Abstract
Fixation reactions reduce the concentration of soluble phosphorus (P) and affect crop growth in alkaline calcareous soils. In lab and greenhouse studies, phosphoric acid (PA) or diammonium phosphate (DAP) were evaluated at various P rates (0, 18, 36 and 54 mg kg−1 soil), either as non-mix (designated as NM-PA and NM-DAP, respectively) or after premixing with farmyard manure (FYM) at 400 mg kg−1 soil (designated as PM-PA and PM-DAP, respectively). The amended soil was incubated at 25 °C and 70% water holding capacity for 7 weeks; thereafter, 32P dynamics were measured using the Freundlich kinetic model. A greenhouse study was also conducted using the same thirteen treatments (as used in incubation experiment) and wheat cultivar (Galaxy 2013) was grown following standard agronomic practices. The results showed that application of PM-PA at the highest rate, which caused maximum change in Pr (ΔPr = 59%) in laboratory condition, also produced maximum P uptake by grain (190.3 mg pot−1) and grain yield (44.1 g pot−1) of wheat in greenhouse experiment. Similarly, regression analysis showed that an increase in Pr values caused a corresponding increase in crop parameters. The results suggested that pre-mixing P fertilizer with FYM could be a viable technique to increase P supply and enhance productivity of wheat in alkaline calcareous soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive cropping system in today agriculture is greatly dependent on the use of chemical fertilizers for achieving higher crop yields to meet the food demand of growing population (Klinglmair et al. 2015). Phosphorus (P) is one of the vital plant nutrients and a major limiting factor for crop production owing to widespread deficiency in arable soils (Elser et al. 2007). It has been found that approximately 220 million tons of phosphate rock (PR) is annually mined out whereas accessible reserves of PR are estimated as 69 billion tons all around the globe (Treadwell 2017). Phosphate fertilizers are applied to meet the nutritional demand and compensate its export from soil by the crop harvest (Gilbert 2009). In calcareous soils, recovery of the applied fertilizer rarely exceeds 20%, mostly due to conversion of soluble P into plant-unavailable forms. The soil reactions result in reduced availability of the applied fertilizer to crops, especially at later growth stages. An appropriate P management is required to increase crop utilization of the applied P fertilizer (Hashmi et al. 2017).
Mixing of water-soluble P fertilizers with animal manures has been found effective in increasing P-use efficiency and crop productivity. The manure provides a temporary shield around inorganic P ions, reduces soil reactions and improves P availability to the growing crop. Additionally, mineralization of organic matter provides plant nutrients and improves physicochemical properties of agricultural soils (Odlare et al. 2008). The decomposition process also excretes organic acids which help mobilize soil-bound nutrients (Sharma et al. 2013). However, application of manure as an alternative to chemical fertilizer has certain limitations such as limited availability at farm level and/or its slow nutrient releasing nature (Sharpley and Moyer 2000). The use of manure as a sole P source may also imbalance the addition of other elements and often encounter environmental problems (Hafiz et al. 2016). Mixing inorganic P fertilizers with organic sources seems a feasible approach to maintain optimum P supply for higher crop uptake (Abril et al. 2007).
Irrespective of the source, P application increases plant-available orthophosphate ions (H2PO4−, HPO4=) in soil solution (Messiga et al. 2012). The post-fertilization reactions transfer most of plant-available P onto the soil solids, leaving only a fraction (< 1%) in the soil solution. The release of P ions from solid phase is controlled by diffusion process, and hence, quantifying P release from solid phase to soil solution is important (Frossard et al. 2011, Messiga et al. 2012). The diffusible P ions (Pr) are regulated by the difference in concentration gradient at soil solid-solution interfaces and can be elaborated by accounting the dilution of 32P ions in soil-solution. This technique capable to differentiate P ions already present in the soil solution (Cp) from those entering the solution. This process helps elucidate P fluxes in soil by using the Freundlich kinetic equation (Pr = vCpwtp with Pr < PrLIMIT). This empirical model accurately assesses the size of plant-available pool in soil (Achat et al. 2010, Frossard et al. 1994) and simulates changes in P supply in soil due to fertilizer addition (Stroia et al. 2011).
The present study was conducted to elucidate the effects of mixing phosphate fertilizer with FYM on P availability in soil, P uptake, P-use efficiency and yield of wheat grown in a calcareous (~ 6% CaCO3) soil. The mechanistic approaches as well as applied approaches were found important to appropriately manage organic and inorganic fertilizer sources for optimum wheat productivity.
Materials and methods
Experimental soil
For laboratory incubation and greenhouse experiments, surface soil (0–20 cm) was collected from experimental farm at the Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad (31° 23′ 54.8″ N and 73° 02′ 02.1″ E, 184 m above sea level). The mean monthly temperature reaches as high as 48°C in June and drops down to 4.8 °C in January, while the mean annual rainfall is 375 mm. The soil is a Calcisol with protocalcic nature according to World Reference Base (WRB) classification as it contains carbonates/bicarbonates of calcium and magnesium. Such soils exist in the regions prevailing with high temperature and lesser precipitation/rainfall than the evapotranspiration. Hence, soils pertaining to such environment may also be designated as Aridisols owing to their development in regions of extreme temperature and low rainfall.
Physicochemical analyses of soil, fertilizer and farmyard manure
Soil was air-dried, passed through a 2-mm sieve and stored in plastic containers under laboratory conditions. The soil was analyzed for different physicochemical properties including: soil texture, water-holding capacity (WHC), pH, Electrical Conductivity (EC), plant-available potassium, Olsen P, organic matter (OM) and other parameters (i.e. carbonates, soluble Ca2+ + Mg2+) using standard methods (Table 1) as described by Hashmi et al. (2017).
The composition of used inorganic fertilizers was also determined. Diammonium phosphate (DAP) is a compound fertilizer, it contained N (18%) and P (18%) while its saturated extracts produced alkaline pH (8.1). Phosphoric acid (PA) used in the study was agricultural grade (purity = 57%, pH = 1.0) containing 18% P. The farmyard manure (FYM) contained 0.69% N, 0.1% P and 0.55% K on dry weight basis.
Treatments for lab and greenhouse experiment
Treatments included zero P control, three P fertilizer levels (18, 36 and 54 mg P kg−1) applied either as diammonium phosphate (DAP) or phosphoric acid (PA); phosphate fertilizers were applied either as non-mix (designated as NM-DAP and NM-PA) or after pre-mixing with farmyard manure (FYM) at 400 mg kg−1 (designated as PM-DAP and PM-PA). The FYM was ground and passed through a 2-mm sieve. The mixing was carried out using a blender and water was sprayed to keep moisture content at 10% to avoid dust and make the mixture homogenous. There were thirteen treatments in total with three replicates. During soil preparation, the proposed quantity of FYM was also applied to control and non-mix P fertilizer treatments in order to balance organic matter and nutrient content of soil.
Quantifying diffusible P ions in the soil-solution system
After applying amendments, 500 g soil of each treatment was placed in plastic containers which were arranged in a completely randomized design with three replicates. The soil was incubated for seven weeks at 25 ± 1 °C maintaining the soil moisture at 70% water-holding capacity (WHC) (Yu et al. 2013). A separate sample was used to estimate moisture content for expressing results on oven dry basis.
At the end of incubation, fifteen soil suspensions for each soil were separately prepared. These suspensions involved five P concentrations (0, 10, 25, 50 and 100 mg P as KH2PO4 kg−1 soil) and three time intervals (3, 30 and 300 min) after introducing 32P spike. These also contained 0.1 ml of toluene (as biocide) to avoid microbial activity. The final liquid to solid ratio of 10:1 was obtained by adding distilled water wherever required. The soil suspensions were gently shaken using a revolving shaker for 40 h to achieve steady state condition. The isotopic labelling of P ions in the soil solution was carried out by introducing 0.25 ml of carrier-free 32P radioactive solution (to create radioactivity of 0.1–0.2 MBq). At desired time intervals (3, 30 and 300 min), 5 ml of the suspension was filtered through a disposable syringe filter (0.2 μm) and the filtrate was obtained to analyze for concentration of P in soil solution (Cp) and radioactivity remaining in the solution. The dataset thus achieved for Cp (μg P ml−1) at various reaction intervals (t min) was utilized for quantifying the gross amount of diffusible P ions (Pr, mg P kg−1 soil) by using the Freundlich kinetic equation as follows (Chardon and Blaauw 1998):
The v, w and p parameters were estimated using non-linear regressions, constructed from the experimental dataset. The v parameter is the Pr value at time t = 1 min and for Cp = 1 μg P ml−1; w describes the non-linear increase in Pr with Cp; and p is the non-linear increase in Pr with time (t). This equation is also capable of estimating Pr value for much longer periods (Fardeau 1993). The PrLIMIT value is generally considered lower than the soil inorganic P content (Morel et al. 1994).
Parameterization of fitted kinetic model and assessing ΔPr values
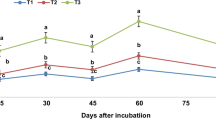
The model equation was developed by plotting the dataset of fast reactions for Cp (mg P L−1), Pr (mg P kg−1 soil) and reaction intervals (t = 3, 30 and 300 min) (Fig. 1). The parameterization of fitted model equation were carried out to extract the desired information as provided in Table S1 and Figure S1 (Cp = 0.5 mg P L−1; t = 1 week, 1 month and 1 year) and Fig. 2 (Cp = 0.5 mg P L−1; t = 1 min). Actually, the treated soil was incubated for 7 weeks, and thereafter, the soil was analysed for Pr values. In this way, the sorbate (applied P) may react with sorbent (soil) surface during incubation time (7 weeks) which could be considered for calculation. Therefore, parameterization of the fitted equation as given in Fig. 2 accounted for time period (49 days+1 min) for reaction. The proposed solution P (Cp = 0.5 mg P L−1) is usually present in arable soil.
The change in Pr (ΔPr) for a given treatment (in relation with a reference treatment) is determined by Pr value of the treatment relative to the reference one, e.g. the ∆Pr for amended soil (relative to control) may be calculated using the following empirical expression:
The ∆Pr can be quantified for various time periods, i.e. 1 week, 1 month and 1 year, and for various concentrations of P ions in solution ranging from 0.1 to 3 mg P L−1 (natural range of solution P in arable soils). The ∆Pr can also be computed for the pre-mix treatments in relation to the respective non-mix application.
Measurement of yield attributes of wheat
Pot experiment was conducted in greenhouse at NIAB under controlled conditions with an average mean temperature of 21 °C and relative humidity of 53% at the time of sowing in November, 2016. Generally, during the experimental season (November, 2016–April, 2017), the mean minimum temperature at night for two months (December–January) was as low as 4 °C while the mean maximum was as high as 20 °C during these months, whereas the day temperature raised up to 39 °C during the month of crop maturity (April, 2017). This study aimed at evaluating the effects of different P fertilizer treatments for P uptake, yield and yield parameters of wheat. The experiment was laid out in completely randomized design (CRD) containing the same thirteen treatments as used for the incubation studies and contained three replications. During the fall cropping season (first week of November 2016), eight seeds of wheat cultivar Galaxy 2013 were sown in each pot containing 5 kg soil and the seedlings were thinned out to four after germination. The doses of Nitrogen (150 mg kg−1 soil) as urea, potassium (60 mg kg−1 soil) as potassium sulphate and zinc (10 mg kg−1 soil) as zinc sulphate were also applied to each pot at sowing. The plants were harvested at maturity (160 days after sowing) in second week of April. The samples taken from the field were threshed manually to record yield parameters, i.e. grain yield and biological (grain + straw) yield.
Phosphorus uptake by plant (grain + straw) and P use efficiency (PUE) were determined for different treatments using the following equations (Syers et al. 2008):
Data analysis and statistics
For incubation study, statistical analysis was performed using SPSS Statistical Software. For each treatment, the standard deviations and estimates of the v, w, and p parameters (for obtaining a fitted Freundlich kinetic equation) and other associated statistics were determined using the maximum likelihood method. This is also known as a nonlinear regression optimization method. For greenhouse study, the data obtained were subjected to analysis of variance (ANOVA) using statistical programme (Statistix 8.1) for windows and significant differences in the treatment means were compared using Tukey’s HSD at 5% level of probability.
The Pearson’s correlation coefficients were determined to assess the nature of relationships between the diffusible P (Pr) and crop parameters. In this regard, step-wise regression analyses were performed by selecting the crop parameters that were most relevant to the increasing Pr values.
Results
Transformation of P ions in soil
The soil samples were analyzed for Pr and Cp values for the three reaction intervals of isotopic dilution and the dataset were fitted using the Freundlich kinetic model. The result described here corresponds to only one regression between Pr and Cp values of control treatment (Fig. 1), whereas parameters of fitted model equations for all the thirteen regressions are presented in Table S1. The results showed that an increase in Pr value was observed with the increase in Cp and reaction time. In previously zero P control soil, the Cp values at steady state condition ranged from 0.07 to 5.91 mg P L−1 (for initial solution P concentration of 0 to 100 mg P L−1), whereas the corresponding Pr values also changed with the change in Cp and time of the reaction elapsed. The maximum Cp (5.91 mg L−1 of the control soil) produced corresponding Pr values of 19.24, 29.76 and 38.70 mg P kg−1 soil for the isotopic dilution time of 3, 30 and 300 min, respectively. The nonlinear regression yielded values of parameters (v, w, and p) required for fitted kinetic model equation (Fig. 1).
The v, w, and p parameters significantly differed for control (zero P), pre-mix and non-mix fertilizer treatments (Table S1). The v parameter varied from 5.08 mg P kg−1 in soil treated with zero P (control) to 6.12 mg P kg−1 in soil treated with highest P rate (54 mg kg−1 soil) as PM-PA. The values of p parameter indicated greater effects of dilution time for pre-mix treatments as compared to non-mix fertilizer amendment. The higher p value in pre-mix treatments exhibited higher availability of P in soil with the passage of time. The p value was recorded lowest (0.139) in the control while highest (0.163) in PM-PA treatment (Table S1).
Variation in P availability under different fertilizer amendments
The relative change in Pr (ΔPr) was calculated for understanding the consequence of P dynamics in fertilized treatment relative to un-fertilized control (Table S1) or any other referred treatment. The Figure S1 elaborates the relationship between ΔPr and Cp (up to 3 mg P L−1) for some reaction intervals, i.e. 1 week (10080 min), 1 month (40320 min) and 1 year (525600 min). In general, fertilized treatments produced higher Pr value than that of un-fertilized control while the pre-mix treatments produced higher Pr values than their respective non-mix treatments. Relative to control, the highest rate of PM-PA produced maximum ΔPr value (59%) followed by that of PM-DAP (52%) while the lowest rate of NM-DAP produced the minimum value (13%), for a reaction interval of 1 year and Cp of 0.5 mg P L−1 (Table S1). Across all the periods of isotopic dilution and explored Cp, the ΔPr values (relative to control) were found positive for all the treated soils. The positive value was obtained due to higher Pr values for treated soils than that of control (Table S1 and Figure S1).
Wheat yield and phosphorus-use efficiency
Irrespective P source or mode of application, wheat grain and biological yields significantly increased with increasing P levels and pre-mix treatments produced higher yield over non-mix treatments (Table 2). In fertilized treatments, the maximum grain and biological yields (44.1 and 96.6 g pot−1, respectively) were obtained at the highest application of PM-PA while the minimum values (30.9 and 81.1 g pot−1, respectively) were recorded at the lowest application of NM-DAP; these values were still significantly higher than that of un-fertilized control (20.1 and 56.4 g pot−1, respectively) (Table 2). Under non-mix fertilizer application, the maximum grain yield (41.1 g pot−1) was produced by NM-PA followed by NM-DAP (40.7 g pot−1) applied at the highest rate (54 mg P kg−1 soil).
The total P uptake by aerial part (grain+straw) was also significantly affected by fertilizer treatments (Table 2); however, pre-mix treatments were found effective in enhancing P accumulation. The maximum P uptake by grain (190.3 mg pot−1) was recorded at the highest rate of PM-PA application followed by PM-DAP (180.8 mg pot−1). The lowest P uptake by grain (51.4 mg pot−1) was observed in the control treatment. In general, non-mix treatments showed lower P uptake when compared with the corresponding pre-mix treatments. In non-mix treatments, maximum P uptake by grain (150.7 mg pot−1) was recorded in NM-PA applied at the highest rate (54 mg P kg−1 soil), whereas the minimum (82.5 mg P pot−1) was observed for NM-DAP applied at the lowest rate (18 mg P kg−1 soil).
The P fertilizer-use efficiency (PUE) was highest (24%) in PM-PA applied at the highest rate; this was statistically at par with PM-PA applied at 18 and 36 mg P kg−1 soil and PM-DAP at 54 mg P kg−1 soil (Table 2). In general, PM-PA showed higher PUE compared to the respective levels of PM-DAP. The lowest PUE (16.5%) was observed at the highest P applied as NM-DAP and it was generally increased with the decrease in P rates.
Discussion
Significance of using the Freundlich kinetic model in quantifying diffusible P ions in soil
Phosphorus in soil-solution and that associated with soil-solid in easily detachable form is considered as plant-available or diffusible P (McBeath et al. 2005, Moradi et al. 2012). Addition of soluble P fertilizer instantly increases concentration of P ions in the soil solution, however, a rapid decrease occurs in solution P either because of plant uptake or transfer onto soil solids (Arvieu and Bouvier 1974), leaving behind a small amount (1% only) of soluble P required by the plant during growth. A gradual release of P ions held by solid phase is therefore required for maintaining P concentration in soil-solution (Hinsinger 2001). Owing to key role of diffusible P in plant nutrition, its accurate assessment is important in precise fertilizer management. The Freundlich kinetic model has been used to quantify diffusible P ions (designated as Pr) for forest soils (Achat et al. 2010), grasslands (Stroia et al. 2007, 2011) and croplands (Morel et al. 2000).
As shown in the present study, the Pr values are affected by the reaction time of applied P with soil surface (usually expressed as time of isotopic dilution) and concentration of P in soil solution (Cp values) (Fig. 1 and Table S1). The diffusion of P ions into soil solution continues until reaching an equilibrium, however, earlier studies have shown limitation to achieve equilibrium even after the reaction time of 44 days (Morel and Fardeau 1991) and 100 days (Barrow and Shaw 1975). Fast reactions are therefore considered in this study to obtain fitted model equation and the values of constants (v, p and w) for further utilization (Sharpley and Ahuja 1983). The fitted kinetic equation was further used to simulate Pr values for longer time periods, i.e. from 1 month to 1 year. The authenticity of the simulated values were ascertained by achieving a strong correlation (R2, 0.99) against experimental Pr values, such comparison in earlier finding also confirmed our results (Messiga et al. 2012, Morel et al. 2014).
Improving diffusible P ions on mixing P-fertilizer with farmyard manure
In calcareous soils, very low efficiency of P fertilizers demands effective management skills to achieve higher plant recovery of applied fertilizers. In the present study, 45-day incubation of FYM-mixed phosphate fertilizer was found to produce higher change in Pr values (ΔPr) over non-mix fertilizer treatments. An increase in P rates (18, 36 and 54 mg P kg−1 soil) also caused an increase in ΔPr values whereas the highest rate of PM-DAP and PM-PA produced maximum ΔPr values of 52 and 59 mg P kg−1 soil, respectively, while, similar rate of non-mix fertilizers, i.e. NM-DAP and NM-PA produced relatively lower ΔPr values, i.e. 27 and 35 mg P kg−1 soil, respectively (Table 2). Therefore, appropriate fertilizer management is important for enhancing P availability to cereal crops grown on alkaline calcareous soils (Hashmi et al. 2017). In a similar study involving 32P labelling technique, plant-available P was analyzed in soil incubated with FYM-amended phosphate fertilizers, where manure-amended PA and DAP (applied at 36 mg P kg−1) produced higher values (114 and 97 mg kg−1 soil, respectively) while un-amended PA and DAP produced lower values (80 and 58 mg kg−1 soil, respectively) in respect of plant available or exchangeable P.
Reaction time is also a key determinant in model based assessment as diffusion is a slow process and hence cumulative content of diffusible P is increased with the passage of time (Morel et al., 2000; Akhtar et al., 2019). In this way, one year reaction time was found to produce higher diffusible P (59 mg P kg−1 soil in PM-PA treatment) while one week reaction time produced lower value (44 mg P kg−1 soil in PM-PA). Higher Pr values in pre-mix treatments have been attributed to the presence of more P as easily detachable (Von Wandruszka 2006) and earlier studies also reported beneficial impacts of OM on P availability (Odlare et al. 2008). The OM has been found to dissolve native P minerals in soil (Johnson and Loeppert 2006), saturate binding sites (Rosling et al. 2007), reduce calcium activity (Jones and Darrah 1994) and obstruct adsorption phenomena (Lundström et al. 1995); this may result in higher P availability for crop growth (Abril et al. 2007, Pizzeghello et al. 2011, 2014). Consistent with the earlier findings (Yu et al. 2013), the present study also exhibited higher P availability in soil receiving FYM-amended P fertilizers. Other similar studies also linked plant-availability of nutrients to OM application in agricultural soils (Khan et al. 2009, Sihi et al. 2017).
Impact of diffusible P (Pr) ions in soil on wheat productivity
The linear regressions (developed from the experimental dataset) clearly describe that an increase in Pr value (irrespective of treatments) increases yield parameters of wheat crop (Fig. 2). The Pr values significantly correlated (p < 0.05) with the grain yield (r = 0.80) and biological yield (r = 0.77). This shows that the P content associated with soil-solids (Pr values) play key role in crop nutrition and they could be used as an indicator of crop productivity (Grant et al. 2005). Similarly, the scientists have also correlated laboratory information regarding soil fertility to the outcomes in terms crop yields under greenhouse conditions. Some earlier similar correlation included: Pr vs P uptake by crop (Achat et al. 2014), labile P vs crop productivity (Grant et al. 2005) labile P vs P uptake by plant (Barber 1995, Barber and Chen 1990) and L value vs grain/biological yield of wheat (Akhtar et al. 2019). However, some deviations in this regard have been attributed to the role of root exudates having significant role in nutrient solubility or the variability in experimental condition of laboratory and greenhouse studies (Marschner et al. 2011). In present studies, manure-amended fertilizer treatments enhanced P supply from soil and hence improved P uptake and grain yield of wheat grown on alkaline calcareous soils (Nziguheba et al. 1998).
Conclusion
The results of present study suggest combined application of inorganic phosphate with FYM as an effective technique to improve plant-available P in soil and agronomic efficiency of P fertilizer in wheat production. Thus, integrated use of fertilizer sources is recommended for enhancing P uptake and increasing yield parameters of wheat. This technique achieves higher PUE in wheat grown on alkaline calcareous soils, especially when chemical P fertilizer is used in liquid form.
References
Abril A, Baleani D, Casado-Murillo N, Noe L (2007) Effect of wheat crop fertilization on nitrogen dynamics and balance in the Humid Pampas, Argentina. Agric Ecosyst Environ 119:171–176
Achat DL, Bakker MR, Zeller B, Pellerin S, Bienaimé S, Morel C (2010) Long-term organic phosphorus mineralization in Spodosols under forests and its relation to carbon and nitrogen mineralization. Soil Biol Biochem 42:1479–1490
Achat DL, Sperandio M, Daumer M-L, Santellani A-C, Prud'Homme L, Akhtar M, Morel C (2014) Plant-availability of phosphorus recycled from pig manures and dairy effluents as assessed by isotopic labeling techniques. Geoderma 232:24–33
Akhtar M, Ikram W, Mahmood T, Yousaf S, Gillani SMW, Ejaz A (2019) Improving soil phosphorus supply and wheat yield with manure-amended phosphate fertilizer. Exp Agric:1–11
Arvieu J, Bouvier O (1974) Chemical processes in the evolution of phosphates in calcareous soils. Science du Sol 74:207–224
Barber SA, Chen J-H (1990) Using a mechanistic model to evaluate the effect of soil pH on phosphorus uptake. Plant Soil 124:183–186
Barber SA (1995) Soil nutrient bioavailability: a mechanistic approach. John Wiley & Sons
Barrow N, Shaw T (1975) The slow reactions between soil and anions: 2. Effect of time and temperature on the decrease in phosphate concentration in the soil solution. Soil Sci 119:167–177
Chardon W, Blaauw D (1998) Kinetic Freundlich equation applied to soils with a high residual phosphorus content. Soil Sci 163:30–35
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fardeau J (1993) Available soil phosphate: its representation by a functional multiple compartment model [conceptual model, pool, buffering capacity]. Agronomie (France)
Frossard E, Morel J, Fardeau J, Brossard M (1994) Soil isotopically exchangeable phosphorus: a comparison between E and L values. Soil Sci Soc Am J 58:846–851
Frossard E, Achat DL, Bernasconi SM, Bünemann EK, Fardeau J-C, Jansa J, Morel C, Rabeharisoa L, Randriamanantsoa L, Sinaj S (2011) The use of tracers to investigate phosphate cycling in soil–plant systems. In: Phosphorus in Action. Springer, pp 59–91
Gilbert N (2009) Environment: The disappearing nutrient. Nature 461:716–718
Grant C, Bittman S, Montreal M, Plenchette C, Morel C (2005) Soil and fertilizer phosphoru s: Effects on plant P supply and mycorrhizal development. Can J Plant Sci 85:3–14
Hafiz N, Adity SM, Mitu SF, Rahman A (2016) Effect of manure types on phosphorus sorption characteristics of an agricultural soil in Bangladesh. Cogent Food Agric 2:1270160
Hashmi ZUH, Khan MJ, Akhtar M, Sarwar T, Khan MJ (2017) Enhancing phosphorus uptake and yield of wheat with phosphoric acid application in calcareous soil. J Sci Food Agric 97:1733–1739
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Johnson SE, Loeppert RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Khan AA, Jilani G, Akhtar MS, Naqvi SMS, Rasheed M (2009) Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci 1:48–58
Klinglmair M, Lemming C, Jensen LS, Rechberger H, Astrup TF, Scheutz C (2015) Phosphorus in Denmark: national and regional anthropogenic flows. Resour Conserv Recycl 105:311–324
Lundström U, Breemen NV, Jongmans A (1995) Evidence for microbial decomposition of organic acids during podzolization. Eur J Soil Sci 46:489–496
Marschner P, Crowley D, Rengel Z (2011) Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol Biochem 43:883–894
McBeath T, Armstrong R, Lombi E, McLaughlin MJ, Holloway R (2005) Responsiveness of wheat (Triticum aestivum) to liquid and granular phosphorus fertilisers in southern Australian soils. Soil Research 43:203–212
Messiga AJ, Ziadi N, Bélanger G, Morel C (2012) Process-based mass-balance modeling of soil phosphorus availability in a grassland fertilized with N and P. Nutr Cycl Agroecosyst 92:273–287
Moradi N, SADAGHIANI MR, Sepehr E, MANDOULAKANI BA (2012) Effects of low-molecular-weight organic acids on phosphorus sorption characteristics in some calcareous soils. Turk J Agric For 36:459–468
Morel C, Fardeau J (1991) Phosphorus bioavailability of fertilizers: a predictive laboratory method for its evaluation. Fertil Res 28:1–9
Morel C, Tiessen H, Moir J, Stewart J (1994) Phosphorus transformations and availability under cropping and fertilization assessed by isotopic exchange. Soil Sci Soc Am J 58:1439–1445
Morel C, Tunney H, Plénet D, Pellerin S (2000) Transfer of phosphate ions between soil and solution: perspectives in soil testing. J Environ Qual 29:50–59
Morel C, Ziadi N, Messiga A, Belanger G, Denoroy P, Jeangros B, Jouany C, Fardeau J-C, Mollier A, Parent L-E (2014) Modeling of phosphorus dynamics in contrasting agroecosystems using long-term field experiments. Can J Soil Sci 94:377–387
Nziguheba G, Palm CA, Buresh RJ, Smithson PC (1998) Soil phosphorus fractions and adsorption as affected by organic and inorganic sources. Plant Soil 198:159–168
Odlare M, Pell M, Svensson K (2008) Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag 28:1246–1253
Pizzeghello D, Berti A, Nardi S, Morari F (2011) Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric Ecosyst Environ 141:58–66
Pizzeghello D, Berti A, Nardi S, Morari F (2014) Phosphorus-related properties in the profiles of three Italian soils after long-term mineral and manure applications. Agric Ecosyst Environ 189:216–228
Rosling A, Suttle K, Johansson E, van Hees PA, Banfield J (2007) Phosphorous availability influences the dissolution of apatite by soil fungi. Geobiology 5:265–280
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:1
Sharpley A, Ahuja L (1983) Main content area A diffusion interpretation of soil phosphorus desorption. Soil Sci 135:322–326
Sharpley A, Moyer B (2000) Phosphorus forms in manure and compost and their release during simulated rainfall. J Environ Qual 29:1462–1469
Sihi D, Dari B, Sharma DK, Pathak H, Nain L, Sharma OP (2017) Evaluation of soil health in organic vs. conventional farming of basmati rice in North India. J Plant Nutr Soil Sci 180:389–406
Stroia C, Morel C, Jouany C (2007) Dynamics of diffusive soil phosphorus in two grassland experiments determined both in field and laboratory conditions. Agric Ecosyst Environ 119:60–74
Stroia C, Morel C, Jouany C (2011) Nitrogen fertilization effects on grassland soil acidification: consequences on diffusive phosphorus ions. Soil Sci Soc Am J 75:112–120
Syers J, Johnston A, Curtin D (2008) Efficiency of soil and fertilizer phosphorus use. FAO Fertilizer and Plant Nutrition Bulletin 18
Treadwell J (2017) Phosphorus and organic waste management: investigating the potential for phosphorus recovery and recycling. McGill University Libraries
Von Wandruszka R (2006) Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochem Trans 7:1
Yu W, Ding X, Xue S, Li S, Liao X, Wang R (2013) Effects of organic-matter application on phosphorus adsorption of three soil parent materials. J Soil Sci Plant Nutr 13:1003–1017
Funding
This study was financially supported by the Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad; Government College University, Faisalabad, and the Higher Education Commission (HEC) of Pakistan under NRPU Project No. 20-3653/NRPU/R&D/HEC/14/437.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Ikram, W., Akhtar, M., Morel, C. et al. Phosphate fertilizer premixing with farmyard manure enhances phosphorus availability in calcareous soil for higher wheat productivity. Environ Sci Pollut Res 26, 32276–32284 (2019). https://doi.org/10.1007/s11356-019-06468-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06468-1