Abstract
The alkaline nature of biochar provides a potential for soil arsenic (As) mobilization and, hence, enhancing efficiency of As phytoextraction by combining with As hyperaccumulator. To testify the feasibility and potential risk of the above strategy, biochar effect on As transfer in a paddy soil and accumulation in P. vittata was investigated in a pot experiment. By leaching soil (total As concentration 141.17 mg/kg) with simulated acid rain (pH 4.2), As the concentration in leaching eluate increased proportionally with increasing biochar ratio. Coincident with elevated soil As mobility, apparent enhancement in As uptake and translocation in P. vittata was determined with 1–5% biochar amendment after 40 days of plant growth. Furthermore, diffusive gradients in thin film (DGT) technique were employed to characterize any potential risk in vertical downward migration of As at 2-mm resolution. A significantly increasing profile of DGT-As ranging from on average 20 μg/L in CK to 50–100 μg/L in 1–3% biochar treatments was recorded over 0–60 mm depth, with 25–71% lower labile As in the rhizosphere than non-rhizosphere zone with few exceptions. As compared to Chinese quality standard for groundwater (Class IV 50 μg/L), biochar ratio at ≤ 1% was suggested for local water safety while actual application should take the physicochemical characteristic of tested soil into account. Our results demonstrated the biochar-assisted P. vittata phytoremediation can serve as an emerging pathway to enhance efficiency of soil As phytoextraction. The combination of DGT techniques and greenhouse assay provided a powerful tool for evaluating the gradient distribution of heavy metal in rhizosphere and accessing corresponding ecological risk at more precise scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is ubiquitous in the earth’s crust ranging from < 1 to > 100 mg/kg (Cullen and Reimer 1989). Since the process of industrialization and urbanization, arsenic is widely accumulated in aquatic and terrestrial environments caused by anthropogenic activities including mining, metal processing, and consumption of As-laden agrochemicals (Leist et al. 2000). As an unessential element for human beings, arsenic exceedance would trigger damage of the kidney, lung, and liver, even increasing mortality rates through polluted drinking water, rice diet, inhalation, and direct skin contact (Althobiti et al. 2018, Argos et al. 2010, Irem et al. 2019). There are thousands of people suffering from As contamination all around the world, especially in the region of South Asia such as Bangladesh, India, and China (Brammer and Ravenscroft 2009, Moreno-Jimenez et al. 2012, Shah et al. 2019).
Under the pressure of escalating soil As pollution and its consequent threat toward humans, phytoremediation has been increasingly considered as a cost-efficient and environmentally harmonious method (Salt et al. 1998). Pteris vittata L. (P. vittata) is one of the widely acknowledged As-hyperaccumulators that is resistant to As poisoning, with aboveground As concentration being up to 1442–7526 mg/kg (dry weight) (Ma et al. 2001, Niazi et al. 2011). However, the extraction efficacy of P. vittata is limited by growth rate, plant biomass, and soil properties especially As availability in tested sites (Mathews et al. 2010, Singh et al. 2015, Ye et al. 2011). Former researchers have done numerous work to facilitate phytoextraction efficiency (Niazi et al. 2016). For example, the supplement of phosphate rock was identified to promote more As uptake of P. vittata than phosphorous fertilizer (Lessl et al. 2014). Chemical additive such as sodium polyacrylate and water-soluble chitosan were investigated to mobilize As and then expedite As extraction from soil (Yan et al. 2012, Yang et al. 2017). Nevertheless, the above materials mainly consisted of phosphorous-containing substances and other chemical complexes, which imposed potential risks of secondary pollution and eutrophication in water body.
Biochar(BC) is a promising material made by biomass such as farm and forestry wastes via oxygen-depleted thermal degradation (pyrolysis) (Ok et al. 2015). Relative to other common organic amendments for soil, BCs embraced myriad surface functional groups, high cation exchange capacity (CEC), proper alkalinity, and sustainable recalcitrance (Jindo et al. 2014, Kuzyakov et al. 2014, Wu et al. 2012). It has been well established that the utility of BC could mitigate climate change and ameliorate soil physicochemical properties and thus plant growth as well as rehabilitating contaminated soil (Atkinson et al. 2010, Lehmann et al. 2011). Nevertheless, due to its alkaline nature, BCs have been increasingly recognized to enhance soil As mobility, posing potential threats to ecological security (Beesley et al. 2014, Liang et al. 2017, Zeng et al. 2018). From the perspective of phytoextraction, however, this property of BCs could be employed to facilitate an elevated As bioavailability and thus enhance As depletion by its hyperaccumulator as exemplified by Pteris vittata.
Considering the key limitation of extraction efficiency, As availability was chosen as major index to assess contaminant level and actual risk toward organisms and ecosystem instead of total As concentration. Diffusive gradients in thin films (DGT) is an emerging technique to measure heavy metal availability in site, reflecting interface dynamic procedure, metals bioavailability in soil, and bioaccumulation in plants (Zhang and Davison 2015). Different from traditional test methods, DGT mimics the plants uptake based on Fick’s First Law of Diffusion, determining metals availability without element redistribution (Zhang et al. 2001). In recent years, DGT has been implemented to evaluate mobilization, resupply ability, and pertinent kinetic information of soil metals (Egene et al. 2018, Wang et al. 2018). Further, phytoextraction is largely correlated with As activity near rhizosphere while the corresponding researches are still warranted (Hinsinger and Marschner 2006). So this work focuses on study area of rhizosphere and non-rhizosphere to investigate As transportation and availability in the vertical profile.
The objectives of the present work were to (1) elucidate the available As two-dimensional (rhizosphere, non-rhizosphere) distribution in soil with influence of P. vittata and biochar and (2) investigate the possibility of new decontamination solution combining P. vittata with biochar and its potential environmental risk.
Materials and methods
Soil pretreatment and biochar preparation
The soil used in this work was collected from a historically As mining-impacted cropland (29° 39' 38" N, 111° 02' 16" E) adjacent to Shimen Realgar Mine in Changde, Hunan province of China (Fig. 1a). The studied area belonged to subtropical monsoon climate with mean temperature of 28.6 °C and average annual precipitation of 1540 mm predominated by the acid rain. This site has been suffering from critical As contamination with total As concentration in agriculture land ranging from 475.4 to 1229.3 mg/kg (Tang et al. 2016). Soil samples were air-dried at room temperature, mixed thoroughly, and ground. To remove large debris and gravel fraction and get more homogeneous test portion, the soil was passed through a nylon sieve of 0.154 mm (100 meshes) for subsequent chemical analysis (Alloway 2012).
Rice straw, which was the most abundant biomass resource in most areas of southern China, was chosen as the feedstock for biochar production. In this work, the rice straw was obtained from a farmland free of As contamination in Huailiu village (27°36' 39" N, 111°59' 42" E; Loudi, Hunan province of China) (Fig. 1b). After being washed thoroughly and oven-dried at 60 °C, the feedstock was subjected to slow pyrolysis in a muffle furnace (BF51732 BPMC-1, Thermo Scientific, USA) under N2 atmosphere. The temperature was raised by 6 °C min−1 and kept at 450 °C for 1 h. After cooling, the product was crushed to pass a 2-mm sieve and referred to as RBC (Rice straw derived biochar). The basic properties of studied soil, RBC, and their mixtures were tested directly prior to planting (Table 1). Relative to soil, the As content in RBC was fewer and RBC-spiked soils did not show obvious As increase, suggesting that the presence of RBC itself would not introduce much arsenic load.
Reagents
All chemicals used in this work were of analytical grade purity and were dissolved in deionized (DI) water (18.2 MO) (Nanopurewater, Barn-stead). All plastic and glassware were soaked in 10% (v/v) HNO3 for 24 h and then washed by DI water. Sodium arsenate (Na2HAsO4·7H2O) was purchased from Fisher Scientific.
Leaching column experiment
The columns with internal diameter of 5 cm were filled to a height of 18 cm with soil, RBC, and soil amended with RBC (2% and 5%, w/w) respectively, according to the optimal addition ratio in agriculture soil (Matovic 2011). Each treatment was repeated with three identical columns. The columns were leached upwards from their base, with the bottom of columns being packed with filter paper and quartz sand. Based on the average amount of rainfall in monsoon season in Hunan province, 800 ml of acid solution (HNO3:H2SO4 = 1:4, pH = 4.2) simulating local precipitation of acid rain was introduced into the columns by peristaltic pump at a 27.26 ml/min flow rate (1 rpm). The leachate was collected from the outlet and analyzed for As concentration and pH.
Greenhouse assay
Untreated control soil and RBC-amended soil were used for pot experiments. RBC treatments were prepared by mixing the soil with corresponding RBC additional rate in large plastic containers. Plastic pots (18 × 25 cm; d × h) were then filled with 3500 g of substrate and equilibrated at field capacity for four weeks. Each treatment had three replicate pots.
P. vittata plants were collected from Shimen realgar mining area. After 2-week acclimation in its original soil at greenhouse, every two randomly picked plants of similar size were transferred into each pot and kept in an opened glasshouse to make the P. vittata plants grow under almost the same ambient temperature as nature. To provide more detailed information about influence of RBC addition on As contaminated soil, the plant were incubated in soil under two level of RBC (2%, 5% and 1%, 2%, 3%, respectively) (w%) and then harvested at two period (from 20 April to 30 May with mean monthly temperature of 25 °C, from 20 October to 30 November with mean monthly temperature of 14 °C, respectively), which was designated as HA and LA-expt. Soil moisture was maintained around the field capacity with deionized water being added periodically as required during the growing period.
DGT measurement
In this study, the Zr-oxide gel was prepared according to Ding et al. (2011). For assembling the flat DGT probe, the Zr-oxide gel was covered in sequence by diffusive gel (APA gel, 0.80-mm thick) (Scally et al. 2006), and a cellulose nitrate filter membrane (0.13-mm thick, Whatman, 0.45-μm pore size) with an open window of 20 mm × 150 mm (width × length) (Ding et al. 2016).
At end of experiment before plant harvest, DGT devices were inserted into both the rhizosphere and non-rhizosphere soils at water content of 70% and kept for 24 h. The DGT devices were then retrieved from soils and rinsed by deionized water to remove soil residue. The ZnO binding gels were peeled from devices, sliced at 2-mm intervals, and extracted with 0.8 mL of 1 M NaOH for 24 h at room temperature. Arsenic concentration in the extraction was analyzed with an atomic fluorescence spectrometer (AFS6500, Haiguang, Beijing). Detailed procedure for deployment of this flat type of DGT with subsequent processing and calculation can be found elsewhere (Ding et al. 2015, Sun et al. 2014).
Soil and plant analyses
After 40 days growth, P. vittata plants were harvested and washed thoroughly. The plants were then separated into roots, stems, and fronds. Oven-dried fern tissues were grounded and passed through a 1-mm sieve. The total arsenic concentration of plant and soil was analyzed with AFS following microwave-digestion (CEM MARS 6, Matthews, NC, USA) with HNO3/HCl as requirement of EPA Method 3051a (USEPA, 1986). Soil pH was determined in water extract at soil-to-solution ratio of 1:2.5 (1:20 for biochar) by a redox potential depolarization automatic analyzer (FJA-6, Nanjing, China) (China Ministry of Agriculture, NY/T 1377-2007, determination of pH in Soil). The soil available phosphorous was tested by Mehlich 3 method (Mehlich 1984). The cation exchange capacity (CEC) of all soil batch was analyzed by the method described by Liang et al. (2006). To attain the soil buffering capacity, the titration curve was also investigated through description of Xu et al. (2012). Briefly, 2g air-dried samples from each treatment were shaken for 1 h to mix up with the 5 mL solution containing 0, 0.3, 0.5, 1.0, and 1.5 mL of 0.1 M HCl (NaOH), respectively. Then, the suspensions were equilibrated in calorstat for 7 days at 25 °C before pH analysis, followed by daily 2min mixture.
Statistical analysis
All results presented were the average of triplicates ± SD. Differences between treatments were analyzed by one-way analysis of variance (ANOVA) followed by Fisher’s test (p < 0.05) for multiple comparison with SPSS v.21.
Results and discussion
Effect of RBC on As leachability in column test
Frequent acid precipitation in southern China has been contributing to soil acidification, which may consequently induce mobilization of toxic metals in soil substrates (Zhang et al. 2007). In the initial period of acid leaching (the first 20 collections) with a total of 3.15 × 10−3 cmol H+ input (Fig. 2a) or titration with a total of 3 × 10−3 cmol H+ input (Fig. 3), leachate pH of the soils with 2% and 5% RBC (w/w) declined slowly, both of which were consistently higher than that of the unamended control. Afterwards, leachate pH from the soil-2% RBC decreased more steeply with continuous acid leaching while soil-5% RBC still maintained a stable pH higher than 6 (Fig. 2a), indicating a more persistent acid buffering capacity of the soil with higher BC incorporation. Leaching pH of soil was lower than mixture by 0.46–1.74 unit in previous period and went parallel with soil-5% RBC mixture since 17th sampling, which may attribute to the exhausted alkaline component in biochar such as carbonates and the function of soil buffer capacity for acids (Yuan et al. 2011). The pH buffering capacity (pHBC) of media was further calculated through the linear simulation of relationship between acid/base introduction versus corresponding pH with great fitting degrees (Table S1). As a result, the buffering capacity of soil, soil-2% RBC, soil-5% RBC, and RBC were 3.20, 2.94, 3.00, and 1.61 cmol/kg, respectively. Different from previous study (Shi et al. 2018), there was no promotion of soil pHBC under the effect of RBC, which may be credited to the short equilibration time (40 days) in this study compared to other researches (several years).
The eluate As increased proportionally as a function of RBC ratio, with the steep increase occurring within the initial 10 fractions followed by a stable plateau throughout the rest of leaching period (Fig. 2b). The As content in soil-2% RBC mixture ascended rapidly until ninth sample, descending with fluctuation but still keeping a high level beyond 40 μg/L while As of soil-5% RBC mixed media kept rising (up to 123.14 μg/L) and held 5 times arsenic content as much as the control soil column. With few exceptions, leachate As from the RBC-only control was consistently lower than those from the soil control, suggesting the higher labile As in the RBC-amended soil was not derived from RBC itself. Most likely, As fraction of differential lability in the tested soil had been largely changed with RBC application. According to the results by Yin et al. (2017), soil As concentrations associated with water-soluble, surface-adsorbed, and carbonate-bound fractions exhibited consistent increase with RBC amendment, while As complexed by Fe/Al hydrous oxides was markedly reduced. The increased labile pool of As was most probably resulted from the increased soil pH upon RBC application (Fig. 2a), with OH- outcompeting HAsO42−/H2AsO4− for the anion binding sites on Fe/Al hydrous oxides and other soil constituents. This result highlights, on the one hand, the potential risk of soil As mobilization with RBC amendment, especially in areas suffered from persistent acid precipitation and/or soil acidification. While on the other hand, it provides a possibility to improve soil As bioavailability and hence, phytoextractability upon biochar application with As hyperaccumulating plants growth.
Effect of RBC on soil As phytoextraction by P. vittata
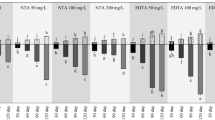
The introduction of RBC contributed to a distinct phytoextraction potential of P. vittata compared to phylotype in native and control soil in HA-expt (Fig. 4a). The As content in frond, stem, and root significantly increased as elevated RBC addition level (p < 0.05) except that As concentrated in stem of soil-5% RBC treatment was lower compared to soil-2% RBC treatment but still higher than control. Specifically, the frond As exhibited significant increase by 22.01–82.78% (p < 0.01) in the RBC treatments than unamended control of HA-expt. This significant promotion caused by RBC at the ratio of 2% and 5% manifested a possibility of advanced phytoextraction using P. vittata combined with RBC. However based on Chinese environmental quality standards for surface water (GB3838-2002), the Class V limit for As in agricultural irrigation water (0.1 mg/L) was partly exceeded by leachate As in the 5% RBC treatment (Fig. 2b), indicating the RBC application ratio in the tested soil should be < 5% for environmental safety. Therefore, less biochar proportion, i.e.,1–3%, was used in the following experiments.
Under distinct RBC ratio, the As accumulation patterns among tissues of P. vittata were altered in LA-expt. With 1–2% RBC amendment, As level in P. vittata root was increased by 3.3–59.85%, in parallel with 1.29–2.10-fold higher As concentration in the aboveground part comparative with control (Fig. 4b). By contrast, both the root and stem As level in P. vittata plants were decreased significantly at higher RBC application ratio (3%) compared to other treatments (p < 0.05), which was accompanied by a 1.27-time higher As concentration in the frond. In comparison, more efficient As transport from root to stem and frond was determined with increasing RBC ratio, with As translocation factor (ratio of As concentration between aboveground tissue and root) increasing from the value of 1.15–3.26 in 1–2% RBC treatment to 3.67 at the presence of 3% RBC. This result elucidated that elevated RBC application can not only increase As uptake by P. vittata but also favor in vivo upward translocation, which was consistent with the higher soil As mobility with increasing RBC ratio (Fig. 2). However, excessive As mobilization due to high rate RBC (3% in this study) deteriorated the uptake capability of P. vittata and consequently the proper incorporation rate of RBC should be taken into consideration in the phytoremediation implementation. Previous studies have shown that P. vittata was featured by its high root exudation composed mainly of phytic acid and oxalic acid (Lessl and Ma 2013, Tu et al. 2004), which could thus cause much enhanced As bioavailability in the highly acidified rhizosphere upon RBC amendment. In view of district translocation rate between As(III) and As(V) in soil-hyperaccumulator system (Wang et al. 2010), whether the RBC treatment has influenced As redox transformation in the rhizosphere of P. vittata is yet to be identified and warrants further investigation.
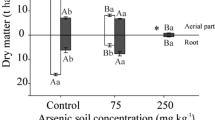
Coinciding with enhanced As uptake and accumulation by P. vittata at the presence of RBC, As removal from RBC-amended soil was increased 1.12–2.28-fold compared to the control. In particular, up to 33.25% of soil As was removed in 1% RBC with P. vittata after 40 days of growth, compared to 14.55% in control, suggesting markedly enhanced phytoextraction efficiency by RBC amendment (Fig. 5). However, the As removal rate of soil-2% RBC showed insignificant variation relative to control. This may be explained by the fact that the phytoremediation was a long-term process (Ali et al. 2013) and the alleviating potential of P. vittata may partly represent as phytostabilization in site instead of phytoextraction (Lessl et al. 2014, Yan et al. 2012), which needed further research with longer culture period and more proper assessment method. The removal rate under the fortification of 1% RBC was 2 times higher than previous study (Niazi et al. 2012). This result highlighted that the mobilization of soil As by alkaline biochar, as identified in the present (Fig. 2) and other works (Beesley et al. 2014, Hartley et al. 2009), can transform into positive promotion on soil As removal by phytoextraction with As hyperaccumulator.
Effect of RBC on As leachability with P. vittata growth based on DGT analysis
The vertical profile of DGT-labile As of both rhizosphere and non-rhizosphere of P. vittata was obtained at end of experiment before harvest (Fig. 6). Without exception, DGT-labile As increased consistently as a function of RBC application ratio, indicating higher As leachability with increasing RBC amount. In particular, apparently higher concentrations of labile As were almost exclusively determined in the non-rhizosphere than rhizosphere, which was in expectation considering the highly efficient As uptake by P. vittata as identified by a number of studies (Caille et al. 2005, McGrath and Zhao 2003, Rascio and Navari-Izzo 2011). Very interestingly, DGT-labile As of non-rhizosphere increased tardily from soil interface, reached an ultimate value around − 20 mm and − 30 mm, respectively, in the treatment below and above 1% RBC ratio, followed by gradual decline. It was demonstrated that As infiltration depth was constrained within the top 20 mm of soil in CK and 1% RBC treatment, while much extended and homogeneous percolation of As throughout the 30mm depth range was recorded at the presence of higher RBC (2–3%). This could be largely resulted from the elevated porosity and coarser soil texture with higher RBC input (Lim et al. 2016), which could facilitate water infiltration and As migration to deeper soil layers with limited root length (Ajayi et al. 2016). The DGT-labile As of rhizosphere and non-rhizosphere soil was significantly increased with increasing RBC ratios (p < 0.01). The maximum values of rhizosphere and non-rhizosphere (minimum values in rhizosphere) in a serial of treatments were 231.77 (114.04), 101.17 (29.39), 92.38 (22.79), and 34.44 (6.95) μg/L, respectively, also diminished with the decreasing amount of RBC amendment. This result signified that P. vittata could partly uptake the extra As but did not surpass the entire As load brought by RBC introduction when relative to rhizosphere in control. Nevertheless, the corresponding minimum values in non-rhizosphere were 128.13, 29.39, 34.69, and 18.74 μg/L, respectively, which was a little variant from the sequence of total soil concentration. This incompatibility was probably consequent upon the opposite impact of biochar. On the one hand, the biochar promoted porosity (Guo 2016), nutrient cycle (Tan et al. 2017), and PGP (plant growth promoting) microbe colonization as well as intercepting pathogens in soil (Zhou et al. 2017) thereby intensifying plant metabolism. On the other hand, biochar immobilized arsenic leading to pernicious influence on plant. To protect groundwater for irrigation, the threshold value for As in groundwater was set at 50 μg/L based on Chinese quality standard for groundwater (Class IV limit) (GB/T14848-2017). As phytoextraction by P. vittata in this work was suggested to be aided with RBC at ratios ≤ 1%, as shown in Fig. 6.
Therefore, for any target field site with specific geological structure and groundwater level, pre-optimization of biochar ratio is much essential before scaled-up application of biochar-aided phytoextraction to secure groundwater quality.
Conclusion
The present study has demonstrated a promising effect of biochar on increasing As uptake by P. vittata, which provides a possible pathway attenuating soil As contamination. Whereas with varying soil property, geological condition as well as root length, pre-optimization of biochar application ratio in field trials is needed to maximize As phytoextraction while avoiding As mobilization into groundwater. Another issue warranting further exploration is how long the promoting effect with biochar can last and the screening of final stabilization method for residual As, which are relating closely to the remediation time frame and reuse type of recovered land soil.
References
Ajayi AE, Holthusen D, Horn R (2016) Changes in microstructural behaviour and hydraulic functions of biochar amended soils. Soil Tillage Res 155:166–175
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals-concepts and applications. Chemosphere 91:869–881
Alloway BJ (2012) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer Science & Business Media, pp 101–104
Althobiti RA, Sadiq NW, Beauchemin D (2018) Realistic risk assessment of arsenic in rice. Food Chem 257:230–236
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Caille N, Zhao FJ, McGrath SP (2005) Comparison of root absorption, translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytol 165:755–761
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Ding S, Jia F, Xu D, Sun Q, Zhang L, Fan C, Zhang C (2011) High-resolution, two-dimensional measurement of dissolved reactive phosphorus in sediments using the diffusive gradients in thin films technique in combination with a routine procedure. Environ Sci Technol 45:9680–9686
Ding S, Han C, Wang Y, Yao L, Wang Y, Xu D, Sun Q, Williams PN, Zhang C (2015) In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res 74:100–109
Ding S, Wang Y, Zhang L, Xu L, Gong M, Zhang C (2016) New holder configurations for use in the diffusive gradients in thin films (DGT) technique. RSC Adv 6:88143–88156
Egene CE, Van Poucke R, Ok YS, Meers E, Tack FMG (2018) Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci Total Environ 626:195–202
GB/T14848-2017 Chinese quality standard for groundwater. National standards of the People’s Republic of China
GB3838-2002 Environmental quality standards for surface water. National standards of the People’s Republic of China
Guo M (2016) Application of biochar for soil physical improvement. In: Guo M, He G , Uchimiya SM (Editors), Agricultural and environmental applications of biochar: advances and barriers. SSSA Special Publication. Soil Science Soc Amer, 677 S Segoe Rd, Madison, Wi 53711 USA, pp. 101-122
Hartley W, Dickinson NM, Riby P, Lepp NW (2009) Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut 157:2654–2662
Hinsinger P, Marschner P (2006) Rhizosphere - perspectives and challenges - a tribute to Lorenz Hiltner 12-17 September 2004 - Munich, Germany. Plant Soil 283: VII-VIII
Irem S, Islam E, Maathuis FJM, Niazi NK, Li T (2019) Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: Effect of soil amendments. Chemosphere 225:104–114
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621
Kuzyakov Y, Bogomolova I, Glaser B (2014) Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific C-14 analysis. Soil Biol Biochem 70:229–236
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota - A review. Soil Biol Biochem 43:1812–1836
Leist M, Casey RJ, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76:125–138
Lessl JT, Ma LQ (2013) Sparingly-soluble phosphate rock induced significant plant growth and arsenic uptake by Pteris vittata from three contaminated soils. Environ Sci Technol 47:5311–5318
Lessl JT, Luo J, Ma LQ (2014) Pteris vittata continuously removed arsenic from non-labile fraction in three contaminated-soils during 3.5 years of phytoextraction. J Hazard Mater 279:485–492
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X, Wu H, Qian Y, Li X, Luo Y (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288
Lim TJ, Spokas KA, Feyereisen G, Novak JM (2016) Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 142:136–144
Ma LQ, Komar KM, Tu C, Zhang WH, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic (vol 409, pg 579, 2001). Nature 411:438–4U3
Mathews S, Ma LQ, Rathinasabapathi B, Natarajan S, Saha UK (2010) Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. Bioresour Technol 101:8024–8030
Matovic D (2011) Biochar as a viable carbon sequestration option: global and Canadian perspective. Energy 36:2011–2016
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416
Moreno-Jimenez E, Esteban E, Penalosa JM (2012) The fate of arsenic in soil-plant systems. In: Whitacre DM (ed) Reviews of Environmental Contamination and Toxicology, vol 215. Reviews of Environmental Contamination and Toxicology, pp 1–37
Niazi NK, Singh B, Van Zwieten L, Kachenko AG (2011) Phytoremediation potential of Pityrogramma calomelanos var. austroamericana and Pteris vittata L. grown at a highly variable arsenic contaminated site. Int J Phytoremediation 13:912–932
Niazi NK, Singh B, Van Zwieten L, Kachenko AG (2012) Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: a long-term study. Environ Sci Pollut Res Int 19:3506–3515
Niazi NK, Bashir S, Bibi I, Murtaza B, Shahid M, Javed MT, Shakoor MB, Saqib ZA, Nawaz MF, Aslam Z, Wang H, Murtaza G (2016) Phytoremediation of arsenic-contaminated soils using arsenic hyperaccumulating ferns. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: Management of Environmental Contaminants, vol 3. Springer International Publishing, Cham, pp 521–545
Ok YS, Chang SX, Gao B, Chung H-J (2015) SMART biochar technology-a shifting paradigm towards advanced materials and healthcare research. Environ Technol Innov 4:206–209
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Scally S, Davison W, Zhang H (2006) Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films. Anal Chim Acta 558:222–229
Shah AH, Shahid M, Khalid S, Natasha SZ, Bakhat HF, Murtaza B, Farooq A, Akram M, Shah GM, Nasim W, Niazi NK (2019) Assessment of arsenic exposure by drinking well water and associated carcinogenic risk in peri-urban areas of Vehari, Pakistan. Environ Geochem Health 41:1–13
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Shi RY, Hong ZN, Li JY, Jiang J, Kamran MA, Xu RK, Qian W (2018) Peanut straw biochar increases the resistance of two Ultisols derived from different parent materials to acidification: A mechanism study. J Environ Manage 210:171–179
Sun Q, Chen J, Zhang H, Ding S, Li Z, Williams PN, Cheng H, Han C, Wu L, Zhang C (2014) Improved Diffusive Gradients in Thin Films (DGT) Measurement of total dissolved inorganic arsenic in waters and soils using a hydrous zirconium oxide binding layer. Anal Chem 86:3060–3067
Tan Z, Lin CSK, Ji X, Rainey TJ (2017) Returning biochar to fields: A review. Appl Soil Ecol 116:1–11
Tang J, Liao Y, Yang Z, Chai L, Yang W (2016) Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J Soils Sediments 16:1519–1528
Tu S, Ma LQ, MacDonald GE, Bondada B (2004) Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction and thiol formation in excised parts of Pteris vittata L. Environ Exp Bot 51:121–131
USEPA (2007) Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, vol. 3051A
Wang X, Ma LQ, Rathinasabapathi B, Liu Y, Zeng G (2010) Uptake and translocation of arsenite and arsenate by Pteris vittata L.: Effects of silicon, boron and mercury. Environ Exp Bot 68:222–229
Wang J, Zeng X, Zhang H, Li Y, Zhao S, Bai L, Su S, Wang Y (2018) Kinetic release of arsenic after exogenous inputs into two different types of soil. Environ Sci Pollut Res 25:12876–12882
Wu W, Yang M, Feng Q, McGrouther K, Wang H, Lu H, Chen Y (2012) Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 47:268–276
Xu R-k, A-z Z, J-h Y, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments 12:494–502
Yan X, Zhang M, Liao X, Tu S (2012) Influence of amendments on soil arsenic fractionation and phytoavailability by Pteris vittata L. Chemosphere 88:240–244
Yang J, Yang J, Huang J (2017) Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean – a field case. Ecol Eng 109:35–40
Ye W-L, Khan MA, McGrath SP, Zhao F-J (2011) Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ Pollut 159:3739–3743
Yin D, Wang X, Peng B, Tan C, Ma LQ (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zeng X, Xiao Z, Zhang G, Wang A, Li Z, Liu Y, Wang H, Zeng Q, Liang Y, Zou D (2018) Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J Anal Appl Pyrolysis 132:82–93
Zhang H, Davison W (2015) Use of diffusive gradients in thin-films for studies of chemical speciation and bioavailability. Environ Chem 12:85–101
Zhang H, Zhao FJ, Sun B, Davison W, McGrath SP (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Zhang J-E, Ouyang Y, Ling D-J (2007) Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67:2131–2137
Zhou H, Zhang D, Wang P, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Crowley D, van Zwieten L, Pan G (2017) Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: a meta-analysis. Agric Ecosyst Environ 239:80–89
Acknowledgement
This work was supported by National Natural Science Foundation of China (No.41977108), Fok Ying Tung Education Foundation for Young Teachers in Higher Education Institutions of China (No.151029), Hunan Young Talents Supporting Program (2017RS3032), and Cultivation Program for National Excellent Youth Science Foundation of Hunan Normal University (XP4180201).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Zheng, C., Wang, X., Liu, J. et al. Biochar-assisted phytoextraction of arsenic in soil using Pteris vittata L. Environ Sci Pollut Res 26, 36688–36697 (2019). https://doi.org/10.1007/s11356-019-06688-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06688-5