Abstract
Purification effects of constructed rapid infiltration system with two main fillers (coarse sand or medium coarse sand) and different addition proportion (5%, 10%, or 15%) modifiers (sponge iron, blast furnace slag, or zeolite) on rainwater runoff were studied through filter column tests. A set of constructed rapid infiltration system test device was designed, which included 9 rainwater filter columns. The test results showed that the permeability of artificial fillers blended with modifiers could have the promotion with varying degrees. There were differences in the characteristics of the modifiers, so the artificial fillers blended with different modifiers had a significant difference for the purification effects on each pollutant. In view of the overall situations, the pollutant removal effects of artificial fillers with two or more modifiers had a smaller gap, and the reduction effects were good, ranging from 38.95 to 46.25% when the main filler is coarse sand and from 46.29 to 49.46% while main filler is medium coarse sand. It was worth noting that the artificial fillers blended with sponge iron showed a slight harden after prolonged used; however, it had little influence on the permeability and water purification effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rainwater plays a very important role in urban water cycle and regional water cycle system. It can adjust and supplement groundwater resources and improve urban ecological environment. The acceleration of urbanization has resulted in a sharp increase in impervious land area. A lot of impervious land area hinders infiltration of rainwater, causes the runoff total quantity increases, and shortens the confluence time. They exacerbate the hydrologic and environmental problems of urban waterlogging and non-point source pollution (Li et al. 2016a). At present, low impact development (LID) has been widely recognized as a technical system of urban water sustainable management and control (Davis 2005). The system can effectively control the total runoff, runoff peak, and runoff pollution through the infiltration, storage, purification, adjustment, and transfer of rainwater (Nguyen et al. 2019). As a typical LID technology, constructed rapid infiltration facilities (include seepage wells, seepage ponds and so on) are mainly applied to the surrounding green spaces of buildings, roads, and parking lots in the building district. If the influent water quality is poor, the rainwater should be pre-processed by planting grass, vegetation buffer, or rain garden before infiltration (Ministry of Housing and Urban-Rural Development of the People’s Republic of China 2015). At present, experts and scholars in the industry have done a lot of research on LID technology, such as bioretention (Wang et al. 2016b), infiltration pavement (Kamali et al. 2017), and green roof (Berardi et al. 2014), but there are few studies on constructed rapid infiltration facilities. The constructed rapid infiltration facilities are widely used in the treatment of rural domestic sewage, urban domestic sewage, and highway drainage (Fang et al. 2018; Wang et al. 2010), but less in rainwater treatment.
In response to the above situations, based on the filter column tests, this research mainly studies the infiltration and purification effects of constructed rapid infiltration facilities with two main fillers (coarse sand or medium coarse sand) and different addition proportions (5%, 10%, or 15%) modifiers (sponge iron, blast furnace slag, or zeolite) on rainwater runoff. This study expects to recommend several high-efficiency artificial fillers suitable for constructed rapid infiltration facility.
Materials and methods
Natural survey of the study area
The study area is located in Xi’an City, Shaanxi Province, in the Guanzhong Basin in the middle of the Yellow River Basin. In winter and spring, climate is affected by the northwest airflow, which is cold and drier. In summer and autumn, climate is affected by the southeast airflow. It is hot and rainy, and the rainfall is unevenly distributed. The annual average temperature in the plain area of the whole city is 13 °C. The average rainfall in all districts and counties of the city is 537.5~1028.4 mm, and the average annual precipitation in the urban area is 583.7 mm. The average annual rainfall days of all districts and counties in the city is 88~105 days, the average annual rainfall days in urban areas is 96.6 days, and the longest continuous rain days are 13~19 days (Li et al. 2016b).
Test device
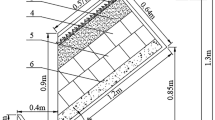
The test device, containing 18 filter columns, is located at the campus of Xi’an University of Technology, Xi’an, China. Each filter column has 120-cm height and 30 cm of diameter. From top to bottom, the filter column is 15-cm aquifer, 90-cm artificial filler layer, and 15-cm drainage layer. The artificial filler was composed of two parts mixed in different proportions (volume ratio). These two parts included main filler (sand) and modifiers (zeolite, blast furnace slag, sponge iron). Different artificial fillers were prepared by adding 5%, 10%, and 15% zeolite, blast furnace slag, and sponge iron into main fillers.
The physical and sectional drawings of the test device are shown in Figs. 1 and 2.
Test scheme
Proportion scheme of artificial fillers
Artificial fillers were prepared by mixing main fillers and modifiers in different proportions. The specific mixing scheme is shown in Table 1.
Influent water quantity design scheme
In this study, three recurrence periods of 0.5a, 1a, and 2a were chosen, and the duration of rainfall was 90 min. The main purpose of this study was to serve the actual project, so according to the actual project demand and relevant information, the confluence ratio was set at 150:1. In practical projects, the underlying surface includes 86.36% concrete impervious surface and 13.64% green space (Table 2).
Finally, the three design water quantities were determined: 40.5 L, 135 L, and 225 L.
Influent water quality design scheme
Constructed rapid infiltration facilities are generally used in roadside, park green space, and green space of building district to absorb rainwater runoff that cannot be absorbed by itself. Because the water quality difference of three kinds of underlying surface rainwater runoff was relatively large, artificial simulated rainwater was prepared according to three kinds of water quality concentration: high, medium, and low. The pollutants were considered including chemical oxygen demand (COD), nitrate nitrogen (NO3-N), ammonia nitrogen (NH3-N), total nitrogen (TN), total phosphorus (TP), copper ion (Cu2+), zinc ion (Zn2+), and cadmium ion (Cd2+). The water distribution concentration of each pollutant is determined as shown in Table 3.
Design of test scheme
The combination of water quantity (large, medium, and small) and water quality (high, medium, and low) was determined. The test scheme is shown in Table 4.
Each filter column would be tested nine times (test 1 to test 9). The inlet time, outlet time, and overflow start time would be recorded for each filter column.
Sample detection methods
The pollutants detected in this study include COD, NO3-N, NH3-N, TN, TP, Cu, Zn, and Cd. The detection methods for each pollutant are shown in Table 5.
Physicochemical properties of fillers
Physical properties of artificial fillers
In this study, the permeability coefficient of fillers was tested. The results showed that the variation range of permeability coefficient was 2.160 to 15.954 cm/min. That was to say, the permeability coefficients of these 18 kinds of fillers were all within the range of 10−3~10−4 m/s.
Physicochemical properties of modifiers
There were three modifiers used in this study: sponge iron, blast furnace slag, and zeolite.
Sponge iron is produced by reduction of iron scale below the melting temperature. It has similar composition to ordinary iron filings, and is composed of pure iron and Fe3C and other impurities (Mn, Cr, Ni, CaO, MgO, etc.). Its surface is porous, rough, and spongy, so it has larger surface area, higher porosity, and faster iron dissolution rate than ordinary iron (Li et al. 2016c). Because the electrode potential of Fe3C and other impurity particles is higher than that of Fe, when sponge iron is in electrolyte solution, it forms numerous corrosive micro batteries. The hydrates of Fe2+ formed by electrode reaction and Fe3+ formed by further oxidation have strong adsorption-flocculation activity (Zafarani et al. 2014).
Blast furnace slag is a by-product discharged from blast furnace during pig iron smelting. In terms of chemical composition, blast furnace slag belongs to silicate material. The chemical composition of blast furnace slag is similar to that of ordinary Portland cement, mainly silica (SiO2), calcium oxide (CaO), magnesium oxide (MgO), alumina (Al2O3), etc. (Cui et al. 2014). SiO2 exists in a crystal structure (SiO4)4− in a solution, and this special porous crystal structure can promote exchange and adsorption between ions. The blast furnace slag is mostly composed of an amorphous glass body, which is a phase separation glass body. The separated continuous phase is a calcium-rich phase with poor chemical stability, which is the main source of blast furnace slag activity (Westholm 2010).
Zeolite is a water-bearing aluminosilicate mineral, which composed of four parts of SiO2, Al2O3, H2O, alkali, and alkaline earth metal ions. It has a three-dimensional framework of silica-oxygen tetrahedron and alumina-oxygen tetrahedron. Its main component is SiO2. Because these silicon (aluminum) oxygen tetrahedrons are interconnected by oxygen atoms at the vertex, and the aluminum atoms have only a negative charge of trivalence lower than that of the surrounding oxygen, the aluminum-oxygen tetrahedron exhibits a negative charge to the outside world. In general, alkali metal and alkaline earth metal ions are used to balance the negative electricity. The combination of these ions with aluminosilicate is weak, which shows great fluidity and is easy to be replaced by other cations. In addition, there are many even-sized holes and channels inside the zeolite. The holes connect with each other through open channels, which makes the zeolite have a huge specific surface area, up to 400−80 m2/g (Zhou and Boyd 2014).
Results and discussion
The data obtained from the nine experiments carried out on 18 filter columns was analyzed. Under different influents and concentrations, the effects of main filler type, modifier type, mixing ratio of main filler and modifier on permeability, and pollutant purification of artificial fillers were studied.
Permeability analysis of different artificial fillers
The permeability of the artificial filler is evaluated by the permeability coefficient of the artificial filler, the effluent time of each soil column, the beginning time of the overflow, and the total amount of the overflow.
As shown in Figs. 3 and 4, the permeability coefficient of the filler increased obviously after adding the modifier, and the permeability coefficient of the coarse sand + modifier was higher than that of the medium coarse sand + modifier. The addition ratio of modifier has a significant effect on the permeability coefficient of artificial filler. The main filler is coarse sand. The permeability coefficient of the artificial filler with 10%, 30%, and 45% modifier increased by 2.512 cm/min, 5.954 cm/min, and 9.668 cm/min, respectively, compared with the artificial filler without modifier. The main filler is medium coarse sand. The permeability coefficient of the artificial filler with 10%, 30%, and 45% modifier increased by 0.913 cm/min, 2.273 cm/min, and 4.644 cm/min, respectively, compared with the artificial filler without modifier.
It can be seen from Table 6 that after the addition of the modifier, the average water discharge time of the artificial filler will be advanced to different extents, and the average start overflow time and the average overflow flow have different degrees of delay and decrease. This indicates the permeability of artificial fillers blended with modifiers would be improved with varying degrees.
Purification effects of different artificial fillers on pollutants
Purification effects of different artificial fillers on COD
As can be seen from Figs. 5 and 6, the average about COD load reduction rates of artificial fillers with coarse sand and medium coarse sand as main fillers were 24.37% and 22.29% respectively. Medium coarse sand has no advantage. After filling sponge iron, the removal rate of COD by artificial fillers with coarse sand and medium coarse sand as main fillers decreased significantly, from 24.74%, 19.28% to 13.45% and 12.49% respectively. Compared with 1# and 2#, the load reduction rate of artificial fillers 5# and 6# with blast furnace slag as modifier increased by 2.71% and 3.62% respectively. Compared with 1# and 2#, the load reduction rate of artificial fillers 7# and 8# with zeolite as modifier increased significantly by 5.45% and 9.52% respectively. Load reduction rate of COD decreases in varying degrees by blending the mixing ratio of blast furnace slag and zeolite. When the blending ratio of the main filler is 90%, and the blast furnace slag and zeolite are respectively 5%, the removal effects of the artificial filler on COD are the highest, which is 31.86% (coarse sand) and 33.91% (medium coarse sand), respectively. Compared with 1# and 2#, the artificial fillers 15#, 17#, 16#, and 18# which increase the blending ratio of the modifier have little change in COD removal effects, and there has even been a decline. In summary, 90% main filler +5% blast furnace slag +5% zeolite was the best for COD load reduction compared with other blending schemes.
Because the constructed rapid infiltration facilities mainly use natural river sand and large particle modifier as filler, the microbial content is less. The removal of COD mainly depends on the adsorption of the filler. The reason why the artificial filler with sponge iron as a modifier has a poor effect on COD load reduction may be that the sponge iron itself has poor adsorption performance for COD. After 10% of the main filler was replaced by sponge iron, the permeation rate of the artificial filler was increased, and the contact time between the filler and the pollutant was shortened, so the reduction effects were reduced. The reason for the improvement of the removal efficiency of artificial fillers whose modifier is blast furnace slag or zeolite may be that the better adsorption of COD by zeolite and blast furnace slag can compensate for the decrease of the reduction rate caused by the shorter contact time. Sponge iron needs acidic environment to remove COD. Blast furnace slag and zeolite can remove COD in neutral environment. The experimental environment was neutral, so the adsorption capacity of the sponge iron to COD was poor.
Purification effects of different artificial fillers on NO3-N
As can be seen from Figs. 7 and 8, the average about NO3-N load reduction rates of artificial fillers with coarse sand and medium coarse sand as main fillers were 25.48%, 26.84% respectively. Medium coarse sand has no advantage. After the addition of the modifier, the effects of the artificial filler on the removal of NO3-N was significantly improved. Especially, the removal efficiency of NO3-N by artificial filler with only sponge iron was improved obviously. The main filler was coarse sand and medium coarse sand, which increased from 20.66%, 22.82% to 30.86% and 30.85% respectively. Reducing the blending ratio of sponge iron will reduce the removal effects of NO3-N. The artificial filler with coarse sand as main filler decreased by 3.93%~5.36%, and the artificial filler with medium filler coarse sand decreased by 2.83%~3.77%. The modifier was blast furnace slag, and the artificial fillers with coarse and medium coarse sand were increased from 20.66% and 22.82% to 26.11% and 25.65%, respectively. Reducing the blending ratio of blast furnace slag has little effects on the removal effects of NO3-N, and the variation range is between 0.61%~1.34% (coarse sand) and 0.21%~0.72% (medium coarse sand). The modifier was zeolite, and the artificial fillers with coarse and medium coarse sand were increased from 20.66% and 22.82% to 25.65% and 27.23%, respectively. Reducing the blending ratio of zeolite has little effects on the removal effects of NO3-N, and the variation range was between 0.88%~1.28% (coarse sand) and 0.78%~1.09% (medium coarse sand). The removal efficiency of NO3-N was not improved obviously by increasing the mixing ratio of modifiers, and even decreased. The main reason may be that the proportion of modifier increases, the permeability of artificial fillers was improved, the contact time between pollutants and fillers was shortened, and finally the removal effects were reduced. In summary, 90% primary filler +10% sponge iron was the best for NO3-N load reduction.
The artificial filler added with sponge iron had a good effect on the removal of NO3-N, and its strong reducibility was an important reason. The products of sponge iron to reduce NO3-N are ammonia nitrogen, nitrous nitrogen, nitrogen, and other nitrogen-containing gases (Tang et al. 2007). Studies had shown that ammonia nitrogen was the main product, and the main reactions are as follows:
A small amount of NO2-N was produced in the reaction, which was very unstable and easy to be reduced by iron and its corrosives. Therefore, there is no need to worry about the accumulation of NO2-N. Under the premise that the blending ratio of the modifier was constant, increasing the dosage of sponge iron would increase the removal effects of NO3-N. The main reason is that the increase of the dosage of sponge iron not only increases the total surface area of sponge iron, but also enables sponge iron to provide more reaction sites. At the same time, it also increases the number of free iron ions that can participate in the reaction, increases the reaction opportunity between NO3-N and sponge iron, and ultimately improves the removal effects of NO3-N (Rodríguez-Maroto et al. 2009).
The adsorption of nitrate nitrogen by blast furnace slag and zeolite is mainly physical adsorption. The intermolecular force between adsorbent and adsorbate plays a major role in the adsorption process. The removal of nitrate nitrogen by blast furnace slag and zeolite mainly depends on the adsorption of fillers. Solid-liquid adsorption can be divided into three stages: fast adsorption, slow adsorption, and adsorption equilibrium. The first stage is the diffusion of pollutants from liquid membrane to the surface of filler (membrane diffusion), the second stage is the diffusion process of pollutants in the pore of filler particles, and the third stage is the adsorption process of pollutants on the inner surface of filler particles. The rate of adsorption reaction is very fast. The total adsorption process is mainly controlled by the first and second stages. Adsorption reaction is controlled by membrane diffusion at the beginning and internal diffusion at the end of adsorption (Wang et al. 2014).
Purification effects of different artificial fillers on NH3-N
As can be seen from Figs. 9 and 10, the average about NH3-N load reduction rates of artificial fillers with coarse sand and medium coarse sand as main fillers were 15.91%, 28.19% respectively. Medium coarse sand showed great advantage. The artificial filler that only used sponge iron as a modifier had a negative load reduction rate for NH3-N. The reason may be that sponge iron can reduce NO3-N to NH3-N, so that the content of NH3-N in artificial rainwater is greatly increased, and finally the effluent concentration is greater than the influent concentration. The effects of artificial fillers 5# and 6# using only blast furnace slag as modifier on NH3-N load reduction were not much different from that of 1# and 2#, which increased by − 0.67% and 5.29% respectively. The artificial fillers 7# and 8# using only zeolite as a modifier have a significant effect on the reduction of NH3-N load compared with 1# and 2#, which increased by 10.16% and 14.65%, respectively. Reducing the blending ratio of sponge iron will increase the NH3-N load reduction effects. 9# and 11# are compared with 3#, and the reduction rates are increased by 11.27% and 33.13%, respectively. Compared with 4#, the reduction rates of 10# and 12# increased by 62.68% and 55.15% respectively. Reducing the blending ratio of blast furnace slag will reduce the NH3-N load reduction effects. 11# and 12# are reduced by 23.93% and 3.50% respectively compared with 5# and 6#. Reducing the blending ratio of the zeolite also reduces the NH3-N load reduction effects. The reduction rates of 9# and 10# are reduced by 56.62% and 5.33%, respectively, compared with 7# and 8#. Ninety percent of primary filler +5% blast furnace slag +5% zeolite had the best effects on NH3-N load reduction, 41.25% (coarse sand) and 52.57% (medium coarse sand). The load reduction rate of NH3-N will be reduced by increasing the blending ratio of the modifier. In summary, 90% primary filler +5% blast furnace slag +5% zeolite was the best for NH3-N load reduction compared with other blending schemes.
Sponge iron can reduce NO3-N to ammonia nitrogen, nitrous nitrogen, nitrogen, and other nitrogen-containing gases due to its own reducing property. Some studies had shown that the main product of NO3-N reduction is ammonia nitrogen. The production of ammonia nitrogen accounts for 68%~83% of the reduction of nitrate (Li et al. 2016c).
The removal of ammonia nitrogen by blast furnace slag is mainly achieved by adsorption. Some studies have shown that (Zhao et al. 2016) blast furnace slag has a certain ability to remove cations, and its surface may have negative charges, mainly relying on electrostatic attraction for adsorption.
The diameter of zeolite hole is 0.6~1.5 nm, and the diameter of zeolite channel is 0.3~1 nm, and the diameter of NH3-N is 0.286 nm, which indicates that ammonia nitrogen can be directly absorbed into the interior of zeolite. Moreover, the zeolite itself has a strong cation exchange property. In zeolite, a strong electric field is formed around the negative charge of oxygen frame and the cations balanced with it. This electric field is prone to generate strong electrostatic attraction and has strong adsorption capacity. This adsorption capacity is a selective adsorption with molecular sieve properties, and has strong adsorption properties for ammonia nitrogen with strong polarity and diameter smaller than the pore diameter of the zeolite (Zhou and Boyd 2014). In summary, zeolite can remove ammonia nitrogen from water by ion exchange of ionic ammonia and adsorption of molecular ammonia.
Purification effects of different artificial fillers on TN
As can be seen from Figs. 11 and 12, the average about TN load reduction rates of artificial fillers with coarse sand and medium coarse sand as main fillers were 20.79%, 23.44% respectively. Medium coarse sand has a slight increase compared with coarse sand. The artificial fillers 7# and 8# using only zeolite as the modifier have the most obvious improvement on the removal of TN, which is 4.93% and 8.15% higher than that of 1# and 2#, respectively. Artificial fillers 5# and 6#, which only use blast furnace slag as a modifier, were increased by 0.69% and 4.01%, respectively, compared with 1# and 2#.The artificial fillers 3# and 4# which only use sponge iron as a modifier were less effective than 1# and 2#, and even the removal effects were degraded. Compared with other mixing schemes, 90% main filler +10% zeolite and 90% main filler +5% blast furnace slag +5% zeolite mixing schemes had better load reduction effects on TN. The reduction rates were 25.52% (7#), 28.32% (8#), 25.01% (13#), and 27.86% (14#) respectively. Increasing the blending ratio of the modifier did not show a significant advantage over other blending schemes. In summary, the blending scheme of 90% primary filler +10% zeolite and 90% primary filler +5% blast furnace slag +5% zeolite had good effect on TN load reduction.
Sponge iron reduces NO3-N to ammonia nitrogen, nitrous nitrogen, nitrogen, and other nitrogen-containing gases. The main product is ammonia nitrogen. That is to say, sponge iron only converts the form of nitrogen and does not remove nitrogen. Therefore, the removal of TN by sponge iron was poor. The blast furnace slag and zeolite adsorb the nitrogen in the storm water runoff by physical adsorption and chemical adsorption, instead of converting the form of nitrogen. From the above test results, it could be seen that the removal efficiency of nitrogen by artificial fillers with these two modifiers was better than that of sponge iron.
Purification effects of different artificial fillers on TP
It can be seen from Figs. 13 and 14 that the average value of the TP load reduction rate of the artificial filler with medium coarse sand and coarse sand as the main filler is 82.92% and 72.76%, respectively, and the coarse sand is obviously superior to the coarse sand. Compared with pure sand (1# and 2#), the removal efficiency of TP by artificial fillers with sponge iron had been greatly improved. For medium coarse sand (2) and coarse sand (1), they increased from 67.63% and 51.84% to more than 90% respectively. Reducing the proportion of sponge iron had little effects on the removal of TP, probably because 5% of sponge iron could meet the requirements for phosphorus removal. At the same time, it could be seen from the above figure that the artificial filler with the addition of sponge iron had a load reduction rate of more than 90% for TP, which could also prove the above conjecture from the side. The reduction effects of the artificial filler added only with zeolite and blast furnace slag had not changed much or even decreased to some extent. In summary, 90% main filler +10% sponge iron was a better solution to remove phosphorus from storm water runoff.
In the artificial rainwater prepared, phosphorus was mainly present in the form of orthophosphate, and the ionization tendency of orthophosphate ions was greater than that of water and it was acidic. In neutral or acidic water, sponge iron and the new ecologically produced Fe3+ can react chemically with PO43-.The specific reaction process was as follows (Xue et al. 2018):
Since FePO4·3H2O and Fe3(PO4)2 had a small solubility, they were easily adsorbed on the surface of the artificial filler.
The blast furnace slag contained a large amount of metal oxides, and these active substances had a strong chemisorption effects on orthophosphate ions in the rainwater runoff. However, these materials are wrapped in the interior of the blast furnace slag and could not be well contacted with orthophosphate ions hydrochloride in the runoff. Wang (2016a) has studied the adsorption performance of blast furnace slag. The results show that the blast furnace slag has strong adsorption performance for cations, and the adsorption performance for anions is weak, especially for the adsorption of strong polar ions. The results of this study also explain the poor effects of blast furnace slag on the removal of orthophosphate ions in this study. The orthophosphate ion has a negative charge, and it is known from the above that the zeolite is negatively charged due to its structure. Because of the mutual repulsion of the same charge, the adsorption performance of zeolite on orthophosphate ion is poor. In addition, replacing the same volume of the main filler with the modifier will improve the permeability of the artificial filler to a certain extent and shorten the contact time between filler and pollutant. These may be the main reasons for the decline in the effects of the reduction.
Purification effects of different artificial fillers on Cu2+
It could be seen from Figs. 15 and 16 that the effects of artificial fillers with coarse sand and medium coarse sand as the main filler on Cu2+ were almost the same, which was 47.66% and 47.87%, respectively. Compared with 1# and 2#, the artificial fillers 3# and 4# with only sponge iron added, the load reduction rate increased by 20.27% and 8.68%. Compared with 1# and 2#, the artificial fillers 5# and 6#with only blast furnace slag added, the load reduction rate increased by 4.28% and 2.26%. Compared with 1# and 2#, the artificial fillers 7# and 8# to which only zeolite was added the load reduction rate reduced by 3.69% and 8.82%, respectively. The 5% sponge iron in 3# and 4# was replaced by the same volume of zeolite (9#, 10#), which had a large difference in Cu2+ load reduction effects, and 9# was reduced by 2.04% compared with 3#, and #10 was increased by 6.03% compared with #4. The 5% sponge iron in 3# and 4# was replaced by the same volume of blast furnace slag (11#, 12#), which had a significant improvement in Cu2+ load reduction effects, and #11 was increased by 8.63% compared with 3#, and #12 was increased by 11.72% compared with #4. In the artificial filler of double modifier, the mixing scheme of blast furnace slag + zeolite was the worst, which was 54.89% and 53.52%, respectively. Increasing the blending ratio of the modifier has no significant advantage over other blend fillers. In summary, 90% primary filler +5% sponge iron +5% blast furnace slag is a better solution to remove copper ions from storm water runoff.
For sponge iron, since the standard electrode potential φθ(Cu2+/Cu) = 0.340 > φθ(Fe2+/Fe) = − 0.440, Cu2+ can be removed by the displacement reaction. At the same time, as the test progresses, the pH value of the solution increased, the concentration of OH− in the solution increases, Fe(OH)2 and Fe(OH)3 flocs were formed, and Cu2+ in the adsorption solution was formed. Cu2+ could also form OH− to form a blue flocculent precipitated Cu(OH)2 which was poorly soluble in water. CaO in the blast furnace slag was easily dissolved in the aqueous solution, and exists in the form of Ca2+ in the solution, which can form CaCO3 under alkaline conditions, and then adsorb the heavy metal ions thereon (Wang et al. 2016a). Finally, CaCO3 and heavy metal ions adsorbed on CaCO3were adsorbed onto the blast furnace slag. Previous studies (Duan and Su 2011) shown that at pH 7, the removal rate of heavy metal ions from blast furnace slag is also considerable. The zeolite itself has a cation exchange capacity. The heavy metal ions themselves are positively charged and can be removed by cation exchange with the zeolite.
Purification effects of different artificial fillers on Zn2+
It can be seen from Figs. 17 and 18 that the artificial filler with medium coarse sand as the main filler has better Zn2+ removal effects than coarse sand. Artificial filler with modifier could improve the removal efficiency of Zn2+. Compared with 1# and 2#, the load reduction rate of Zn2+ by artificial fillers 3# and 4# using only sponge iron as a modifier was increased by 45.30% and 34.39%, respectively. Compared with 1# and 2#, the load reduction rate of Zn2+ by artificial fillers 5# and 6# using only blast furnace slag as a modifier was increased by 20.09% and 10.30%, respectively. Compared with 1# and 2#, the load reduction rate of Zn2+ by artificial fillers 7# and 8# using only zeolite as a modifier were increased by 26.48% and 12.93%, respectively. Therefore, the artificial filler added with sponge iron has a better effect on the removal of Zn2+ than the artificial filler with the other two modifiers. The three mixing schemes of 90% main filler +5% sponge iron +5% blast furnace slag, 70% main filler +10% sponge iron +10% blast furnace slag +10% zeolite, and 55% main filler +15% sponge iron +15% blast furnace slag +15% zeolite all had a load reduction rates of over 90%, and the load reduction rate of Zn2+ by the mixed filler of 90% main filler +10% sponge iron is over 85%. Therefore, increasing the blending ratio of the modifier did not significantly improve its ability to remove Zn2+. In summary, the two mixed fillers, 90% main filler +10% sponge iron and 90% main filler +5% sponge iron +5% blast furnace slag, could better meet the purification requirements of zinc ions.
For sponge iron, since the standard electrode potential φθ(Fe2+/Fe) = − 0.440 > φθ(Zn2+/Zn) = − 0.762, Zn2+ cannot be removed by the displacement reaction, but the removal rate of Zn2+ was still very high as shown in Fig. 18. The reason may be that with the continuous reaction, the pH value of the solution increases, the content of OH− in the solution increases, and the formation of Fe(OH)3 flocculant, so that the Zn2+ in the solution can be removed (Luo et al. 2011). Zn2+ also reacted with OH− to form Zn(OH)2, which was poorly soluble in water.
Purification effects of different artificial fillers on Cd2+
From Figs. 19 and 20, it can be seen that there is a certain gap between the load reduction effects of artificial fillers with coarse sand and medium coarse sand as the main fillers, which are 37.95% and 41.24% respectively. Compared with 1# and 2#, the removal effects of Cd2+ by artificial fillers 3# and 4# using only sponge iron as a modifier were improved by 4.50% and 3.14%, respectively. Compared with 1# and 2#, the artificial fillers containing only blast furnace slag (#5, #7) or zeolite (#6, #8) has no obvious effects on the reduction of Cd2+ load, and even the effects of reduction has declined. The range of change was between − 7.09 and 0.44%. The load reduction effects of artificial filler that with coarse sand as main filler and two modifiers added on Cd2+ were not much different, which were 39.34%, 42.07%, and 40.59% respectively. The differences between the three are also small compared with 3#. The load reduction effects of artificial filler that with medium coarse sand as main filler and two modifiers added on Cd2+ were different, and the artificial filler of sponge iron + blast furnace slag as modifier is better than the other two, which is 7.79% and 8.11% higher, respectively. Compared with 4#, these three had obvious advantages, and the reduction rates were 5.29%, 13.08%, and 4.97% respectively. Increasing the blending ratio of the modifier did not increase the ability of the artificial filler to remove Cd2+. In summary, the artificial filler of 90% primary filler +5% sponge iron +5% blast furnace slag had a better purification effects on Cd2+.
For sponge iron, since the standard electrode potential φθ(Cd2+/Cd) = − 0.403 > φθ(Fe2+/Fe) = − 0.440, it is said that iron is more active than cadmium, and iron can reduce cadmium ions from rainwater runoff by reduction reaction. At the same time, as the test progresses, the pH value of the solution increases, and the concentration of Fe2+ and Fe3+ in the solution also rise to a very high concentration, and Fe(OH)2 and Fe(OH)3 flocs were easily formed, so the removal rate of Cd2+ was increased. Cadmium ions could also be removed by precipitation of Cd(OH)2 formed with OH− (Li et al. 2011).
Comprehensive analysis of purification effects of different artificial fillers on pollutants
The comprehensive reduction rate of pollutant load (mean value of each pollutant load reduction rates) is used to evaluate the comprehensive removal performance of each artificial filler.
Table 7 shows that the comprehensive reduction rate of pollutants load by artificial filler with coarse sand as the main filler was 39.67%. The comprehensive reduction rate of pollutant load by artificial filler with medium coarse sand as main filler was 43.76%. Medium coarse sand was 4.09% higher than coarse sand, which has certain advantages. The comprehensive reduction rates of pollutants load by pure sand were 32.34% (coarse sand) and 35.64% (medium coarse sand) respectively. The load reduction rates of artificial fillers with 10% modifier was 35.46%~45.23% (coarse sand) and 38.03~49.46% (medium coarse sand). The comprehensive reduction rates of pollutant load by artificial fillers with 30% modifier addition were 44.44% and 46.87% respectively. The comprehensive reduction rates of pollutant load by artificial filler with 45% modifier were 46.25% and 46.29% respectively. It could be seen from the above data that when the proportion of modifiers increased from 10 to 30% and 45%, the comprehensive reduction rates of pollutants load did not increase significantly and did not show obvious advantages. The main reason may be that as the proportion of modifier added increases, the adsorption site, the raw materials reacted with the pollutants, and the cation exchange amount will increase, but the increase of the modifier addition ratio will also improve the permeability of the artificial filler and shorten the contact time between runoff pollutants and the filler. These two points lead to a small change in the overall load reduction effects as the proportion of modifier addition increases. The artificial filler added with two or more modifiers had a small difference in the effects of comprehensive reduction of pollutant load, and the reduction effects were good.
Conclusions
The study of the influence of main filler type, modifier type, mixing ratio of main filler and modifier on the permeability of artificial filler, and the purification effects of pollutants under different influent water volume and concentration concludes that:
The permeability of the filler was improved after adding the modifier, and the permeability of coarse sand + modifier was better than that of medium coarse sand + modifier. As expected, the proportion of modifier had a significant effect on the permeability of artificial filler.
Compared with coarse sand, medium coarse sand has certain advantages in the comprehensive reduction effects of pollutant load. Table 7 shows that the comprehensive reduction rate of pollutants load by artificial filler with coarse sand as the main filler was 39.67%. The comprehensive reduction rate of pollutant load by artificial filler with medium coarse sand as main filler was 43.76%. Medium coarse sand was 4.09% higher than coarse sand. Compared with the pure sand, the artificial filler added with the modifier has a certain degree of improvement on the removal effects of the pollutants. Table 7 shows that the comprehensive reduction rates of pollutants load by pure sand were 32.34% (coarse sand) and 35.64% (medium coarse sand) respectively. The load reduction rates of artificial fillers with 10% modifier was 35.46%~45.23% (coarse sand) and 38.03~49.46% (medium coarse sand). The comprehensive reduction rates of pollutant load by artificial fillers with 30% modifier addition were 44.44% and 46.87% respectively. The comprehensive reduction rates of pollutant load by artificial filler with 45% modifier were 46.25% and 46.29% respectively. The artificial filler added with 30% and 45% modifier did not show a significant advantage in the purification of contaminants compared with the artificial filler with only 10% modifier added.
Since the characteristics of the three modifiers were different, the removal effects of each pollutant were also quite different. The artificial filler with 90% main filler +5% blast furnace slag +5% zeolite has better removal effects on COD, NH3-N and TN. The artificial filler with 90% main filler +10% sponge iron artificial filler has better removal effects on NO3-N, TP, and Zn2+. The artificial filler with 90% main filler +5% sponge iron +5% blast furnace slag has better removal effects on heavy metal ions (Cu2+, Zn2+, Cd2+). Generally, the artificial filler added with two or more modifiers has a small difference in the effects of comprehensive reduction of pollutant load, and the reduction effects were better.
The sponge iron will have a hardener problem after running for some time. The reason for this phenomenon may be that the sponge iron will form iron compound such as iron phosphorus oxides (FePO4·3H2O and Fe3(PO4)2) and iron oxides (Fe2O3 and Fe3O4) on the surface of the filler after long-term operation. The passivation film, composed of these iron compounds, is the main cause of hardening (Pang 2012). In this study, sponge iron was only blended with natural river sand as a modifier, and the hardener phenomenon was not particularly obvious. In the 9 artificial water discharge tests, the artificial fillers added with sponge iron showed good permeability and pollutant removal effects.
References
Berardi U, Ghaffarianhoseini AH, Ghaffarianhoseini A (2014) State-of-the-art analysis of the environmental benefits of green roofs. Appl Energy 115(4):411–428
Cui SP, Liu LL, Chen J, Wang YL, Wang JF, Wang H, Dong SJ (2014) Influence of SiO2/Al2O3 content on structure and hydration activity of granulated blast furnace slag. Key Eng Mater 633:240–244
Davis AP (2005) Green engineering principles promote low-impact development. Environ Sci Technol 39(16):338A–344A
Duan JM, Su B (2011) Removal characteristics of Cd (II) from acidic aqueous solution by modified steel-making slag. Chem Eng J 246:160–167
Fang QL, Xu WH, Yan ZJ, Qian L (2018) Effect of potassium chlorate on the treatment of domestic sewage by achieving shortcut nitrification in a constructed rapid infiltration system. Int J Environ Res Public Health 15(4):670–677
Kamali M, Delkash M, Tajrishy M (2017) Evaluation of permeable pavement responses to urban surface runoff. J Environ Manag 187:43–53
Li JG, Lh W, Li YG, Bi N, Song FF (2011) Cadmium removal from wastewater by sponge iron sphere prepared by hydrogen reduction. J Environ Sci 23(Supplement):S114–S118
Li JK, Jiang CB, Lei TT, Li YJ (2016a) Experimental study and simulation of water quality purification of urban surface runoff using non-vegetated bioswales. Ecol Eng 95:706–713
Li JK, Li HE, Li YJ, Shen B (2016b) LID Technology for urban stormwater runoff purification and utilization-take Xi’an as an example. Science Press, Beijing (in Chinese)
Li T, Zhu YC, Kang X, Long B (2016c) Research on the influencial factors of nitrate nitrogen in micro-polluted source water by sponge iron reduction. Indust Water Treat 36(11):85–89 (in Chinese)
Luo FS, Xu XJ, Li XZ, Qiu M, Wang P, Chen N (2011) Micro-electrolysis treatment of copper smelting of heavy metals in wastewater. Technol Water Treat 37(3):100–104
Ministry of Housing and Urban-Rural Development of the People’s Republic of China (2015) Technical guidelines for sponge city construction-construction of rainwater system for low impact development (trial implementation). China Construction Industry Publishing House, Beijing, pp 37–39 (in Chinese)
Nguyen TT, Ngo HH, Guo WS, Wang XC, Ren NQ, Li GB, Ding J, Liang H (2019) Implementation of a specific urban water management - Sponge City. Sci Total Environ 652:147–162
Pang CC (2012) Pretreatment of fill compaction forming process in interval microelectrolysis. Hebei University of Engineering, Handan (in Chinese)
Rodríguez-Maroto JM, García-Herruzo F, García-Rubio A, Gómez-Lahoz C, Vereda-Alonso C (2009) Kinetics of the chemical reduction of nitrate by zero-valentiro. Chemosphere 74(6):0–809
Tang CL, Zhu YF, Zhang ZQ, Zhang YC (2007) Use of zero-valent iron for nitrate removal from the soil water in loess areas. Acta Sci Circumst 27(8):1292–1299 (in Chinese)
Wang DB, Zhang ZY, Li XM, Zheng W, Yang Q, Ding Y, Zeng TJ, Cao JB, Yue X, Shen TT, Zeng GM, Deng JH (2010) A full-scale treatment of freeway toll-gate domestic sewage using ecology filter integrated constructed rapid infiltration. Ecol Eng 36(6):827–831
Wang HB, Gui H, Yang WR, Li D, Tan W, Yang M, Barrow CJ (2014) Ammonia nitrogen removal from aqueous solution using functionalized zeolite columns. Desalin Water Treat 52(4-6):753–758
Wang Z, Huang GH, An CJ, Chen L, Liu JL (2016a) Removal of copper, zinc and cadmium ions through adsorption on water-quenched blast furnace slag. Desalin Water Treat 48:1):1–1)14
Wang JL, Zhang PP, Yang LQ, Huang T (2016b) Cadmium removal from urban stormwater runoff via bioretention technology and effluent risk assessment for discharge to surface water. J Contam Hydrol 185-186:42–50
Westholm LJ (2010) The use of blast furnace slag for removal of phosphorus from wastewater in Sweden - a review. Water 2(4):826–837
Xue R, Xu J, Gu L, Pan LH (2018) Study of phosphorus removal by using sponge iron adsorption. Water Air Soil Pollut 229(5):161
Zafarani HR, Bahrololoom ME, Javidi M, Mohammad HS, Javad T (2014) Removal of chromate ion from aqueous solutions by sponge iron. Desalin Water Treat 52(37-39):7154–7162
Zhao DH, Qiu QB, Wang YN, Huang M, Wu YH, Liu XJ, Jiang T (2016) Efficient removal of acid dye from aqueous solutions via adsorption using low-cost blast-furnace slag. Desalin Water Treat 57(58):1–10
Zhou L, Boyd CE (2014) Total ammonia nitrogen removal from aqueous solutions by the natural zeolite, mordenite: a laboratory test and experimental study. Aquaculture 432(1):252–257
Funding
This research was financially supported by the National Natural Science Foundation of China (no. 51879215) and the Key Research and Development Project of Shaanxi Province (2017ZDXM-SF-073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Rs., Li, Jk., Guo, C. et al. Filler improvement and purification effects of constructed rapid infiltration facility. Environ Sci Pollut Res 26, 33654–33669 (2019). https://doi.org/10.1007/s11356-019-06462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06462-7