Abstract
Organophosphorus pesticides are effective, cheap, and used extensively but can harm aquatic organism and human health. Here, water quality criteria (WQCs) for dichlorvos (DDVP) and malathion (MAL) were derived. Nine aquatic organisms native to China were used in toxicity tests. Published toxicity data for aquatic organisms native and non-native to China were also analyzed. DDVP and MAL WQCs were derived using (log-normal model) species sensitivity distributions. Species sensitivity distribution curves indicated native and non-native species have different sensitivities to DDVP. The sensitivities of native and non-native species to MAL were not different because non-native species data for fewer than eight genera were available, so further research is required. The results indicated that native species need to be considered when deriving WQCs. The criteria maximum concentration (CMC) and criteria continuous concentration (CCC) were 1.33 and 0.132 μg/L, respectively, for DDVP, and 0.100 and 0.008 μg/L, respectively, for MAL. The CMCs for DDVP and MAL derived using ETX 2.0 software and species sensitivity ranks were different from the CMCs obtained using the SSD method because of parameter uncertainties. The DDVP and MAL WQCs were significantly lower than Chinese surface water quality standard thresholds. The results provide basic data for revising these thresholds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPs) are currently the most widely used pesticides in China. OPs are very effective against a wide range of organisms and are cheap (Chen et al. 2016; Wang 2015). The widespread application of large amounts of OPs such as dichlorvos (DDVP) and malathion (MAL) has led to environmental media in large areas being contaminated with these chemicals (Xu et al. 2012). DDVP was detected at 80% of sites in the Pearl River Estuary at which pesticide contamination was assessed (Zhou et al. 2013). Gao et al. (2009) found MAL in 43.5% of surface water samples collected in 2003 and 2004 from > 600 sites in seven major river basins and three major internal river drainage areas in China. OPs in soil can migrate into aquatic systems in runoff (particularly from sloping fields). OPs can persist in the environment and can accumulate in and harm aquatic organisms (Ellison 2012; Guo et al. 2014). In addition, OPs can biomagnify through the food chain (Cholewa et al. 2015; Li et al. 2015). However, the standard thresholds for some OPs (including DDVP and MAL) in the current Chinese “Surface Water Environmental Quality Standard” (MEP 2002) are no longer appropriate for protecting aquatic organisms in either the short or long term (Zhu et al. 2017). Appropriate water quality criteria (WQCs) therefore need to be developed.
Appropriate WQCs are important for protecting and managing the aquatic environment. Good practical and theoretical understandings of the environmental behaviors and toxicities of OPs are required for an environmental protection agency to develop water quality standards (Wang et al. 2016). Unfortunately, managing OPs in aquatic systems in China is difficult because of a lack of data on the toxicities of OPs to aquatic organisms (Zhu et al. 2016). Research on WQCs started late in China and has mainly relied on data from developed countries, particularly the USA (Wu et al. 2010). However, data from outside China may not be appropriate for the Chinese environment because of the differences between native and non-native species (Zheng et al. 2017a; Zhang et al. 2017b). Data on the toxicities of DDVP and MAL to a small number of aquatic organisms found in China are available (Chen et al. 2016; Zhu et al. 2016), but toxicity tests have been performed using few taxa native to China. In United States Environmental Protection Agency (US EPA) guidelines (USEPA 1984) and other guidelines, it is recommended that WQCs for some toxicants should be derived using data for species native to the region of interest. Therefore, there is an urgent need for DDVP and MAL WQCs to be developed using data for aquatic organisms native to China.
This study was focused on DDVP and MAL, which are used in large quantities and are frequently detected in aquatic environmental media (Chen et al. 2016). OP WQCs for freshwater aquatic organisms were derived following US EPA guidelines using at least eight genera with a specified taxonomic diversity (Mu et al. 2013; Qin et al. 2014b). In this study, 17 acute and six chronic toxicity experiments were performed using nine organisms (from several taxa) native to China. The experiments were performed using three fish species (Pseudorasbora parva, Misgurnus anguillicaudatus, and Gobiocypris rarus), a planktonic crustacean (Daphnia magna), a benthic crustacean (Neocaridina denticulata), an insect (Chironomus plumosus), an annelid (Limnodrilus claparedeianus), an amphibian (Bufo gargarizans), and a mollusk (Cipangopaludina cahayensis). The log-logistic species sensitivity distribution (SSD) method developed by the Netherlands National Institute for Public Health and the Environment was used to evaluate the toxicity test data and the other data (RIVM 2007; Jin et al. 2014; Yi et al. 2016). Differences in the sensitivities of species native and not native to China were assessed. The WQCs of DDVP and MAL for freshwater aquatic organisms were derived according to the SSD method based on the log-logistic model.
The aims of the work were as follows: (1) to extend the DDVP and MAL toxicity databases, (2) to compare the sensitivities of native and non-native species to DDVP and MAL, and (3) to derive WQCs for the toxicities of DDVP and MAL to aquatic organisms. The results will benefit environmental risk assessments for DDVP and MAL and will improve the management of DDVP and MAL in natural aquatic environments in China.
Materials and methods
Toxicity data collection and OP selection
Published ecotoxicity data for OPs were obtained by searching the ECOTOX database (http://cfpub.epa.gov/ecotox), the Elsevier publication database (http://www.sciencedirect.com), and the China Knowledge Resource Integrated Database (http://www.cnki.net). The keywords included dichlorvos, malathion, toxicity, risk assessment, water quality criteria, criterion maximum concentration (CMC), criterion continuous concentration (CCC), toxicity endpoints, environmental exposure, detection, and concentration.
The median effect concentration (EC50) and median lethal concentration (LC50) were used as the acute toxicity measurement endpoints. The geometric mean was used if a number of toxicity data were available for one species.
Test chemicals and organisms
The DDVP and MAL used in the study were purchased from Spectrum Analysis Technology Co. (Beijing, China). The basic physicochemical properties of DDVP and MAL are shown in Table 1.
Tap water was dechlorinated by aerating it before being used as the dilution water in an experiment. The aerated tap water was at pH 8.08 ± 0.18 and had a dissolved oxygen concentration of 8.69 ± 0.16 mg/L, a total organic carbon concentration of 0.43 ± 0.13 mg/L, a conductivity of 353 ± 1 μS/cm, and a hardness (as CaCO3) of 150 ± 0.10 mg/L. The algae growth medium (M11) used in the study contained 100 mg/L NaNO3, 10 mg/L K2HPO4, 75 mg/L MgSO4·7H2O, 40 mg/L CaCl2·2H2O, 20 mg/L Na2CO3, 6 mg/L FeC6H5O7, and 1 mg/L Na2EDTA·2H2O and was at pH 8.0 (Lv and Ma 2013).
According to US EPA guidelines, WQCs such as CMCs must be derived from data for at least eight families of aquatic animals in three different phyla (Stephen et al. 1985). In this study, published DDVP and MAL ecotoxicity data were used, and new data were acquired by performing acute DDVP toxicity tests using eight aquatic species native to China and acute MAL toxicity tests using nine aquatic species native to China. The test organisms were obtained from Chaolai Market (Chaoyang, Beijing). D. magna (< 24 h old) from in-house cultures at the Chinese Research Academy of Environmental Sciences were used. Before a toxicity test was performed, the test organisms (except D. magna) were acclimatized in aerated tap water in the laboratory for at least 7 days (Zhang et al. 2017b). The toxicity tests were performed following Organisation for Economic Co-operation and development standard guidelines (OECD 2016).
General test conditions
The formal test concentrations used in the main toxicity tests were determined after performing preliminary experiments. The acute toxicity tests were performed using a static test method, and the chronic toxicity tests were performed using a semi-static method with the test solutions being completely replaced once every two days (OECD 1992, 2012; Zhang et al. 2017a; Wang et al. 2013a). Triplicate toxicity tests were performed at each formal test concentration, one blank control test was performed for DDVP, and one blank control test and one co-solvent control test were performed for MAL. Each test chamber contained seven organisms (Yin and Cai 2013;OECD 1992). The test conditions were described in detail by Yan et al. (2012). The tests were performed using a 14-h light, 10-h dark photoperiod. Each test chamber was immersed in a water bath kept at 22 ± 2 °C. The temperature, dissolved oxygen concentration, and pH in each test chamber were measured each day during each acute toxicity test and at least once each week during each chronic toxicity test. The mortality rates for the blank control and co-solvent control groups were < 10%.
Acute toxicity tests
Each acute toxicity test lasted 48 or 96 h. Test organisms with similar activities and body sizes were used in each test. The mean body length and wet weight were recorded. In the preliminary experiments, tests were performed at a series of concentrations, each a factor of ten different from the next, to allow the concentration ranges used in the full tests to be determined. Using the preliminary experiment results, at least five concentrations between the minimum concentration at which all the organisms died and the maximum concentration at which all the organisms survived were selected for use in the full tests. In a test, P. parva or M. anguillicaudatus were placed in 4 L of a test solution in a 5-L beaker covered with a watch glass for 96 h. D. magna were placed in a 200-mL beaker containing 100 mL of test solution for 48 h. C. plumosus were placed in a 1-L beaker containing 300 mL of test solution for 48 h. L. claparedeianus were placed in 25 mL of test solution in a glass culture dish for 96 h. B. gargarizans were placed in a 2-L beaker containing 1 L of test solution for 96 h. C. cahayensis were placed in a 2 L beaker containing 1 L of test solution for 96 h. G. rarus were placed in 5 L of test solution for 96 h. As specified in Organisation for Economic Co-operation and Development standard 202 (OECD 2004), the test endpoint for D. magna was 48-h EC50 based on immobilization. D. magna unable to swim within 15 s of the test vessel being gently agitated were considered to be immobilized, even if they could still move their antennae. The test endpoint for C. plumosus was the 48-h LC50 based on the survival rate, and the test endpoint for the other aquatic species was the 96-h LC50 based on the survival rate.
The test organisms were not fed during the acute tests. The test organisms were observed daily, and any abnormal behavior was recorded. Dead organisms were removed promptly to avoid the water becoming contaminated. The dissolved oxygen concentration was > 60% of saturation throughout each test. The DDVP and MAL concentrations were within ± 20% of the mean measured concentrations throughout the toxicity tests. The test organism parameters and concentrations used in the main tests are shown in Table S1.
Chronic toxicity tests
Chronic toxicity tests using P. parva
The chronic toxicity tests using P. parva lasted 28 days, and the 28-day EC20s were calculated. In each test, P. parva were placed in a 60-L beaker containing 50 L of test solution. The test solution was completely replaced every 2 days. When replacing the solution, the solution in the beaker was siphoned off and then fresh solution was poured in slowly. This avoided the test organisms being mechanically damaged. During each test, the fish were fed Artemia cysts (supplied by Tianjin Fengnian Aquaculture Co., Tianjin, China) once each day. The toxicity endpoint was the survival rate. Chronic toxicity tests were performed using seven test concentrations (0.000, 0.075, 0.113, 0.169, 0.253, 0.380, and 0.570 mg/L for DDVP and 0.000, 0.266, 0.400, 0.600, 0.900, 1.350, and 2.025 mg/L for MAL). A test was performed in triplicate at each concentration. A water control test was performed for DDVP, and a water control test and a co-solvent control test were performed for MAL. The DDVP and MAL concentrations were determined at the beginning and end of each test.
Chronic toxicity tests using N. denticulata
Each N. denticulata chronic toxicity test lasted 28 days, and the 28-day EC20 was calculated. The shrimps were fed with Artemia cysts (Tianjin Fengnian Aquaculture Co.) once each day, and the test solution was replaced every 2 days. The water was replaced in the same way as described above for the P. parva chronic toxicity tests. The toxicity endpoint was the same as used for the P. parva chronic toxicity tests. The test chemical concentrations used for the N. denticulata chronic toxicity tests were determined from acute toxicity data published by Zhu et al. (2017). Chronic toxicity tests were performed using seven test concentrations (0.000, 0.991, 1.388, 1.943, 2.720, 3.808, and 5.332 μg/L for DDVP and 0.000, 7.755, 15.200, 21.280, 29.792, 41.709, and 58.392 μg/L for MAL). Each test was performed in triplicate. A clean water control test was performed for DDVP, and a clean water control test and a co-solvent control test were performed for MAL. The DDVP and MAL concentrations were determined at the beginning and end of each test.
Chronic toxicity tests using D. magna
Each chronic toxicity test using D. magna (< 24 h old) lasted 21 days, and the 21-day EC20 was calculated. The test organisms were cultured in M4 medium under controlled conditions (OECD 211, 1998). For each test, D. magna were added to a 200-mL beaker containing 100 mL of test solution. The organisms were transferred to fresh medium every 2 days, and newborn juveniles in each beaker were counted and removed every 2 days. A second test vessel was prepared for each test, and the parent animals were transferred to it using a glass pipette when the medium was renewed. During a test, D. magna were fed each day with green algae (Scenedesmus obliquus) at a concentration of 1.0 × 105 cell/mL. The toxicity endpoint was the total number of offspring. The chronic toxicity test concentrations used in the DDVP tests were 0.000, 0.040, 0.080, 0.160, 0.320, 0.640, and 1.28 μg/L, and chronic toxicity test concentrations used in the MAL tests were 0.000, 0.060, 0.120, 0.240, 0.480, 0.960, 1.92, and 3.84 μg/L. Each test was performed in triplicate. A clean water control test was performed for DDVP, and a clean water control test and a co-solvent control test were performed for MAL. The DDVP and MAL concentrations were determined at the beginning and end of each test.
Chemical analyses
The DDVP and MAL concentrations in solution at the beginning and end of each test were determined by high-performance liquid chromatography using an Agilent 1200 instrument (Agilent Technologies, Santa Clara, CA, USA). A sample was passed through a 0.45-μm membrane; then, the sample was analyzed by high-performance liquid chromatography using a ZORBAX Eclipse XDB-C18 column (150 mm long, 0.3 mm i.d., 5 μm particle diameter; Agilent Technologies). The mobile phase was a 70:30 v/v mixture of methanol and water, and the flow rate was 1.0 mL/min. The column temperature was 25 °C. An ultraviolet detector was used, and the wavelength was 230 nm for DDVP and 225 nm for MAL.
The DDVP and MAL method detection limits were 0.1 and 0.4 μg/L, respectively. A test solution containing DDVP or MAL at a concentration lower than the detection limit was passed through a solid-phase extraction system using a previously described method (Zheng et al. 2017b, 2017c). The extract was then evaporated to 1 mL and analyzed. The mean DDVP recoveries were 94.70–109.99%, and the measured concentrations in the acute and chronic toxicity test solutions were 92.59–106.81% of the expected concentrations. The mean MAL recoveries were 96.54–102.82%, and the measured concentrations in the acute and chronic toxicity test solutions were 109.68–115.82% of the expected concentrations. The DDVP and MAL concentrations before and after a water replacement were generally < 20% different, which met the toxicity test requirements (OECD 1994).
Statistical analyses and criteria derivation
Linear regression analyses were performed to calculate the 48-h EC50 and 96-h LC50 values and the 95% confidence intervals. As recommended by the US EPA, chronic toxicity was assessed using the EC20 and the geometric mean of the no observable effect concentration and lowest observable effect concentration, which was only used when the EC20 could not be calculated for the genus of interest (USEPA 2009, 2013, 2016). The chronic toxicity data were calculated using SPSS 18.0 software and were expressed as 21-day EC20 values for D. magna and 28 d-EC20 values for P. parva and N. denticulata. Independent-samples t tests were used to identify significant differences between sets of data. The t tests were performed using SPSS 18.0 software.
The HC5 values (5% hazard concentrations, at which 5% of the aquatic organisms could have been negatively affected by exposure to the chemical of interest) were derived from the DDVP and MAL SSD curves using a log-logistic distribution model (Awkerman et al. 2008; Dyer et al. 2008). ETX 2.0 software (also used by the Netherlands National Institute for Public Health and the Environment (Van Vlaardingen et al. 2005)) and the species sensitivity rank (SSR) (Stephen et al. 1985) were used to verify these estimates. All toxicity data were determined using Origin Lab 9.0 software to construct SSD curves. The CMC was defined as the HC5 value divided by an assessment factor of 2 (Yi et al. 2016). The CCC was extrapolated from the acute data to chronic data based on the final acute chronic ratio (FACR) because of a lack of chronic toxicity data (Dong et al. 2017).
Results and discussion
Toxicities of DDVP and MAL to aquatic organisms native to China
The acute toxicity values for DDVP for eight aquatic species and for MAL for nine aquatic species are shown in Table 2. No mortality was observed in the control groups. The acute toxicity tests showed that D. magna was the most sensitive species to DDVP (48-h EC50 = 2.87 μg/L), and the next most sensitive species (in decreasing sensitivity order) were N. denticulata, L. claparedeianus, P. parva, M. anguillicaudatus, B. gargarizans, C. plumosus, and C. cahayensis. C. cahayensis was the least sensitive species (96-h LC50 = 3.64 × 103 μg/L). In a previous study, the 48-h LC50 of DDVP for D. magna was found to be 1.59 × 102 μg/L, about 55 times higher than the 48-h EC50 found here (Saler and Saglam 2005a). The different results would have been caused by the different toxicity endpoints used in the studies. In the study by Saler and Saglam, the endpoint was lethality. The acute toxicity tests showed that D. magna was the most sensitive species to MAL (48-h EC50 12 μg/L), and the next most sensitive species (in decreasing sensitivity order) were N. denticulata, G. rarus, B. gargarizans, P. parva, C. cahayensis, M. anguillicaudatus, C. plumosus, and. L. claparedeianus. L. claparedeianus was the least sensitive species (96-h LC50 = 4.13 × 104 μg/L). In a previous study, the 48-h LC50 of MAL for D. magna was found to be 1.20 × 102 μg/L, 10 times higher than the 48-h EC50 found here (Saler and Saglam 2005b). Like for DDVP, the different results would have been caused by the different toxicity endpoints used in the studies. Our data indicated that DDVP is more toxic than MAL to D. magna. P. parva and C. cahayensis had similar sensitivities to MAL. L. claparedeianus was the species least sensitive to MAL (96-h LC50 = 4.13 × 104 μg/L).
The chronic toxicity test results are shown in Table 3. The 28-day EC20 for P. parva, 28-day EC20 for N. denticulata, and 21-day EC20 for D. magna were 1.26 × 102, 1.41, and 0.87 μg/L, respectively, for DDVP and 4.73 × 102, 14.30, and 1.13 μg/L, respectively, for MAL. D. magna was the most sensitive species to chronic exposure to both DDVP and MAL.
Published data on the toxicities of DDVP and MAL to freshwater organisms
Published data on the acute toxicities of DDVP and MAL to freshwater organisms were collected and are shown in Tables S2–S5. Acute DDVP toxicity values for 26 species native to China and acute MAL toxicity values for 30 species native to China were found. Chronic toxicity data for DDVP and MAL are not shown because few values were available. Acute DDVP and MAL toxicity data for species not native to China are shown in Tables S6.
All calculated species mean acute values (SMAVs) for the toxicity of DDVP to 26 species (six fish, eight crustaceans, five amphibians, three insects, and four mollusks) and for the toxicity of MAL to 30 species (15 fish, nine crustaceans, three mollusks, one amphibian, and two insects) are shown in Table 4. The most sensitive organism to DDVP was found to be Daphnia pulex, which had a SMAV of 0.068 μg/L, and the least sensitive species was Bufo melanostictus, which had a SMAV of 5.16 × 104 μg/L. The most and least sensitive species to MAL were Gammarus pulex and L. claparedeianus, which had SMAVs of 1.01 and 4.13 × 104 μg/L, respectively. Planktonic crustaceans were generally relatively sensitive to both DDVP and MAL, so it could be used as biological indicators of OP contamination of aquatic environments. It can be seen from Table 4 that invertebrates are more sensitive than vertebrates to DDVP and MAL. Like other insecticides, DDVP and MAL more strongly affect invertebrates, particularly insects and plankton, than vertebrates. It is therefore very important to assess the harm OPs can cause to invertebrates.
It can be seen from the toxicity data shown in Table 4 that the SMAVs for fish species vary widely, indicating that different fish species have different relative sensitivities to DDVP and MAL. In a previous study, P. parva was found to be more sensitive than other fish species to the brominated flame retardant tetrabromobisphenol A, and the LC50 was 0.860 mg/L (Yang et al. 2012). Wang et al. (2013b) also found that P. parva is particularly sensitive to some organic contaminants, particularly pesticides such as triazophos (LC50 < 0.01 mg/L). In contrast, we found that P. parva is not particularly sensitive to DDVP (LC50 = 3.73 mg/L) or MAL (LC50 = 12.4 mg/L). The contrasting toxicity values may be explained by tetrabromobisphenol A and triazophos being very toxic but DDVP and MAL not being very toxic. OP pesticides inhibit acetylcholinesterase (Pope 1999), and there is concern about the potential for OPs in surface water to pose risks to non-target organisms. The chemical-specific sensitivities of particular organisms might be attributed to different contaminants having different modes of action (Raimondo et al. 2010; Wu et al. 2015; Yan et al. 2013).
Differences in the sensitivities of species native and not native to China to DDVP and MAL
Data on the toxicities of DDVP and MAL to species native and not native to China
Toxicity data for species native and not native to China acquired in this study and from previous publications are shown as a box plot in Fig. 1, and the data are shown in detail in Tables 3 and S2–S5. Fish native to China are more sensitive than fish not native to China to DDVP (the 96-h LC50s for native and non-native fish were 2.23 × 102–9.41 × 102 and 2.70 × 102–2.88 × 104 μg/L, respectively). However, crustaceans not native to China are more sensitive than crustaceans native to China to DDVP (the 96-h LC50s for native and non-native crustaceans were 0.068–1.22 × 102 and 0.400–4.00 μg/L, respectively). This could be because data for eight species native to China (including planktonic crustaceans and benthic crustaceans) but only three species not native to China (all benthic crustaceans) were used. The sensitivities of mollusks and insects to DDVP were also different for species native and not native to China. The acute toxicity of DDVP to amphibians native and not native to China could not be compared because insufficient data for species not native to China were available.
Fish native and not native to China had similar sensitivities to MAL except for Alonella spp. (the 96-h LC50s for native and non-native species were 71.6–1.87 × 104 and 34.3–2.31 × 104 μg/L, respectively). The data for crustaceans not native to China were only for benthic crustaceans, and these were found to be more sensitive than native benthic crustaceans (except G. pulex) to MAL. However, native planktonic crustaceans were found to be more sensitive than benthic crustaceans to MAL. Amphibians not native to China were found to be more sensitive than native amphibians to MAL. The toxicities of MAL to other species native and not native to China could not be compared because insufficient data on MAL toxicities to species not native to China were available.
Independent-samples t tests are usually used to identify significant differences between sets of data (Borja et al. 2000). Differences between the toxicities of DDVP and MAL to species native and not native to China were identified by performing independent-samples t tests. A significant difference was found between the DDVP toxicities to species native and not native to China (n1 = 26, n2 = 19, P = 0.002 (i.e., < 0.05)) but no significant difference was found between the MAL toxicities to species native and not native to China (n1 = 30, n2 = 18, P = 0.358 (i.e., > 0.05)).
Overall, species native to China were found to be less sensitive than species not native to China to both DDVP and MAL but the relative sensitivities of native and non-native species to DDVP and MAL varied.
DDVP and MAL SSDs for species native and not native to China
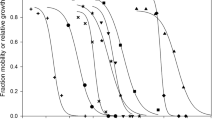
The DDVP and MAL SSDs for species native and not native to China are shown in Fig. 2. Few chronic toxicity data for DDVP and MAL were available, so SSD curves were constructed using acute toxicity data (shown in detail in Tables 4 and S2–S5).
The DDVP SSD for species native to China was to the right of the DDVP SSD for non-native species when the cumulative probability was < 0.5. HC5 values were determined from the fitted curves to allow the sensitivity distributions of the native and non-native species to be compared (Fig. 2). The HC5 for native aquatic organisms (2.66 μg/L) was about eight times higher than the HC5 for non-native aquatic organisms (0.324 μg/L), indicating that aquatic organisms native to China are less sensitive than non-native aquatic organisms to DDVP. The SSD curves for native species were left of the curves for non-native species when the cumulative probability was > 0.50, suggesting that aquatic organisms native to China are more sensitive than non-native aquatic organisms to DDVP. These results were consistent with the results of a study performed by Zheng et al. (2017a) in which native and non-native species were found to have different sensitivities to As(III) in terms of the SSD. We concluded that there were differences between the SSD curves for native and non-native species, particularly for the HC5 of DDVP.
Unlike for DDVP, the MAL SSD curves for species native and not native to China were not markedly different when the cumulative probability was < 0.1. This agreed with the results of independent-samples t tests. The SSD curve for species native to China was right of the curve for non-native species when the cumulative probability was > 0.1, indicating that non-native aquatic organisms are more sensitive than native aquatic organisms to MAL. The HC5 values for native aquatic organisms (0.430 μg/L) and non-native aquatic organisms (0.398 μg/L) were of the same order of magnitude. The data for non-native species were more variable than the data for native species and did not meet the requirement (in guidelines published in 1985) that data should be available for at least eight genera with a specified taxonomic diversity. This introduced some uncertainty into our conclusions, and further research will be required to resolve this.
The different sensitivities of organisms native and not native to China to DDVP and MAL may be attributed to differences in the species composition and the numbers of toxicity data used in the SSD analyses. The DDVP toxicity data for fish accounted for 23% and 53% of the toxicity data used in the native and non-native SSD analyses, respectively. The DDVP toxicity data were for five taxonomic groups of native species and only four taxonomic groups of non-native species. The MAL toxicity data for fish accounted for 77% and 47% of the data used in the non-native and native SSD analyses, respectively. The MAL toxicity data were for five taxonomic groups of native species and only three taxonomic groups of non-native species. Other factors, such as the organism age, could have affected the test results and therefore affected the comparisons of the data for native and non-native species, and further research will be required to address this.
Differences in sensitivity distributions for species native and not native to China have been assessed in some studies. Wu et al. (2015) found that SSDs did not indicate statistically significant differences between native and non-native species. Zheng et al. (2017a) found that SSDs for As(III) indicated a statistically significant difference between the sensitivities of native and non-native species to As(III) (P = 0.029 (i.e., < 0.05)). However, no difference was found between the sensitivities of native and non-native species to As(V) (P = 0.194 (i.e., > 0.05)). No statistically significant difference between the sensitivities of native and non-native species to 2,4-dichlorophenol was found by Jin et al. (2011). Differences in the sensitivities of native and non-native species to a potential toxicant may depend on the nature of the toxicant. Further research will be required to address this. There are therefore uncertainties in and risks associated with using toxicity data for species not native to China when developing WQCs for aquatic organisms in China. Toxicity data for native species should be used to derive WQCs for China. Indeed, in US EPA guidelines, it is recommended that only toxicity data for species native to an area of interest should be used to develop WQCs for that area to minimize uncertainties associated with differences in natural history, aquatic system characteristics, taxonomic groups, habitats, and geographical distributions of species (Stephen et al. 1985). It has been recommended that ecotoxicological data for only aquatic organisms indigenous to Australia or regions adjoining Australia should be used when using the SSD method to derive aquatic ecological benchmarks for Australia (ANZECC and ARMCANZ 2000; Hose and Brink 2004). Wu et al. (2015) found marked differences between the SSD curves for phenanthrene constructed using data for species native and not native to China.
Only aquatic organisms that are native to and widespread in China should be taken into consideration when deriving WQCs for China. Data for some alien species (e.g., Oncorhynchus mykiss) (Lower 2008), however, should be included because cultures of these species have been used widely in toxicological tests performed in China.
Derivation of DDVP and MAL WQCs
Acute toxicity tests were performed using nine aquatic species native to China, and acute DDVP toxicity data for 39 other aquatic organisms (20 native and 19 not native to China) and acute MAL toxicity data for 43 other aquatic organisms (25 native and 18 not native to China) were used. The acute DDVP and MAL toxicity data were used to generate cumulative sensitivity probability distributions using a log-logistic distribution model (Fig. 2) (Yi et al. 2016).
The acute SSD curves for native and non-native species are shown in Fig. 2. Insufficient chronic DDVP and MAL toxicity data were available for chronic SSD curves to be drawn. The HC5 values were calculated from the log-logistic SSD curves to allow DDVP and MAL WQCs to be derived (Zheng et al. 2017b; MEP 2017). The calculated DDVP and MAL HC5 values for aquatic organisms native to China were 2.66 and 0.430 μg/L, respectively. The final CMCs were calculated by dividing the HC5 values by a factor of 2 to allow for uncertainties involved in calculating the HC5 values (Stephen et al. 1985; Chen et al. 2016; Yan et al. 2012). The estimated DDVP and MAL CMCs were 1.33 and 0.215 μg/L, respectively.
Insufficient chronic DDVP and MAL toxicity data were available for the process described above to be used to calculate chronic toxicity WQCs. It is stated in US EPA guidelines that a CCC can be calculated by dividing the CMC by the FACR if inadequate chronic data are available. We used both acute and chronic toxicity data for P. parva, N. denticulata, and D. magna to calculate DDVP and MAL FACRs. The FACR was defined as the geometric mean of the species acute–chronic ratio. The species acute–chronic ratios and FACRs are shown in Table 5. The chronic safety threshold was calculated by dividing the HC5 by the FACR. The estimated DDVP and MAL CCCs were 0.100 and 0.008 μg/L, respectively.
We also calculated DDVP and MAL CMCs using ETX 2.0 (as used by the Netherlands National Institute for Public Health and the Environment) (Van Vlaardingen et al. 2005)) (see Fig. S1) and using the SSR method (Stephen et al. 1985) (see Table S6). The DDVP CMCs estimated using ETX 2.0 and the SSR method were 0.214 and 0.080 μg/L, respectively. The MAL CMCs estimated using ETX 2.0 and the SSR method were 0.890 and 0.535 μg/L, respectively. The DDVP CMCs derived using ETX 2.0 and the SSR method were higher than the DDVP CMCs calculated using the SSD method, whereas the MAL CMCs derived using ETX 2.0 and the SSR method were lower than the MAL CMCs calculated using the SSD method. The method and fitting equation used in the ETX 2.0 software are not specified. HC5 values can only be obtained by inputting toxicity data through the operating interface, and correlation coefficients, root-mean-square errors, and other parameters characterizing the fitting curve are not provided. The SSR method is typically controlled by the four most sensitive genera in the sensitivity distribution and the number of genera in the dataset because the fifth percentile of the distribution is calculated, meaning toxicity values for other species are ignored (Zheng et al. 2017a). These uncertainties lead to variability when estimating HC5 values. Nevertheless, the results given using the SSR method and ETX 2.0 software were in the same order of magnitude as the results of the SSD curve method using a log-logistic model.
A previous study gave MAL CMC and CCC values of 170 and 28 ng/L, respectively (Palumbo et al. 2012). These results were similar to our results. The US EPA calculated that the MAL CCC is 0.1 μg/L, which is 12.5 times higher than the CCC we calculated (USEPA 1986). DDVP CMC and CCC values have not been calculated by the relevant organization in Canada, the European Union, or the Netherlands, possibly because DDVP and MAL are used less in these regions than in others. Our results are therefore a welcome addition to the toxicity data available for DDVP and MAL around the world.
The DDVP and MAL CMCs of 1.33 and 0.215 μg/L, respectively, were calculated taking into account the acute toxic effects of DDVP and MAL on aquatic organisms. These values can be used to evaluate the risks posed by short-term exposure to DDVP or MAL. The DDVP and MAL CCCs (0.100 and 0.008 μg/Lm, respectively) were calculated taking into account the chronic toxic effects of DDVP and MAL on aquatic organisms. The CCCs can be used to evaluate the risks posed by long-term exposure to DDVP or MAL. The CMCs and CCCs (except the MAL CCC) were higher than were found in a study performed by Qin et al. (2014a), in which the DDVP and MAL CMCs were 0.447 and 0.085 μg/L, respectively, and the DDVP and MAL CCCs were 0.030 and 0.056 μg/L, respectively. The current Chinese surface water quality standard thresholds (MEP 2002) for DDVP and MAL are both 50 μg/L. This is markedly higher than the WQCs suggested by our results. Rapid agricultural development in China has led to great increases in the amounts of pesticides produced and used. However, DDVP and MAL standard thresholds have not been updated to meet the need to manage pesticide use to protect the environment and are not appropriate for protecting aquatic organisms in either the short or long term. The current Chinese freshwater threshold for DDVP (50 μg/L) is not appropriate for protecting six sensitive species (D. pulex, Ceriodaphnia dubia, Simocephalus serrulatus, Lymnaea stagnalis, D. magna, and N. denticulata) according to the SMAVs (Table 4). The entire populations of the four most sensitive species (D. pulex, C. dubia, S. serrulatus, and L. stagnalis), which have SMAVs < 1 μg/L, could be destroyed if the Chinese freshwater threshold for DDVP is not reassessed. The situation is the same for MAL. The SMAVs for the seven most sensitive species (G. pulex, C. dubia, D. pulex, Simocephalus vetulus, S. serrulatus, D. magna, and Daphnia carinata) were lower than the MAL threshold (50 μg/L). These species will be harmed by MAL if the Chinese freshwater threshold for MAL is not reassessed.
There is an urgent need to reevaluate the Chinese surface water quality standards for DDVP and MAL. Useful information on which to base revised DDVP and MAL standards is provided here.
Conclusions
Crustaceans were found to be more sensitive than the other test organisms to DDVP and MAL. The SSD curves indicated that there are significant differences between the sensitivities of species native and not native to China to DDVP but not to MAL. However, the MAL data for species not native to China did not meet the requirement that eight genera with a specified taxonomic diversity should be included, so the MAL results were rather uncertain. Further research will be required to address this. Our results support the conclusion that native species should be taken into account when deriving WQCs for an area of interest. The DDVP and MAL CMCs were 1.33 and 0.215 μg/L, respectively, and the DDVP and MAL CCCs were 0.1 and 0.008 μg/L, respectively. These DDVP and MAL WQCs were markedly lower than the current Chinese surface water quality standard thresholds; therefore, the existing Chinese water quality standard thresholds for DDVP and MAL urgently need reevaluation. The data presented here will be useful for revising the DDVP and MAL standards.
References
ANZECC (2000) ARMCANZ. Australian and New Zealand guidelines for fresh and marine water quality, 1st edn. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra
Awkerman JA, Raimondo S, Barron MG (2008) Development of species sensitivity distributions for wildlife using interspecies toxicity correlation models. Environ Sci Technol 42:3447–3452
Borja A, Franco J, Pérez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Pollut Bull 40:1100–1114
Chen H, Zhu J, Li Z, Chen A, Zhang Q (2016) The occurrence and risk assessment of five organophosphorus pesticides in river water from Shangyu, China. Environ Monit Assess 188:614
Cholewa R, Beutling D, Budzyk J, Pietrzak M, Walorczyk S (2015) Persistent organochlorine pesticides in internal organs of coypu, Myocastor coypus. J Environ Sci Health B 50:590–594
Dong L, Zheng L, Yang S, Yan Z, Jin W, Yan Y (2017) Deriving freshwater safety thresholds for hexabromocyclododecane and comparison of toxicity of brominated flame retardants. Ecotoxicol Environ Saf 139:43–49
Dyer SD, Versteeg DJ, Belanger SE, Chaney JG, Raimondo S, Barron MG (2008) Comparison of species sensitivity distributions derived from interspecies correlation models to distributions used to derive water quality criteria. Environ Sci Technol 42:3076–3083
Ellison CA (2012) Assessing the human health risks of exposure to organphosphorus pesticides
Gao J, Liu L, Liu X, Zhou H, Lu J, Huang S, Wang Z (2009) The occurrence and spatial distribution of organophosphorous pesticides in Chinese surface water. Bull Environ Conam Toxicol 82:223–229
Guo Q, Tian H, Mao XX, Huang T, Gao H, Ma JM, Wu JN (2014) Ecological risk assessment of organophosphorus pesticides in aquatic ecosystems of Pearl River Estuary. Environ Sci 35:1029–1034 (in Chinese)
Hose GC, Brink PJVD (2004) Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data. Arch Environ Contam Toxicol 47:511–520
Jin XW, Zha JM, Xu YP, Wang ZJ, Kumaran SS (2011) Derivation of aquatic predicted no-effect concentration (PNEC) for 2,4-dichlorophenol: comparing native species data with non-native species data. Chemosphere 84:1506–1511
Jin X, Wang Y, Jin W, Rao K, Giesy JP, Hollert H, Richardson KL, Wang Z (2014) Ecological risk of nonylphenol in China surface waters based on reproductive fitness. Environ Sci Technol 48:1256–1262
Li CC, Huo SL, Xi BD, Yu ZQ, Zeng XY, Zhang JT, Wu FC, Liu HL (2015) Historical deposition behaviors of organochlorine pesticides (OCPs) in the sediments of a shallow eutrophic lake in Eastern China: roles of the sources and sedimentological conditions. Ecol Indic 53:1–10
Lower N (2008) The effects of contaminants on various life-cycle stages of Atlantic Salmon (Salmo salar L.). University of Portsmouth, Portsmouth
Lv A, Ma W (2013) Comparison on growth situations of three kinds of algae under two kinds of media. Meteorol Environ Res 4:43–45
MEP (2002) Environmental quality standards for surface water. Ministry of Environmental Protection of the People’s Republic of China, GB 3838
MEP (2017) Technical guideline for deriving water quality criteria for the protection of freshwater aquatic organisms, HJ 831
Mu JL, Wang Y, Zhang ZF, Wang J (2013) Marine water quality criteria for cadmium with a view to protecting aquatic life in China and ecological risk assessment. Acta Oceanol Sin 35:137–146
OECD (1992) Guidelines for Testing of Chemicals “Fish, Acute Toxicity Test (203)”. Paris: OECD. Adopted 17th July
OECD (1994) OECD Guidelines for the Testing of Chemicals. Organization for Economic Cooperation and Development
OECD (2004) Guidelines for Testing of Chemicals “Daphnia sp., Acute Immobilization Test (202)”. Paris: OECD. Adopted 13th April
OECD (2012) Guideline for the testing of chemicals: Daphnia magna reproduction test, No. 211
OECD (2016) OECD Guidelines for the Testing of Chemicals, Organization for Economic Co-operation and Development
Palumbo AJ, Tenbrook PL, Fojut TL, Faria IR, Tjeerdema RS (2012) Aquatic life water quality criteria derived via the UC Davis method: I. organophosphate insecticides. Rev Environ Contam Toxicol 216:1–49
Pope CN (1999) Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B 2:161–181
Qin L, Zhou T, Chen X, Zhang Y, Zeng H, Cao Y, Liu Z (2014a) Derivation of aquatic life water quality criteria for five organophosphorus pesticides in freshwater. Environ Chem 8:1269–1275 (in Chinese)
Qin LM, Zhang YH, Cao Y, Yan ZG, Zeng HH, Liu ZT (2014b) Screening native freshwater molluscs for establishing aquatic life criteria. J Agro-Environ Sci 33:1791–1801 (in Chinese)
Raimondo S, Jackson CR, Barron MG (2010) Influence of taxonomic relatedness and chemical mode of action in acute interspecies estimation models for aquatic species. Environ Sci Technol 44:7711–7716
RIVM (2007) Guidance for the derivation of environmental risk limits within the framework of international and national environmental quality standards for substances in the Netherlands (INS). Revision 63:1016–1021
Saler S, Saglam N (2005a) Acute toxicity of DDVP (Dichlorvos) on Daphnia magna Straus, 1820. Pak J Biol Sci 8:40–42
Saler S, Saglam N (2005b) Acute toxicity of malathion on Daphnia magna Straus, 1820. J Biol Sci 5:297–299
Stephen CE, Mount DI, Hansen DJ, Gentile JR, Chapman GA, Brungs WA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. United States Environmental Protection Agency. Office of Research and Development, Washington
USEPA (1984) Ambient Water Quality Criteria for Arsenic-1984. United States Environmental Protection Agency, Washington
USEPA (1986) Quality Criteria for Water, 1986. United States Environmental Protection Agency, Washington
USEPA (2009) National Recommended Water Quality Criteria. United States Environmental Protection Agency, Washington
USEPA (2013) Update of ambient water quality criteria for ammonia [J]. United States Environmental Protection Agency, 2013
USEPA (2016) Update of ambient water quality criteria for cadmium [J]. United States Environmental Protection Agency, 2016
Van Vlaardingen P, Traas T, Wintersen A, Aldenberg T (2005) ETX 2.0. A program to calculate hazardous concentrations and fraction affected, based on normally distributed toxicity data. National Institute for Public Health and the Environment (RIVM), Bilthoven (Rapport 601501028)
Wang LJ (2015) Impacts of organic phosphorus pesticide in water environment for aquatic animals and human and its detection. J Fujian Fish 37:338–344 (in Chinese)
Wang XN, Liu ZT, Yan ZG, Zhang C, Wang WL, Zhou JL, Pei SW (2013a) Development of aquatic life criteria for triclosan and comparison of the sensitivity between native and non-native species. J Hazard Mater 260(Complete):1017–1022
Wang XN, Liu ZT, Yan ZG, Zhang C, He L, Meng SS (2013b) Species sensitivity evaluation of Pseudorasbora parva. Environ Sci 34:2329–2334 (in Chinese)
Wang T, Zhou Y, Bi C, Lu Y, He G, Giesy JP (2016) Determination of water environment standards based on water quality criteria in China: limitations and feasibilities. J Environ Sci 57:127–136
Wu F, Meng W, Zhao X, Li H, Zhang R, Cao Y, Liao H (2010) China embarking on development of its own national water quality criteria system. Environ. Sci Technol 44:7992–7993
Wu JY, Yan ZG, Liu ZT, Liu JD, Liang F, Wang XN, Wang WL (2015) Development of water quality criteria for phenanthrene and comparison of the sensitivity between native and non-native species. Environ Pollut 196:141–146
Xu ZL, Dong JX, Yang JY, Wang H, Jiang YM, Lei HT, Shen YD, Sun YM (2012) Development of a sensitive time-resolved fluoroimmunoassay for organophosphorus pesticides in environmental water samples. Anal Methods 4:3484–3490
Yan ZG, Zhang ZS, Wang H, Liang F, Li J, Liu HL, Sun C, Liang LJ, Liu ZT (2012) Development of aquatic life criteria for nitrobenzene in China. Environ Pollut 162:86–90
Yan ZG, Wang H, Wang YZ, Zhang YH, Yu RZ, Zhou JL, Kenneth MYL, Liu ZT (2013) Developing a national water quality criteria system in China. Water Policy 15:936–942
Yang S, Yan Z, Xu F, Wang S, Wu F (2012) Development of freshwater aquatic life criteria for Tetrabromobisphenol a in China. Environ Pollut 169:59–63
Yi C, Yu S, Song T, Li Y, Liu H, Zhang X, Su G, Bing L, Yu H, Giesy JP (2016) Site-specific water quality criteria for aquatic ecosystems: a case study of pentachlorophenol for Tai Lake, China. Sci Total Environ 541:65–73
Yin HT, Cai LM (2013) Chemical Testing method: biological system effect volume [M]. China Environmental Publishing House, China (in Chinese)
Zhang L, Wei C, Song M (2017a) Comparison of biota species sensitivity distributions to nonylphenol in China and the United States. International Conference on Biological Engineering and Pharmacy
Zhang YH, Zang WC, Qin LM, Zheng L, Cao Y, Yan ZG, Yi XL, Zeng HH, Liu ZT (2017b) Water quality criteria for copper based on the BLM approach in the freshwater in China. PLoS One 12:e0170105
Zheng L, Liu Z, Yan Z, Yi X, Zhang J, Zhang Y, Xin Z, Yan Z (2017a) Deriving water quality criteria for trivalent and pentavalent arsenic. Sci Total Environ 587:68–74
Zheng L, Zhan Y, Yan Z, Zhang J, Li L, Yan Z, Zhang Y, Xin Z, Wu J, Liu Z (2017b) Derivation of predicted no-effect concentration and ecological risk for atrazine better based on reproductive fitness. Ecotoxicol Environ Saf 142:464–470
Zheng L, Liu ZT, Yan ZG, Zhang YH, Yi XL, Zhang J, Zheng X, Zhou JY, Zhu Y (2017c) pH-dependent ecological risk assessment of pentachlorophenol in Taihu Lake and Liaohe River. Ecotoxicol Environ Saf 135:216–224
Zhou M, Shi L, Li QS, Yang X (2013) The concentration and seasonal variation of organophosphorus pesticide residues in the Pearl River estuary. China Environ Sci 33:312–318 (in Chinese)
Zhu Y, Wang F, Zhang Y, Cao Y, Zeng H, Liu Z (2016) A preliminary study on species sensitivity analysis of inhibition effect of three organophosphorus pesticides on acetylcholinesterase in aquatic organisms. Asian J Ecotox 11:211–218 (in Chinese)
Zhu Y, Cao Y, Zhang YH, Zeng HH, Qin LT, Yan ZG, Zheng L, Liu ZT (2017) Toxicity of organophosphorus pesticides to Neocaridina denticulate and species sensitivity analysis. China Environ Sci 37(7):45–753 (in Chinese)
Acknowledgments
This study was financially supported by the National Science and Technology Project of Water Pollution Control and Abatement of China (grant no. 2017ZX07602−003), the National Natural Science Foundation of China (grant no. 51268008), the National Natural Science Foundation of China (grant nos. 21677001 and 21207002), and the Natural Science Foundation of Anhui Province, China (grant no. 1708085MB50).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 472 kb)
Rights and permissions
About this article
Cite this article
Ding, Tt., Zhang, Yh., Zhu, Y. et al. Deriving water quality criteria for China for the organophosphorus pesticides dichlorvos and malathion. Environ Sci Pollut Res 26, 34622–34632 (2019). https://doi.org/10.1007/s11356-019-06546-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06546-4