial chemicals, ALT sensitivity could be limited (Brautbar and Williams 2002; Cave et al. 2010b). We therefore hypothesized that the higher ALT cutoffs used previously may have reduced the number of cases of suspected NAFLD, and this may, in turn, have reduced the power to detect industrial chemicals possibly associated with suspected NAFLD. Thus, the objective of the current study is to analyze associations between environmental chemical exposures and suspected NAFLD in adult NHANES 2003–2004 participants, using the lower ALT cutoffs proposed by Prati et al., in an effort to detect additional chemicals dose-dependently associated with increased odds ratios for ALT elevation and suspected NAFLD.
Materials and methods
Study design and participants
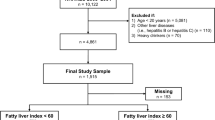
Adult participants from NHANES 2003–2004 were evaluated in this cross-sectional study which was approved by the University of Louisville Institutional Review Board. The following exclusion criteria were utilized: age < 18 years, positive serum hepatitis B surface antigen, positive serum hepatitis C antibody, elevated transferrin saturation (> 60% for males and > 50% for females), and alcohol consumption ≥ 20 g/day for males and ≥ 10 g/day for females. The maximum final sample size was 4582 and has been described previously.
Pollutants
All pollutant data posted by the National Center for Health Statistics (NCHS) prior to December 2008 were accessed and downloaded. This data included 196 pollutants from 17 subclasses. Subclasses comprised of serum or blood organochlorine insecticides; metals including lead, cadmium, and mercury (total and inorganic); volatile organic compounds (VOCs), non-dioxin-like PCBs; dioxin-like compounds including polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and coplanar PCBs; perfluorinated chemicals (PFCs); polybrominated diphenyl ethers (PBDEs); and cotinine. In addition, urinary total (elemental plus inorganic) mercury; heavy metals; total arsenic and speciated arsenics; polyaromatic hydrocarbons; phthalates; organophosphate insecticides; perchlorates; environmental phenols; and iodine were also included. The full list of chemicals belonging to each of these 17 subclasses, as well as the laboratories conducting these measurements and their methods with lower limit of detection (LLOD) have been described previously (Cave et al. 2010a). Only pollutants with a 60% or greater detection rate (111 of 196 pollutants) were evaluated to avoid bias in estimation among those below the limit of detection. Importantly, not all pollutants were measured in every NHANES subject. For most pollutant subclasses, data were collected from a random one-third subsample of subjects (Cave et al. 2010a). Lipid (cholesterol and triglyceride) and creatinine adjustment were appropriately made for pollutants as previously described (Cave et al. 2010a).
Outcome variables and statistical methods
In the current study, elevated ALT was defined by the cutoffs proposed by Prati et al. (> 30 IU/L for men and > 19 IU/L for women) (Prati et al. 2002). The prevalence of ALT elevation was determined in 4582 subjects from various demographic groups based on sex, age, race, and body mass index (BMI). Statistical significance was determined by the chi-square test.
Individuals are likely to be exposed to multiple pollutants within a subclass; thus, the cumulative subclass measure was obtained by summing the ranks according to the magnitude of detectable levels of each pollutant within that subclass (Cave et al. 2010a). For each pollutant subclass, subjects were stratified into quartiles by their cumulative exposure rank with the first quartile representing subjects with the lowest levels. Multivariate-adjusted odds ratios for ALT elevation were then determined across increasing quartiles of chemical exposure using the 1st quartile as the reference group by using logistic regression models. Multiple pollutants have previously been associated with obesity and insulin resistance in NHANES, so the analysis was conducted with adjustments for both BMI and homeostasis model assessment of insulin resistance (HOMA-IR) (Lee et al. 2007a; Lee et al. 2007b). Fasting glucose and insulin were measured in only a subset of NHANES participants, and adjusting for obesity and HOMA-IR further reduces the sample size to 2211 subjects. Because most of the pollutant subclasses were measured only in a subset of this sample, the maximum sample size used for determination of associations was further reduced (Cave et al. 2010a). Adjustments were also made for age, sex, race, and poverty income ratio (PIR). Estimates of the main results were calculated accounting for stratification and clustering, and adjusting for age, race, and PIR using a previously described logistic model (Cave et al. 2010a). p values were determined both with (ptrend-adj) and without (ptrend) adjustment for multiple comparisons.
For subclasses yielding significant results, these analyses were repeated for the individual chemicals within that subclass. Subjects with detectable levels of individual pollutants were ranked and placed into quartiles, and compared with a reference group which consisted of either (i) subjects with levels below the LLOD or (ii) individuals in the first quartile of exposure level.
Finally, chi-squared tests were used to determine whether demographic characteristics (sex, race, age, and BMI) were associated with high levels of exposure to selected subclasses of compounds associated with elevated ALT. All statistical analyses were performed using the survey procedures, SURVEYFREQ and SURVEYLOGISTIC, from SAS 9.1 (SAS Institute Inc., Cary, NC, USA). A p value of 0.05 or less was used to determine statistical significance.
Results
Demographic information
After applying the exclusion criteria, a total of 4582 adult subjects remained, and their demographic information is presented in Table 1. The percentage of females was slightly higher than the percentage of males (52.2% vs. 47.8%, respectively). The mean age was 47.2 ± 21.2 years with a range from 18 to 85 years. Non-Hispanic Whites accounted for 72.3% of the population. Body weights, as defined by NIH guidelines, were fairly evenly distributed between normal weight (31.5%), overweight (34.3%), and obese (32.5%) with very few subjects being underweight (1.7%).
Prevalence of unexplained ALT elevation
Out of the 4582 remaining adult subjects, 1561 subjects had unexplained ALT elevation or suspected NAFLD (Table 1). After incorporating the NHANES sampling weights for these individuals, this corresponded to 37.6% of the adult US population, or 68.9 million people. As shown in Table 1, ALT elevation was slightly more common in females than males (42.9% vs. 31.8%, p = 0.002). Compared to non-Hispanic Whites, ALT elevation was more common in Hispanics, while non-Hispanic Blacks had a lower prevalence of ALT elevation (37.8% vs.47.7% vs. 28.4%, p < 0.001). Moreover, ALT elevation was most prevalent during the 5th and 6th decades (p = 0.022). With regard to body weight, compared to normal weight participants, ALT elevation was more prevalent in overweight and obese participants (25.3% vs. 38.9% vs. 49.0%, respectively, p < 0.001).
Pollutant subclass results
Measures of association were based on a maximum of the 2211 participants with HOMA-IR scores as described previously (Cave et al. 2010a). Out of the 17 NHANES categorized pollutant subclasses investigated, only two were associated with significant dose-dependent increased adjusted odds ratios for ALT elevation (Table 2). These subclasses included (i) serum lipid-adjusted organochlorine insecticides/pesticides and (ii) blood lead, mercury, and cadmium. For these classes, the adjusted odds ratios for the highest exposure quartile, compared to the lowest, were 3.5 (95% CI 2.3–5.5, ptrend-adj < 0.001) and 1.6 (95% CI 1.2–2.3, ptrend-adj = 0.015) respectively.
Individual pollutant results
Eleven individual pollutants from these two subclasses were subsequently analyzed (Table 3). Whole blood lead (99.6%) and total mercury (92.5%) had extremely high detection rates and also demonstrated increased adjusted odds ratios for ALT elevation (lead: ORadj = 3.3 comparing 4th quartile vs. unexposed, 95% CI 0.3–34.2, ptrend-adj = 0.026; and mercury: ORadj = 2.2 comparing 4th quartile vs. unexposed, 95% CI 1.4–3.3, ptrend-adj < 0.001). Cadmium, the third member of this subclass, was not associated with increased odds ratios for ALT elevation.
Eight organochlorine insecticides (or their metabolites) were present at detection rates ranging from 61.7 to 99.9%, and three of these were associated with increased adjusted odds ratios for ALT elevation (Table 3). These included the bioaccumulated chlordane constituents, heptachlor epoxide, and trans-nonachlor, as well as the related insecticide dieldrin. The adjusted odds ratios for the highest exposure quartile compared to unexposed subjects were 3.1 (95% CI 1.3–5.0, ptrend = 0.001), 1.6 (95% CI 0.6–3.8, ptrend = 0.05), and 2.6 (95% CI 1.3–7.2, ptrend = 0.007), respectively, for these chemicals. After adjusting for multiple comparisons, trans-nonachlor was no longer statistically significant (ptrend-adj = 0.093). All five of the other insecticides analyzed from this class showed a trend toward increased odds ratios for ALT elevation across quartiles (ptrend-adj ≤ 0.095). These included the chlordane metabolite, oxychlordane (ORadj = 2.7 comparing 4th quartile vs. unexposed, 95% CI 0.9–8.2, ptrend = 0.053); dichlorodiphenyldichloroethylene (DDE) (ORadj = 1.7 comparing 4th quartile vs. unexposed, 95% CI 0.9–2.9, ptrend = 0.062); hexachlorobenzene (ORadj = 1.4 comparing 4th quartile vs. unexposed, 95% CI 1.0–2.0, ptrend = 0.075); hexachlorocyclohexane (ORadj = 1.7 comparing 4th quartile vs. unexposed, 95% CI 0.9–3.5, ptrend = 0.082); and dichlorodiphenyltrichloroethane (DDT) (ORadj = 1.2 comparing 4th quartile vs. unexposed, 95% CI 0.7–1.9, ptrend = 0.095). Of the eight analyzed insecticides, the DDT metabolite, DDE, had the highest median lipid-adjusted serum concentration (1535 ng/g) in the 4th quartile.
Next, demographic risk factors for organochlorine insecticide and metal exposures were determined. Both older age and higher BMI, but neither race/ethnicity nor sex, were associated with increased odds for having lipid-adjusted organochlorine insecticide levels in the highest quartile (Table 4). Age had the most pronounced effect, with 58.4% of subjects aged 70 years and older having insecticide levels in the highest quartile, compared to only 1.0% of subjects age < 30 years old (p < 0.001). A greater percentage of obese subjects (25.7%) were in the highest insecticide exposure quartile compared to normal weight subjects (11.5%) (p = 0.002). Demographic factors associated with higher whole blood metal levels included older age, having a lower BMI, and being a non-Hispanic Black male (Table 5). Again, age seemed to have the most pronounced effect, with only 8.7% of subjects < 30 years old falling in the highest blood metal concentration quartile, compared to 40.1% of subjects aged 70 years and older. However, in contrast to organochlorine insecticide exposure, the percentage of subjects in the highest quartile of blood metal concentration were subjects with lower BMI (30.3% for lean subjects compared to 19.4% for obese subjects, p = 0.004).
Discussion
Using the ALT reference range proposed by Prati et al., the prevalence of suspected NAFLD was 37.6% in adult NHANES 2003–2004, compared to 10.6% in our prior study (Cave et al. 2010a). Although this prevalence value is much higher, it is however in line with current estimates for the prevalence of suspected NAFLD in western countries and worldwide (20–30%), as well as reported prevalence in the US population (30–46%) (Le et al. 2017; Perumpail et al. 2017; Ruhl and Everhart 2015; Williams et al. 2011). With regard to demographics, suspected NAFLD was more common with females (sex); Hispanics (race/ethnicity); middle age; and with increasing BMI. These results were concordant with our previous study. However, the race/ethnicity differences were lower in the current study when compared to our previous study which used higher ALT cutoffs. In the current study, when compared to non-Hispanic White participants, non-Hispanic Blacks and other groups had 16% and 12% lower levels of NAFLD prevalence, respectively, while Hispanics had 24% higher levels of NAFLD prevalence. In the previous study, non-Hispanic Blacks and other populations had 44% and 1% lower levels of NAFLD respectively and Hispanics had 86% higher levels of NAFLD when compared to non-Hispanic White participants. This observation is broadly consistent with the distribution of loss of function alleles of the NASH gene, namely, patatin-like phospholipase A3 (PNPLA3) within groups that identified with Hispanic ethnicity; and supported the concept that PNPLA3 gene dysfunction can worsen NAFLD and NASH (Hernaez et al. 2013; Rotman et al. 2010).
With regard to environmental pollutants, the present analysis demonstrated that lead and mercury exposures were associated with dose-dependent increased adjusted odds ratios for ALT elevation which was consistent with our earlier reports (Cave et al. 2010a). The biologic plausibility that these metals could play a causal role in the development of liver diseases including NAFLD have also been reported by other groups (Kang et al. 2013; Lin et al. 2014; Yorita Christensen et al. 2013). On the contrary, the present study identified that organochlorine insecticides were associated with significantly increased odds ratios for ALT elevation which was not observed when using the higher ALT cutoffs. Nonetheless, in the previous study, the odds ratio for ALT elevation was higher in the third quartile of organochlorine insecticide exposure compared to unexposed subjects, but this trend did not reach statistical significance (Cave et al. 2010a). However, due to the low number of cases of ALT elevation noted (63/587), the first analysis could have been underpowered to detect a significant difference. In comparison, 202 cases of ALT elevation occurred in the present analysis in the organochlorine insecticide group, and this may have potentially increased statistical power.
Organochlorine insecticides are categorized as persistent organic pollutants due to their intact chemical integrity and resistance to degradation and metabolism. These lipid-soluble and poorly metabolized chemicals tend to bioaccumulate in the adipose tissue and other organs of living organisms including marine wildlife and humans (Kim et al. 2014; Pedro et al. 2017). Indeed, the current study demonstrated that obese subjects, who are at risk for NAFLD, also have the highest levels of serum lipid-adjusted organochlorine insecticides. Organochlorine insecticides include DDT and its metabolite, DDE; cyclodienes such as technical chlordane, a synthetic mixture comprising of over 50 chemicals with major components (60–85%) including cis- and trans-chlordane, hexachlor, heptachlor and trans-nonachlor, and their bioaccumulated metabolites (heptachlor epoxide and oxychlordane). Additional compounds in this class include dieldrin, aldrin, and endrin as well as caged structures such as mirex and chlordecone. Because of the toxic impact of DDT on the ecosystem, particularly wildlife endangerment (Carson and Darling 1962), the chlorinated cyclodienes were produced commercially as alternatives to DDT. These chemicals were officially banned in 2001 at the Stockholm Convention on Persistent Environmental Pollutants (Porta and Zumeta 2002). Importantly, even though these insecticides have been banned from commercial production and use, they continue to persist in the environment (Turusov et al. 2002). For example, although DDT was banned from use in the US in 1972, 99.7% of NHANES subjects had detectable levels of the DDT metabolite, DDE. Furthermore, the organochlorine insecticides analyzed in this study continue to contaminate the US food supply, and US DDT per capita daily consumption was estimated to be greater than 250 ng/day (Schecter et al. 2010). Also, there are still considerable serum levels of chlordane metabolites including oxychlordane and trans-nonachlor in the US population according to the Fourth National Report on Human Exposure to Environmental Chemicals published by the Centers for Disease Control and Prevention (CDC), utilizing the NHANES datasets (1999–2004 and 2005–2010) (Anonymous 2018). Notably, in the current study, increased pesticide body burden was associated with increased age, implicating that bioaccumulation occurred over time.
An appreciable number of studies have reported associations of organochloride insecticide exposures with neurotoxicity, cancer, as well as negative effects on endocrine and metabolic health (De Coster and van Larebeke 2012; Kamel et al. 2007; Lee et al. 2007a, b; Wahlang 2018). Organochlorine insecticides also have well-documented hepatotoxicity, especially at high doses (Al-Eryani et al. 2015; Freire et al. 2015; Ogata and Izushi 1991; Reuber 1978a, b). Therefore, serum organochlorine insecticides could be previously unrecognized mediators of liver disease in the general US adult population. While high-level exposures (e.g., acute poisonings) to these chemicals are sufficient to cause liver disease, the current data implicated that chronic low-level exposures, particularly occurring on a background of obesity or genetic susceptibility, may also contribute to the genesis and progression of liver diseases including NAFLD. Indeed, epidemiologic findings from human observational studies and toxicological studies using animal models have demonstrated that organochlorines, particularly chlordane and its metabolites, are associated with metabolic disorders that encompass NAFLD (Ji et al. 2016; Liu et al. 2017b; Rosenbaum et al. 2017; Wang et al. 2017).
One of the most compelling data on the hepatic effects of organochlorine insecticide exposures in human subjects stemmed from an extremely high chlordecone exposure in 32 plant workers in Virginia (Guzelian et al. 1980). Although liver enzymes were repeatedly normal in these workers, many had hepatomegaly which eventually led to liver biopsy in 12 cases. These biopsies revealed mild steatosis, mild portal inflammation and fibrosis, glycogenated nuclei, and lipofuscin accumulation. Additionally, organochlorine insecticides have also been associated with metabolic dysfunction including hyperglycemia, hypertriglyceridemia, diabetes, and the metabolic syndrome in epidemiologic studies (Kim et al. 2014; Lee et al. 2007a, b). Even though mechanisms of hepatotoxicity are not clearly defined in animal models, organochlorine insecticides have long been recognized as endocrine-disrupting chemicals through their interaction with steroid hormones receptors such as estrogen, androgen, and thyroid receptors (De Coster and van Larebeke 2012). Organochlorine insecticides also induce hepatic expression of cytochrome P450s by activating xenobiotic receptors that can also influence hepatic energy metabolism such as the pregnane-xenobiotic receptor, constitutive androstane receptor, and aryl hydrocarbon receptor (De Coster and van Larebeke 2012; Kiyosawa et al. 2008). Emerging new hypotheses for organochlorine insecticide mechanistic actions contributing to NAFLD development or progression include inhibition of the epidermal growth factor receptor (EGFR) signaling, similar to PCBs (Hardesty et al. 2018). It was recently demonstrated that chlordane and one of its most bioaccumulated components, trans-nonachlor, are potent EGFR inhibitors that diminished EGFR phosphorylation, a crucial step in maintenance of normal signaling processes (Hardesty et al. 2018). Another recently proposed mechanism of action for organochlorine insecticides is their ability to influence the gut-liver axis by modulating intestinal microbiota and altering bile acid metabolism (Liu et al. 2017a).
The current study is not without limitations and potential problems are inherent to the study design. Firstly, ALT levels in the general population may not rise to cross diagnostic thresholds in NAFLD, particularly in populations exposed to industrial chemicals. This is because the rise may either be transient and occurred early in the process or the entire exposed population may have lower ALT expression than unexposed individuals. Secondly, some of these chemicals have been proposed to be diet-induced obesogens that act in conjunction with high fat diet to mechanistically cause NAFLD (Wahlang et al. 2019), and adjusting for BMI may reduce the significance of association with ALT elevations. In the current cross-sectional analysis, higher BMI was associated with both suspected NAFLD and increased pesticide levels, and perhaps unadjusted BMI analysis may yield some insight into the potential obesogenic effect of these chemicals. However, this will need further investigation such as cohort studies to establish temporal trends between obesogen exposure and NAFLD/obesity incidences. Another limitation is that the “true” normal range for ALT levels in adult NHANES is largely unknown, and no causal inferences can be obtained from the current study. Furthermore, age was not taken into consideration as a factor that could drive both increased chemical exposures as well as increased ALT elevation. It is well known that persistent chemicals bioaccumulate over time, and age often dictates length or duration of chronic chemical exposure. Additionally, age is known to influence NAFLD development and progression (Estes et al. 2018), and increased age could possibly subject the liver to higher risks of environment insults; therefore, future studies should take this into consideration. However, a major strength of the study is that it utilized the lower ALT reference range suggested by Prati et al. which has been rigorously validated, albeit in a different population cohort.
Conclusion
In summary, the current study demonstrated that organochlorine insecticides/pesticides, lead, and mercury were present in the serum of nearly all US adults in 2003–2004. These common pollutants were associated with significant dose-dependent increased adjusted odds ratios for ALT elevation in subjects whose ALT elevations were not explained by viral hepatitis, hemochromatosis, or alcoholism. The results suggested a possible interaction between low-level environmental pollutant exposures and the development of liver disease and suspected NAFLD in the general US adult population, dependent on age and race. Importantly, the data from the current study contributes to the body of literature on the associations between insecticide exposures and liver disease and implicates that non-occupational, low-level exposures may pose an even bigger problem for liver disease in the general US population. Future studies employing animal exposure models at doses relevant to human exposures are needed to confirm the causal role of these environmental pollutants, primarily organochlorine insecticides, in NAFLD initiation, development, and progression.
Funding support
This research was supported in part by the National Institute of Environmental Health Sciences [R35ES028373, P42ES023716, T32ES011564]; the National Institute of General Medical Sciences [P20GM113226]; and the National Institute on Alcohol Abuse and Alcoholism [P50AA024337].
Abbreviations
- ALT:
-
Alanine aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- DDE:
-
Dichlorodiphenyldichloroethylene
- DDT:
-
Dichlorodiphenyltrichloroethane
- EGFR:
-
Epidermal growth factor receptor
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LLOD:
-
Lower limit of detection
- MeHg:
-
Methylmercury
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NCHS:
-
National Center for Health Statistics
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- PBDEs:
-
Polybrominated diphenyl ethers
- PCBs:
-
Polychlorinated biphenyls
- PCDDs:
-
Polychlorinated dibenzo-p-dioxins
- PCDFs:
-
Polychlorinated dibenzofurans
- PFCs:
-
Perfluorinated compounds
- PIR:
-
Poverty income ratio
- TASH:
-
Toxicant-associated steatohepatitis
References
Aguilera-Mendez A (2019) Nonalcoholic hepatic steatosis: a silent disease. Rev Med Inst Mex Seguro Soc 56:544–549
Al-Eryani L, Wahlang B, Falkner KC, Guardiola JJ, Clair HB, Prough RA, Cave M (2015) Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol 43:482–497. https://doi.org/10.1177/0192623314549960
Anonymous (2018) Fourth national report on human exposure to environmental chemicals. Updated Tables, Volume Two. Centers for Disease Control and Prevention (CDC). U.S. Department of Health and Human Services
Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, Cave MC (2019) Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut 247:1055–1063. https://doi.org/10.1016/j.envpol.2019.01.064
Brautbar N, Williams J 2nd (2002) Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health 205:479–491. https://doi.org/10.1078/1438-4639-00175
Carson R, Darling L (1962) Silent spring. Houghton Mifflin; Riverside Press, Boston; Cambridge, Mass
Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G (2010a) Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ Health Perspect 118:1735–1742. https://doi.org/10.1289/ehp.1002720
Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, Bon Homme M, McClain CJ (2010b) Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology 51:474–481. https://doi.org/10.1002/hep.23321
Cave M, Falkner KC, Henry L, Costello B, Gregory B, McClain CJ (2011) Serum cytokeratin 18 and cytokine elevations suggest a high prevalence of occupational liver disease in highly exposed elastomer/polymer workers. J Occup Environ Med 53:1128–1133. https://doi.org/10.1097/JOM.0b013e31822cfd68
Clair HB, Pinkston CM, Rai SN, Pavuk M, Dutton ND, Brock GN, Prough RA, Falkner KC, McClain CJ, Cave MC (2018) Liver disease in a residential cohort with elevated polychlorinated biphenyl exposures. Toxicol Sci 164:39–49. https://doi.org/10.1093/toxsci/kfy076
Clark JM (2006) The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40(Suppl 1):S5–S10. https://doi.org/10.1097/01.mcg.0000168638.84840.ff
Clark JM, Brancati FL, Diehl AM (2003) The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98:960–967. https://doi.org/10.1111/j.1572-0241.2003.07486.x
De Coster S, van Larebeke N (2012) Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health 2012:713696. https://doi.org/10.1155/2012/713696
Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB (2008) Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 103:2263–2271. https://doi.org/10.1111/j.1572-0241.2008.02034.x
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ (2018) Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67:123–133. https://doi.org/10.1002/hep.29466
Freire C, Koifman RJ, Koifman S (2015) Hematological and hepatic alterations in Brazilian population heavily exposed to organochlorine pesticides. J Toxicol Environ Health A 78:534–548. https://doi.org/10.1080/15287394.2014.999396
Guardiola JJ, Beier JI, Falkner KC, Wheeler B, McClain CJ, Cave M (2016) Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. Toxicol Appl Pharmacol 313:47–56. https://doi.org/10.1016/j.taap.2016.10.001
Guzelian PS, Vranian G, Boylan JJ, Cohn WJ, Blanke RV (1980) Liver structure and function in patients poisoned with chlordecone (Kepone). Gastroenterology 78:206–213
Hadizadeh F, Faghihimani E, Adibi P (2017) Nonalcoholic fatty liver disease: diagnostic biomarkers. World J Gastrointest Pathophysiol 8:11–26. https://doi.org/10.4291/wjgp.v8.i2.11
Hardesty JE, Al-Eryani L, Wahlang B, Falkner KC, Shi H, Jin J, Vivace BJ, Ceresa BP, Prough RA, Cave MC (2018) Epidermal growth factor receptor signaling disruption by endocrine and metabolic disrupting chemicals. Toxicol Sci 162:622–634. https://doi.org/10.1093/toxsci/kfy004
Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB, Genetics of Obesity-Related Liver Disease C, Nguyen T, Kamel IR, Bonekamp S, Eberhardt MS, Clark JM, Kao WH, Speliotes EK (2013) Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol 11:1183–1190 e2. https://doi.org/10.1016/j.cgh.2013.02.011
Ji G, Xu C, Sun H, Liu Q, Hu H, Gu A, Jiang ZY (2016) Organochloride pesticides induced hepatic ABCG5/G8 expression and lipogenesis in Chinese patients with gallstone disease. Oncotarget 7:33689–33702. https://doi.org/10.18632/oncotarget.9399
Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP (2007) Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol 26:243–250. https://doi.org/10.1177/0960327107070582
Kang MY, Cho SH, Lim YH, Seo JC, Hong YC (2013) Effects of environmental cadmium exposure on liver function in adults. Occup Environ Med 70:268–273. https://doi.org/10.1136/oemed-2012-101063
Kim KS, Lee YM, Kim SG, Lee IK, Lee HJ, Kim JH, Kim J, Moon HB, Jacobs DR Jr, Lee DH (2014) Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 94:151–157. https://doi.org/10.1016/j.chemosphere.2013.09.066
Kiyosawa N, Kwekel JC, Burgoon LD, Dere E, Williams KJ, Tashiro C, Chittim B, Zacharewski TR (2008) Species-specific regulation of PXR/CAR/ER-target genes in the mouse and rat liver elicited by o, p'-DDT. BMC Genomics 9:487. https://doi.org/10.1186/1471-2164-9-487
Kwo PY, Cohen SM, Lim JK (2017) ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 112:18–35. https://doi.org/10.1038/ajg.2016.517
Lazo M, Selvin E, Clark JM (2008) Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med 148:348–352
Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH (2017) Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 12:e0173499. https://doi.org/10.1371/journal.pone.0173499
Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR Jr (2007a) Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 30:622–628. https://doi.org/10.2337/dc06-2190
Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR Jr (2007b) Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetologia 50:1841–1851. https://doi.org/10.1007/s00125-007-0755-4
Liangpunsakul S, Chalasani N (2004) Relationship between unexplained elevations in alanine aminotransferase and serum leptin in U.S. adults: results from the third National Health and Nutrition Examination Survey (NHANES III). J Clin Gastroenterol 38:891–897
Liangpunsakul S, Chalasani N (2005) Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III). Am J Med Sci 329:111–116
Lin YS, Ginsberg G, Caffrey JL, Xue J, Vulimiri SV, Nath RG, Sonawane B (2014) Association of body burden of mercury with liver function test status in the U.S. population. Environ Int 70:88–94. https://doi.org/10.1016/j.envint.2014.05.010
Liu Q, Shao W, Zhang C, Xu C, Wang Q, Liu H, Sun H, Jiang Z, Gu A (2017a) Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ Pollut 226:268–276. https://doi.org/10.1016/j.envpol.2017.03.068
Liu Q, Wang Q, Xu C, Shao W, Zhang C, Liu H, Jiang Z, Gu A (2017b) Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci Rep 7:46339. https://doi.org/10.1038/srep46339
Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL (2017) Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine (Baltimore) 96:e6770. https://doi.org/10.1097/MD.0000000000006770
Ogata M, Izushi F (1991) Effects of chlordane on parameters of liver and muscle toxicity in man and experimental animals. Toxicol Lett 56:327–337
Pedro S, Boba C, Dietz R, Sonne C, Rosing-Asvid A, Hansen M, Provatas A, McKinney MA (2017) Blubber-depth distribution and bioaccumulation of PCBs and organochlorine pesticides in Arctic-invading killer whales. Sci Total Environ 601-602:237–246. https://doi.org/10.1016/j.scitotenv.2017.05.193
Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A (2017) Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 23:8263–8276. https://doi.org/10.3748/wjg.v23.i47.8263
Porta M, Zumeta E (2002) Implementing the Stockholm treaty on persistent organic pollutants. Occup Environ Med 59:651–652
Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G (2002) Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 137:1–10
Reuber MD (1978a) Carcinomas and other lesions of the liver in mice ingesting organochlorine pesticides. Clin Toxicol 13:231–256. https://doi.org/10.3109/15563657808988235
Reuber MD (1978b) Carcinogenicity testing of chemicals with particular reference to organochlorine pesticides. Sci Total Environ 10:105–115
Rosenbaum PF, Weinstock RS, Silverstone AE, Sjodin A, Pavuk M (2017) Metabolic syndrome is associated with exposure to organochlorine pesticides in Anniston, AL, United States. Environ Int 108:11–21. https://doi.org/10.1016/j.envint.2017.07.017
Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, Nash CRN (2010) The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52:894–903. https://doi.org/10.1002/hep.23759
Ruhl CE, Everhart JE (2015) Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 41:65–76. https://doi.org/10.1111/apt.13012
Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L (2010) Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect 118:796–802. https://doi.org/10.1289/ehp.0901347
Takyar V, Nath A, Beri A, Gharib AM, Rotman Y (2017) How healthy are the “healthy volunteers”? Penetrance of NAFLD in the biomedical research volunteer pool. Hepatology 66:825–833. https://doi.org/10.1002/hep.29247
Turusov V, Rakitsky V, Tomatis L (2002) Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 110:125–128. https://doi.org/10.1289/ehp.02110125
Wahlang B (2018) Exposure to persistent organic pollutants: impact on women’s health. Rev Environ Health 33:331–348. https://doi.org/10.1515/reveh-2018-0018
Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, Cave MC (2013) Toxicant-associated steatohepatitis. Toxicol Pathol 41:343–360. https://doi.org/10.1177/0192623312468517
Wahlang B, Song M, Beier JI, Cameron Falkner K, Al-Eryani L, Clair HB, Prough RA, Osborne TS, Malarkey DE, Christopher States J, Cave MC (2014) Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol 279:380–390. https://doi.org/10.1016/j.taap.2014.06.019
Wahlang B, Prough RA, Falkner KC, Hardesty JE, Song M, Clair HB, Clark BJ, States JC, Arteel GE, Cave MC (2016) Polychlorinated biphenyl-xenobiotic nuclear receptor interactions regulate energy metabolism, behavior, and inflammation in non-alcoholic-steatohepatitis. Toxicol Sci 149:396–410. https://doi.org/10.1093/toxsci/kfv250
Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, Falkner KC, Prough RA, Kirpich IA, Cave MC (2019) Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep 6:80–94. https://doi.org/10.1007/s40572-019-00232-w
Wang D, Wang X, Zhang P, Wang Y, Zhang R, Yan J, Zhou Z, Zhu W (2017) The fate of technical-grade chlordane in mice fed a high-fat diet and its roles as a candidate obesogen. Environ Pollut 222:532–542. https://doi.org/10.1016/j.envpol.2016.11.028
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140:124–131. https://doi.org/10.1053/j.gastro.2010.09.038
Wong VW, Wong GL, Tsang SW, Hui AY, Chan AW, Choi PC, Chim AM, Chu S, Chan FK, Sung JJ, Chan HL (2009) Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther 29:387–396. https://doi.org/10.1111/j.1365-2036.2008.03896.x
Yorita Christensen KL, Carrico CK, Sanyal AJ, Gennings C (2013) Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003-2004. Int J Hyg Environ Health 216:703–709. https://doi.org/10.1016/j.ijheh.2013.01.005
Zhou JH, Cai JJ, She ZG, Li HL (2019) Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol 25:1307–1326. https://doi.org/10.3748/wjg.v25.i11.1307
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wahlang, B., Appana, S., Falkner, K.C. et al. Insecticide and metal exposures are associated with a surrogate biomarker for non-alcoholic fatty liver disease in the National Health and Nutrition Examination Survey 2003–2004. Environ Sci Pollut Res 27, 6476–6487 (2020). https://doi.org/10.1007/s11356-019-07066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07066-x