Abstract
Particulate matter (PM) contains different chemical substances that have been associated with health effects and an increased risk of mortality due to their toxicity. In this study, fine particulate matter (PM2.5) samples were collected in a region with rural characteristics (Seropédica (Se)) and another with some industries (Duque de Caxias (DC)) (Brazil, RJ). Rats were exposed to PM2.5 extracts daily for 25 days at different dilutions: 10×, 5×, and a concentrated solution (CS). Biochemical analyses were investigated for total antioxidant capacity (ACAP), lipid peroxidation (LPO) levels, reduced glutathione (GSH) concentration, activity of glutamate cysteine ligase (GCL), and activity of glutathione S-transferase (GST). The liver showed a significant increase in GCL (DC-5×, DC-CS and Se-CS) and GST activities (DC-CS and Se-CS) in both regions when compared to the control group. In the renal cortex, GCL activity decreased in most of the tested groups while GST activity increased only in the 5× groups of both regions (DC and Se). In the renal medulla, GCL activity decreased for Se-10× and DC-CS but increased for Se-5×, and GST activity increased in the Se-10×, DC-5×, and DC-CS groups. Lung GCL increased in all groups for both regions. Moreover, this organ also showed an increase in GST activity when higher metal concentrations were present (5× and CS). TBARS levels were increased for all tissues in most tested concentrations. These data indicate that soluble compounds (e.g., metals) from PM2.5 sampled in areas with different pollution indexes can change the redox status and cause damage to different tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of particulate matter (PM) in the air has been associated with the development and worsening of various diseases, increases in hospitalizations, and premature deaths (Lelieveld et al. 2019; Wei and Tang 2018; Wu et al. 2018; Pascal et al. 2014; Pope III 2000). Particulate matter is emitted from biomass, natural sources, fossil burning, and industrial and agricultural activities (Olawoyin et al. 2018; Simkhovich et al. 2008; Bai et al. 2007). It is known that exposure to PM generates different biochemical responses depending on its concentration and composition; its effects are inversely proportional to its size (Araujo et al. 2008). Several studies have shown that exposure to PM can induce (1) redox imbalance with alterations in the antioxidant systems and, consequently, oxidative stress induction (Yi et al. 2011; Araujo et al. 2008; Bai et al. 2007); (2) cytokine production (Rodríguez-Cotto et al. 2014; Fuentes-Mattei et al. 2010); and (3) reduction in alveolar macrophages (Williams et al. 2013). Inhaled PM initiates inflammatory responses in the alveoli, diffusing up and reaching systemic circulation (Polichetti et al. 2009). In the presence of transition metals in PM, oxidative damage may occur through the formation of reactive oxygen species (ROS) and consequent deterioration of cell membranes and protein structure may occur as a result of oxidative stress (Qin et al. 2017; Pisoschi and Pop 2015).
PM contains trace metals and organic and inorganic compounds, and the composition and concentration depend on the region, season, and local activities (Chen et al. 2016; Ku et al. 2016; Li et al. 2014; Mateus et al. 2013). Previous studies indicate that metals can interfere in cellular homeostasis, favoring oxidation reactions depending on their concentrations and interaction capacities with biological systems (Valko et al. 2015; Shi et al. 2004). Furthermore, non-essential metals may reduce the supply of essential metals, thus promoting toxicity in several organs (Cobbina et al. 2015). Redox signaling is essential for cellular processes, and its impairment by ROS and/or metals can compromise cell survival and defense capacity (Kalinowski et al. 2016; Pisoschi and Pop 2015). Fine particulate matter (PM2.5; diameter ≤ 2.5 μm) has received special attention due to its constituents, for example, metals, which have the capacity to interact with biochemical routes and interfere in cellular reactions, causing harm to health (Vreeland et al. 2017; Weber et al. 2016; Mirowsky et al. 2015; Pulido and Parrish 2003).
Soluble fractions of PM2.5 were obtained from two regions of the metropolitan area of Rio de Janeiro (MRRJ), Brazil, which has two different pollution indexes (Massone et al. 2015). These were Duque de Caxias, with prominent industrial activities, and Seropédica, with few industrial activities with rural characteristics (Gioda et al. 2011; Mateus et al. 2013). Even in rural areas, there are high levels of PM (Shridhar et al. 2010), which can compromise the health of the exposed population (Brook et al. 2014).
The purpose of this study was to elucidate the effects of PM2.5 from two regions on the liver, kidneys, and lungs of rats. After the compounds reached the bloodstream, total antioxidant capacity (ACAP), glutamate cysteine ligase (GCL), and glutathione S-transferase (GST) activities, and reduced glutathione (GSH) concentrations, were measured. Malondialdehyde, an oxidative stress marker, was also verified in order to evaluate the oxidative damage of membrane phospholipids.
Materials and methods
Sampling
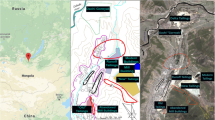
Filters containing PM2.5 were obtained from the Environmental Institute of Rio de Janeiro State (INEA). Two sampling sites were chosen: an area characterized as industrial/urban, Duque de Caxias (22° 42′ 15.5″ S, 43° 18′ 41.3″ W), and another with less pollution index characterized as rural, Seropédica (22° 75′ 76″ S, 43° 68′ 492″ W) (Fig. 1).
The samples (4 filters) were collected between June and July 2012 using high-volume samplers (Energetica, RJ) and fiberglass filters under a flow rate of 1 m3 min−1 for 24 h at 1 filter per week. The mean concentration of PM2.5 was 8.5 ± 4.0 μg/m−3 for Seropédica and 14.4 ± 4.1 μg/m−3 for the industrial/urban area Duque de Caxias (Ribeiro et al. 2016). Metals and other water-soluble elements in PM2.5 were extracted from the filter pools using a saline solution (1 g filter/100 mL of 0.9% saline solution) under magnetic stirring for 1 h at room temperature. Blank filters (1 filter 1 g/mL—BF; without PM2.5), used as a control, were extracted similarly. All extracts were filtered through a 0.45-μm mesh (Macherey-Nagel, Germany) and used as the concentrated solution (CS), which was further diluted 5× and 10× with saline solution. The metals present in the PM2.5 saline extracts were previously analyzed (Ribeiro et al. 2016) using inductively coupled plasma with mass spectrometry (ICP-MS).
Animal model
Four-month-old male heterogenic Wistar rats (weight 300 ± 40 g) were kept at a controlled temperature (24.0 ± 2.0 °C), at the Institute of Biological Sciences (ICB) of the Federal University of Rio Grande (FURG). They were kept in collective cages in light/dark cycles of 12 h and were fed with food and water ad libitum. The experimental protocol was approved by the Ethics Committee of the FURG under protocol (P075/2014).
Groups and experimental protocol
A total of 40 rats were used in our experiments; they were divided into eight groups of five animals. Two groups were considered as controls (CT and BF): one injected only with saline solutions (CT) and the other with blank filter (BF) extracts. The remaining 6 groups were treated with PM2.5 extracts from Seropédica (Se) and Duque de Caxias (DC) as follows: two groups were treated with the concentrated extract solution (DCCS and SeCS), two groups used for the diluted 5× and 10× from concentrated extracts Se5× and DC5×, and Se10× and DC10×. Rats have dosed IP with 1 mL/day of the previously described solutions for a total of 25 days (Ribeiro et al. 2016). All the animals were euthanized 24 h after the last IP administration, and their organs were collected, dried, and weighted (lung, liver, and left kidney), and stored at − 80 °C.
Sample preparations and protein determination
Each organ was homogenized (1:5) in a Tris HCl buffer (1 mM) containing EDTA (2 mM) and MgCl2·6H2O (5 mM) at pH 7.75, and centrifuged at 10,000×g at 4 °C for 20 min. Protein concentration was determined in the supernatant and normalized to 3.37 mg of proteins/ml using a commercial kit (Total Protein, DOLES Ltda).
Total antioxidant capacity against the peroxyl radical
An aliquot of 10 μL of the supernatant at the normalized protein concentration was used to determine the total antioxidant capacity (ACAP) using the generation of fluorochrome from the reaction of diacetate of 2′,7′-dichlorodihydrofluorescein (H2DCFDA, Molecular Probes) which produces ROS (peroxyl radicals) in the sample. Then, 2-methylpropionamidine dihydrochloride (ABAP; 4 mM; Aldrich) was employed as a peroxyl radical generator (7.5 μL). The assays were performed in triplicate with and without ABAP. The reading was carried out in a microplate reader (Victor2, Perkin Elmer Wallac model 1420-042), preheated, and kept at 37 °C. Readings were performed every 5 min for 60 min using excitation and emission wavelengths of 485 and 520 nm, respectively; the results were expressed per relative area of fluorescence (Amado et al. 2009). Using this methodology, a higher relative area meant low ACAP and vice versa.
Lipoperoxidation determination
The amount of LPO was determined by malondialdehyde concentration (MDA) and subsequent reaction with thiobarbituric acid (TBA) in an acidic pH (3.4). This reaction generates a fluorescent compound that emits at 580 nm. It was quantified using a spectrofluorometer (PerkinElmer Wallac Victor2 model 1420-042), at an excitation wavelength of 520 nm and 580 nm (emission). MDA formation was expressed in nanomoles MDA/milligram protein when compared to tetramethoxypropane (TMP, Sigma-Aldrich) which was used as a standard (Oakes and Van Der Kraak 2003).
Glutamate cysteine ligase activity and concentration of reduced glutathione
GCL and reduced GSH were quantified following the protocol described by White et al. (2003). Briefly, this technique evaluates the GSH capacity to react with naphthalene-2,3-dicarboxaldehyde (NDA; Biogen), forming a fluorescent complex owing to glutamyl amino group and cysteine sulfhydryl group of GSH. Reactions occur in a reaction buffer (Tris HCl 100 mM, ATP 40 mM, MgCl2·6H2O 40 mM, sodium borate 20 mM, glutamic acid 20 mM, serine 2 mM, and EDTA 2 mM) at pH 7.75 with sample extract (25 μL). To initiate the reactions, cysteine 2 mM was added with the function of being a substrate for GCL activity and this mixture was incubated for 60 min at room temperature. Thus, this allowed for the quantification of GCL activity and in parallel but without the addition of cysteine. The GSH levels were measured, being the GSH level and GCL activity measured on separate plates and to prevent the reaction. At the end of the 60-min period, cysteine was added to the GSH plate, allowing for the quantification of GSH levels. The reaction was stopped by adding sulfosalicylic acid (200 mM), incubated for 20 min, and then centrifuged for 5 min at 1500 rpm. In each well, 180 μL of the derivatizing solution of NDA was added and incubated for 30 min. The fluorescence intensity was read in a spectrofluorometer (Victor 2, Perkin Elmer) using an excitation wavelength of 480 nm and an emission wavelength of 525 nm.
Determination of glutathione S-transferase activity
The GST activity was determined according to Habig and Jakoby (1981) through the conjugation of 1 mM GSH (Sigma) with 1 mM of 1-chloro-2,4-dinitrobenzene (CDNB; Sigma), using 10 μL of homogenate. This complex is determined by absorbance at 340 nm using an ELISA microplate reader (BioTek ELx808). The GST activity was expressed in nanomoles of CDNB conjugated with GSH per minute per milligram of protein. Briefly, 80 μL of CDNB was added to 3.8 mL of phosphate buffer at 25 °C; 235 μL was incubated with 10 μL of the supernatant. In the preheated (25 °C) reader, 10 μL of GSH 25 mM was added to the respective wells.
Statistical analysis
The data are reported as mean ± SEM. The comparison between groups was performed using one-way ANOVA, followed by post hoc Newman Keuls tests and orthogonal contrasts. Normality and variance homogeneity were checked. In all cases, the significance level was set at p < 0.05 (Zar 2010).
Results
Liver tissue response to PM2.5 extracts
ACAP
Significant reductions in ACAP (higher relative area) in the liver were observed in groups exposed to the 10× dilution and DCCS. In contrast, the Se5× group presented an increment (lower relative area) in total ACAP against peroxyl radicals when compared to controls while the DC5× and SeCS groups presented no change in ACAP (Fig. 2a).
Effects of different concentrations of PM2.5 in the liver tissue. Total antioxidant capacity against peroxyl radical (ACAP) (a), expressed per relative area of fluorescence and inversely proportional values. Lipid peroxidation by TBARS method (b), expressed by quantifying the level, in nanomoles of malondialdehyde (MDA) per milligram of protein. Groups are presented as control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All amounts are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) represented by different letters
TBARS
For all rat groups exposed to PM2.5, liver LPO levels were higher than those of the controls (BF and CT), and this effect was greatest for rats treated with the concentrate CS (Fig. 2b).
GCL
The GCL activity increased with the extract at DC-5× and Se-5× groups when compared to the controls (Fig. 3a), and with DC-CS and the rural concentrate Se-CS. In treatment of 10× groups no significant difference was observed with either diluted extract when compared to the controls (Fig. 3a).
Effects of different concentrations of PM2.5 in the liver tissue. Effect on glutathione cysteine ligase activity (a), expressed by levels of ƴ-glutamyl cysteine per milligram of protein. Reduced glutathione (GSH) levels (b) in nanomoles per milligram of protein. Glutathione S-transferase activity (c), expressed in nanomoles of 1-chloro-2,4-dinitrobenzene (CDNB) conjugated with GSH per minute per protein. Groups are control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All values are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) are represented by different letters. When necessary, analyses were done by orthogonal contrast, represented by lines and *
GSH
Both the DC and Se extracts significantly reduce GSH levels for most groups exposed to PM2.5 polar extracts. The extract exhibited less GSH effect since Se10× and DC10× did not induce significant reduction (Fig. 3b). However, when DC and Se extracts, 5× diluted and SC, are compared, the GSH effect is similar. The reductions in GSH levels (Fig. 3b) are inversely proportional to the GCL activity (Fig. 3a).
GST
GST activity was not affected at 10× for either region or in DC5× (although levels were somewhat higher than those of controls, however, it was significantly higher for Se5×, and both concentrates (SeCS and DCCS) (Fig. 3c).
Kidney tissue responses (cortex and medulla) to PM2.5 extracts
ACAP
The effects on the kidney were evaluated on the cortex and medulla. Effects of PM2.5 extracts on the renal cortex showed a significant decrease in antioxidant capacity (ACAP) (higher relative area) at 10× dilution for both the DC and Se regions (Fig. 4a). However, the effect at this extract concentration (10×) was completely opposite at the renal medulla; ACAP significantly increased when compared to controls (Fig. 4b). No significant ACAP effect was observed at other extract concentrations in the renal cortex or medulla compared to controls (Fig. 4a, b).
Effects of different concentrations of PM2.5 in the renal cortex (a and c) and renal medulla (b and d). Total antioxidant capacity against peroxyl radical (ACAP) (a and b), expressed per relative area of fluorescence and inversely proportional values. Lipid peroxidation by TBARS method (c and d), expressed by quantifying the level, in nanomoles of malondialdehyde (MDA) per milligram of protein. Groups are shown as control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All values are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) is represented by different letters
TBARS
Lipid peroxidation was significantly higher for all extract concentrations except for the rural 10× (Se10×) dilution. A significant increase in LPO for both cortex and medulla was observed at other concentrations (Fig. 4c, d). There is a direct relationship between lipid peroxidation and rural extract concentration in both renal cortex and medulla; however, this relationship is not evident for the DC extract in the renal medulla (Fig. 4c, d).
GCL
The activity of GCL in the renal cortex was significantly reduced by all PM2.5 extract concentrations, except for Se10× (Fig. 5a). This effect was less evident for the rural extract when compared to the industrial extract. However, the effect of extracts on GCL activity in the renal medulla was a reduction by Se10× and DCCS and an increase by Se5× while no significant effect was found for other experimental groups (Fig. 5b).
Effects of different concentrations of PM2.5 in kidney tissue, the renal cortex (a, c, and e) and renal medulla (b, d, and f). Effect on glutathione cysteine ligase activity (a and b) expressed by levels of ƴ-glutamyl cysteine per milligram of protein. Reduced glutathione (GSH) levels (c and d) in nanomoles per milligram of protein. Glutathione S-transferase activity (e and f), expressed in nanomoles of 1-chloro-2,4-dinitrobenzene (CDNB) conjugated with GSH per minute per protein. Groups are control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All values are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) is represented by different letters. When necessary, analyses were done by orthogonal contrast, represented by lines and *
GSH
The levels of GSH were mostly reduced by both extracts (DC and Se) in both kidney cortex and medulla. In the renal cortex, GSH levels were decreased by DC10× and DCCS, as well as by Se5×, compared to those of the controls (Fig. 5c). The greatest reductions in GSH levels were measured in the renal medulla. Significant reductions were determined for all extract concentrations, except for the Se5× group, where there was an opposite increase in GSH concentration when compared to controls (Fig. 5d). Industrial extracts exerted similar GSH reduced levels at all extract concentrations tested, demonstrating a consistent and conserved response to the extract (Fig. 5d).
GST
A significant increase in GST renal cortex activity for 5× extract groups (Fig. 5e) was observed, while GST in the medulla increased in Se10×, and at higher concentrations of the DC extract (DC5×, and DCCS) when compared to controls (Fig. 5f). No significant differences in GST activity was encountered in the cortex or medulla for other extract concentrations (Fig. 5e, f).
Lung tissue responses to PM2.5 extracts
ACAP
Reduced ACAP (higher relative area) in both 10× extract groups, as well as for the DCCS (Fig. 6a). The effect was greater for the DC extracts than for the Se extracts. Total ACAP of the other extract concentration groups was not altered when compared to controls (Fig. 6a).
Effects of different concentrations of PM2.5 in the lung tissue. a Total antioxidant capacity against peroxyl radical (ACAP), expressed per relative area of fluorescence and inversely proportional values. b Lipid peroxidation by TBARS method, expressed by quantifying the level, in nanomoles of malondialdehyde (MDA) per milligram of protein. Groups are control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All values are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) is represented by different letters
TBARS
A significant increase in LPO was determined for most groups exposed to PM2.5 extracts when compared to controls (Fig. 6b); however, no significant difference was observed for DC10× and SeCS.
GCL
The GCL activity was significantly higher in all groups when compared to controls (Fig. 7a). A direct relationship with extract concentration was observed, with the DC extracts exhibiting relatively higher activity than the Se extracts.
Effects of different concentrations of PM2.5 in the lung tissue. Effect on glutathione cysteine ligase (a) expressed by levels of ƴ-glutamyl cysteine per milligram of protein. Reduced glutathione (GSH) levels (b) in nanomoles per milligram of protein. Glutathione S-transferase activity (c), expressed in nanomoles of 1-chloro-2,4-dinitrobenzene (CDNB) conjugated with GSH per minute per protein. Groups are control (CT), blank filter (BF), Seropédica (Se-10×, Se-5×, and Se-CS), and Duque de Caxias region (DC-10×, DC-5×, and DC-CS). All values are expressed as mean ± SEM (n = 5). The significant difference (p < 0.05) is represented by different letters
GSH
Significantly lower levels of GSH were observed in rat lung tissues exposed to PM2.5 extracts (Fig. 7b).
GST
No significant difference in GST activity was observed for the 10× PM2.5 extract dilution (which is the least concentrated extract tested) from either region. Nevertheless, the more concentrated extracts 5× and the CS (Se and DC) exhibited significantly higher GST activity when compared to the controls (Fig. 7c).
Discussion
The main goal of this study was to analyze the systemic toxicity triggered by the different soluble PM2.5 fractions from two distinct regions (Duque de Caxias (DC) and Seropédica (Se) sites). Various redox responses were evaluated after IP exposure on the liver, kidneys, and lungs. For this purpose, exposure to PM was performed according to Ribeiro et al. (2016), where particulate material extracts were administered via IP and the period of 25 days was selected as the number of days in a single month in which people are exposed to outdoor pollution.
The intraperitoneal (IP) way to exposure was used, avoiding the many pro-inflammatory effects related to the inhalation route and covering the different forms of PM entry into the body (Kato et al. 2011; Shrey et al. 2011; Folkmann et al. 2007). This exposure method verify the redox state mediated by PM2.5 compounds in different tissues mediated by PM2.5 compounds, and not by the inflammatory response, and provides an alternative pathway for the arrival of PM into the bloodstream to investigate effects throughout the body. The lung parenchyma is the first stage of filtering airborne particles, where the alveoli and terminal bronchioles are its main functional units (Hussain et al. 2011). In addition, the particulate matter can be absorbed by the gastrointestinal system (Sá et al. 2017; Bellmann et al. 2015) and olfactory bulb (Wang et al. 2017; Costa et al. 2015); all pathways contribute to the toxic effects of PM. Besides that, the particles that reach large airways and are not absorbed by the alveolar epithelium arrive at the gastrointestinal tract through mucociliary transport and swallowed. Then, a part of the soluble fraction can be retained on the lung mucus and driven to the oral cavity and then incorporated into the digestive tract where it can be transferred to the bloodstream and distributed to other organs (Bellmann et al. 2015). The peritoneal surface area and high vascularization allow rapid absorption and delivery for hepatic metabolism through the portal system (Nebendahl 2000). Therewithal, intraperitoneal and intratracheal exposures of PM2.5 have already been compared, obtaining similar genotoxicity in rat lungs (Kato et al. 2011).
These findings on systemic exposure to PM2.5 from both areas showed significant redox changes in major organs involved in detoxification (liver), depuration processes (kidneys), and gas exchange (lungs). Surprisingly, these results showed that, despite lesser concentrations of metals in the Seropédica area, samples from that place also elicited toxic effects similar to the samples coming from the industrial region. PM2.5 polar extracts obtained from two different regions elicit the ability to impair redox balance, evidenced by the results of this study in which the reduction in antioxidant competence, reduction of GSH levels, and onset of LPO are altered. Several toxicological mechanisms could be responsible for these effects, including the fact that some non-essential metals can alter the uptake of essential metals due to the ionic competition on the specific protein binding sites, interfering in the cellular pathways, and inhibiting and/or stimulating enzymatic activity (Cobbina et al. 2015).
One of the questions about the toxicological effects of PM regards the interactions occurring between metals present in atmospheric particles, and biochemical interactions in living organisms. In vivo exposure showed the effects of a polar mixture of inorganic and organic compounds present in airborne PM2.5 extracts at different concentrations, inducing a redox imbalance in different organs. These findings also showed that antioxidant defenses are recruited more in the redox cycle and compromise the maintenance of the redox cell environment. The effects of these extracts clearly vary from organ to organ. Antioxidant defense systems are recruited by cells in unfavorable situations. Because of this, checking the total antioxidant profile shows the modulation of these systems against an increase in ROS and their ability to prevent damage. Moreover, enzymes such as superoxide dismutase (acts against superoxide), catalase, and glutathione peroxidase (decomposes hydrogen peroxide) are crucial for controlling ROS, along with non-enzymatic systems (e.g., GSH), to prevent tissue imbalance and impairment (Valko et al. 2015).
The PM2.5 polar extracts used in this study are composed of a variety of metals (Ribeiro et al. 2016), which leads to biomolecule oxidation above the supported capacity of antioxidant defenses, unleashing hepatic responses, such as lipoperoxidation. These data are in agreement with that found by Araujo et al. (2008), who exposed mice to ultrafine particles by inhalation for 40 days “in situ” under environmental conditions, reporting high LPO in this organ.
Many studies have reported the effects of metals in the liver, indicating cytotoxicity associated with lipoperoxidation (García-Niño and Pedraza-Chaverrí 2014; Messarah et al. 2012), protein oxidation (Boveris et al. 2012), mitochondrial dysfunction (Belyaeva et al. 2011), apoptosis, and DNA damage (Liu et al. 2012). The liver plays a major role in GSH synthesis and reduction, which is a crucial non-enzymatic antioxidant that maintains the redox status and cellular defense to protect against metal toxicity and other organic compounds (Lee et al. 1997). The observed reduction of hepatic GSH content in this study due to PM2.5 polar extract exposure was also supported by similar findings in other studies (Reckziegel et al. 2016; Jihen et al. 2011). We also found that the industrial extract is a more potent GSH reducer than the rural extract since it maintains similar effects even at its most diluted concentration while this effect is lost for diluted rural extracts.
These results also show that higher concentrations of PM2.5 (CS groups from both regions) induced GST activity (Fig. 3c). The increase in GST activity should be analyzed together with increased GCL activity and decreased GSH concentration in the same experimental groups since this tripeptide is a co-substrate of GST. Overall, the results indicate that higher metal concentrations, regardless of the region, may be inducing the results presented here, since according to Ribeiro et al. (2016), these tested extracts have numerous metals measured. That said, these elements activated an oxidative rebalancing response as GCL activation to support GSH requirements by GST, which in turn should lead to GSH depletion.
There is little data about possible damage caused by PM2.5 constituents to the kidney, comprising the cortex and medulla. Antioxidant defense against peroxyl radicals was evident only at low concentrations, Se10× and DC10× groups (Fig. 4a, b). A decrease in ACAP value was found in the kidney region with higher aerobic metabolism (cortex), possibly due to its higher oxygen requirement and antioxidant utilization as a ROS scavenger. Despite the few alterations in the total ACAP of renal tissues by both extracts, the kidneys suffered damage in the lipid membrane when exposed to the mixture of metals contained in PM2.5 as evidenced by the lipid peroxidation profile generated (Fig. 4c, d). The specific ACAP was modulated for each renal region from the control group, as shown by the ACAP values for Se10× for the cortex and medulla (Fig. 4a, b). This means that the induction of the antioxidant system was a compensatory response to avoid oxidative damage, which is confronted in less concentration in the industrial area. The renal medulla has a greater tissue volume, which is representative of its prominent response compared to the renal cortex. Taking this into account, these results agree with the study by Abdelhalim et al. (2015), which found an increase in total ACAP and lipoperoxidation in the whole kidney and also analyzed the liver and lungs of rats exposed to gold nanoparticles by IP administration. In the PM2.5 extracts, a variety of metals were found, and the most relevant were Mg, Zn, Ca, K, Cu, Ga, Pb, Cd, and Sb in the administered solutions (Ribeiro et al. 2016).
Many metals can facilitate the entrance of toxic elements into the intracellular environment by means of transporters, by the formation of metal complexes with membrane proteins, by channel affinity (molecular mimicry), or even by competing for regulatory sites of transmembrane channels (Martinez-Finley et al. 2012). Metals can be biodistributed unevenly in different organs, as demonstrated by Paßlack et al. (2014), where Zn, Mn, and Cu were more concentrated in the liver than in the cortex and renal medulla. However, Cd levels were detected in higher concentrations in the renal cortex and liver when compared to the renal medulla, but metals such as Ba, Cr, Sb, and Pb did not exhibit differences in concentration in these tissues (Paßlack et al. 2014).
These studies suggest that PM2.5 compounds have a higher affinity for a particular cell type. This may explain the antagonistic and random responses found in the cortex and renal medulla; metals and/or increases in ROS production due to PM2.5 seem to interfere, decreasing GCL activity in the renal cortex (Fig. 4a), though GSH levels were only affected by some of the extract concentrations (Fig. 4c, d). Cortex GST activity was slightly modified in most of the experimental groups, but this concurrently occurred with the depletion of GSH (Fig. 5c), advocating for the importance of this enzyme (Fig. 5e). Surprisingly, GST does not appear to vary significantly in the renal medulla, suggesting that other forms of protection pathways are being requested by this tissue. In addition, a reduction in GCL activity was observed in the medulla for the Se10× and DCCS groups. In these same experimental groups, decreased GSH levels and higher GST activities were also verified, demonstrating the importance of GST detoxification systems. Higher GCL/GSH ratios were observed in the renal medulla and 3× more GST activity in the renal cortex than in the medulla. Therefore, these results suggest that GST activity differs between kidney regions, being greater in the cortex; moreover, there exists a concentration of GST expressed at the controls which was shown between regions of the renal parenchyma and also more mobilized by PM2.5 in the medulla than in the renal cortex in most concentrations. Furthermore, the ACAP in the medulla is even higher than that found in the liver (Fig. 2a, b). These data are consonant with the conclusions derived by Zou et al. (2001), who considered the renal medulla as a region with a greater oxidative profile. This is because the O2− important action is in control of medullary blood flow, and inhibition of superoxide dismutase (SOD) or an increase in O2− production reduced the medullary renal blood flow and excretion of water and sodium.
Biochemical analyses of the lungs exposed to PM polar extracts showed that the total ACAP underwent modifications, mainly with more diluted extracts (Se and DC), with the greatest response always due to the DC extracts. There were significant TBARS responses in almost all of the treatments (Fig. 6b). After acute exposure in the cell line A549 to the soluble polar portion of PM10 for 24 h, Yi et al. (2011) reported increased ROS levels and apoptosis, while the activity of antioxidant enzymes such as SOD and catalase (CAT) was reduced. Similar to the liver findings, the depletion in GSH concentrations and the direct relationship between the increase in GCL activity and extract concentration in the lungs for most of the experimental groups suggest a redox imbalance and, more importantly, GSH depletion. The latter has been associated with higher susceptibility, tissue damage, and pathological development (Biswas and Rahman 2009). GSH plays an important role in lung defense against atmospheric oxidizing agents and environmental xenobiotics. Low concentrations of this antioxidant have been associated with lung diseases and inflammatory reactions (Biswas and Rahman 2009; Siedlinski et al. 2008). Riva et al. (2011), using instillation exposure, demonstrated that metals present in PM2.5 can cause similar effects to those reported here, such as increased lipoperoxidation and depletion of GSH contents in mouse lungs. Moreover, the direct relationship between GST activity and extract concentration, significantly established for 5× and CS treatments, should also favor GSH depletion.
Conclusion
These findings showed how IP PM2.5 polar extract exposure affects differently the various organs. Exposure to these extracts revealed susceptible injuries in different organs distributed through the bloodstream triggering redox imbalances and consequently oxidative stress. This is evident since in all of the organs tested, GSH levels were significantly reduced by PM2.5 polar extract exposure. In addition, this was generally accompanied by increases in GST and GCL activities as well as an increase in lipid peroxidation. Many metals present in the polar fractions of particulate matter can induce tissue changes culminating in organ-specific oxidative damage through the interference in cytosolic and mitochondrial oxidative reactions. These findings also show that in some cases, lower PM2.5 concentrations can exert similar effects as the extract concentrate. This is probably due to similarities in PM2.5 chemical composition between the regions and interactions among elements, and the affinity of enzymatic regulatory sites can partially justify the effects verified in these areas with different levels of atmospheric pollution.
References
Abdelhalim, Mohamed A, Kassem A, Al-Ayed MS, bdelmottaleb Moussa SA (2015) The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak J Pharm Sci 28(2):705–712
Amado, Lund L, Garcia ML, Ramos PB, Freitas RF, Zafalon B, Ferreira JLR, Yunes JS, Monserrat JM (2009) A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: application to evaluate microcystins toxicity. Sci Total Environ 407(6). Elsevier B.V.):2115–2123. https://doi.org/10.1016/j.scitotenv.2008.11.038
Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE (2008) Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102(5):589–596. https://doi.org/10.1161/CIRCRESAHA.107.164970
Bai N, Khazaei M, van Eeden SF, Laher I (2007) The pharmacology of particulate matter air pollution-induced cardiovascular dysfunction. Pharmacol Ther 113(1):16–29. https://doi.org/10.1016/j.pharmthera.2006.06.005
Bellmann S, Carlander D, Fasano A, Scimeca JA, Waldman WJ, Tsytsikova L, Canady R, Pereira DIA (2015) Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. WIREs Nanomed Nanobiotechnol 7:609–622. https://doi.org/10.1002/wnan.1333
Belyaeva EA, Korotkov SM, Saris NE (2011) In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: a comparison of copper and mercury with cadmium. J Trace Elem Med Biol 25(SUPPL. 1). Elsevier GmbH):S63–S73. https://doi.org/10.1016/j.jtemb.2010.10.007
Biswas SK, Rahman I (2009) Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Asp Med 30(1–2). Elsevier Ltd):60–76. https://doi.org/10.1016/j.mam.2008.07.001
Boveris A, Musacco-Sebio R, Ferrarotti N, Saporito-Magriñá C, Torti H, Massot F, Repetto MG (2012) The acute toxicity of iron and copper: biomolecule oxidation and oxidative damage in rat liver. J Inorg Biochem 116. Elsevier Inc:63–69. https://doi.org/10.1016/j.jinorgbio.2012.07.004
Brook RD, Robert L, Bard M, Morishita JT, Dvonch L, Wang H-y, Yang C, Spino et al (2014) Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect 2013(March):624–631. https://doi.org/10.1289/ehp.1306595
Chen S-j, Wang J, Wang T, Wang T, Mai B-x, Massey SL (2016) Science of the total environment seasonal variations and source apportionment of complex polycyclic aromatic hydrocarbon mixtures in particulate matter in an electronic waste and urban area in South China. Sci Total Environ 573. Elsevier B.V.:115–122. https://doi.org/10.1016/j.scitotenv.2016.08.101
Cobbina SJ, Chen Y, Zhou Z, Wu X, Feng W, Wang W, Li Q et al (2015) Interaction of four low dose toxic metals with essential metals in brain, liver and kidneys of mice on sub-chronic exposure. Environ Toxicol Pharmacol 39(1). Elsevier B.V.):280–291. https://doi.org/10.1016/j.etap.2014.11.030
Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ (2015) Neurotoxicity of traffic-related air pollution. NeuroToxicology 59:133–139. https://doi.org/10.1016/j.neuro.2015.11.008
Folkmann JK, Risom L, Hansen CS, Loft S, Møller P (2007) Oxidatively damaged DNA and inflammation in the liver of dyslipidemic ApoE-/- mice exposed to diesel exhaust particles. Toxicology 237(1–3):134–144. https://doi.org/10.1016/j.tox.2007.05.009
Fuentes-Mattei E, Rivera E, Gioda A, Sanchez-Rivera D, Roman-Velazquez FR, Jimenez-Velez BD (2010) Use of human bronchial epithelial cells (BEAS-2B) to study immunological markers resulting from exposure to PM(2.5) organic extract from Puerto Rico. Toxicol Appl Pharmacol 243(3):381–389. https://doi.org/10.1016/j.taap.2009.12.009
García-Niño WR, Pedraza-Chaverrí J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69(July):182–201. https://doi.org/10.1016/j.fct.2014.04.016
Gioda A, Amaral BS, Monteiro ILG, Saint’Pierre TD (2011) Chemical composition, sources, solubility, and transport of aerosol trace elements in a tropical region. J Environ Monit 13(8):2134–2142. https://doi.org/10.1039/c1em10240k
Habig WH, Jakoby WB (1981) Glutathione S-transferases (rat and human). Methods Enzymol 77(January):218–231
Hussain M, Madl P, Khan A (2011) Lung deposition predictions of airborne particles and the emergence of contemporary diseases, part-I. Health 2(2):51–59 http://www.thehealthj.com/may_2011/lung_deposition_predictions_of_airborne_particles.pdf
Jihen EH, Sonia S, Fatima H, Tahar SM, Abdelhamid K (2011) Interrelationships between cadmium, zinc and antioxidants in the liver of the rat exposed orally to relatively high doses of cadmium and zinc. Ecotoxicol Environ Saf 74(7). Elsevier):2099–2104. https://doi.org/10.1016/j.ecoenv.2011.06.008
Kalinowski DS, Stefani C, Toyokuni S, Ganz T, Anderson GJ, Subramaniam NV, Trinder D et al (2016) Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim Biophys Acta Mol Cell Res 1863(4). Elsevier B.V.):727–748. https://doi.org/10.1016/j.bbamcr.2016.01.026
Kato T, Totsuka Y, Hasei T, Watanabe T, Wakabayashi K, Kinae N, Masuda S (2011) In vivo examination of the genotoxicity of the urban air and surface soil pollutant, 3,6-dinitrobenzo[ e ]pyrene, with intraperitoneal and intratracheal administration. Environ Toxicol 28(10):588–594. https://doi.org/10.1002/tox.20754
Ku T, Chen M, Li B, Yang Y, Li G, Sang N (2016) Synergistic effects of particulate matter (PM 2.5 ) and sulfur dioxide (SO 2 ) on neurodegeneration via the microRNA-mediated regulation of tau phosphorylation. Toxicol Res 6(1). Royal Society of Chemistry):7–16. https://doi.org/10.1039/C6TX00314A
Lee TK, Li L, Ballatori N (1997) Hepatic glutathione and glutathione S-conjugate transport mechanisms. Yale J Biol Med 70(4):287–300
Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T (2019) Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 40(20):1590–1596. https://doi.org/10.1093/eurheartj/ehz135
Li W, Wang C, Wang H, Chen J, Yuan C, Li T, Wang W et al (2014) Distribution of atmospheric particulate matter (PM) in rural field, rural village and urban areas of Northern China. Environ Pollut 185. Elsevier Ltd:134–140. https://doi.org/10.1016/j.envpol.2013.10.042
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp Toxicol Pathol 64(6). Elsevier GmbH):575–582. https://doi.org/10.1016/j.etp.2010.11.016
Martinez-Finley EJ, Chakraborty S, Fretham SJB, Aschner M (2012) Admit one: how essential and nonessential metals gain entrance into the cell. Metallomics 4(7):593–605. https://doi.org/10.1039/c2mt00185c
Massone CG, Wagener ALR, Abreu HM, Gioda A (2015) Hydrocarbon concentration and source appraisal in atmospheric particulate matter (PM2.5) of an urban tropical area. Environ Sci Pollut Res 22(19):14767–14780. https://doi.org/10.1007/s11356-015-4695-0
Mateus VL, Monteiro ILG, Rocha RCC, Saint’Pierre TD, Gioda A (2013) Study of the chemical composition of particulate matter from the Rio de Janeiro Metropolitan Region, Brazil, by inductively coupled plasma-mass spectrometry and optical emission spectrometry. Spectrochim Acta B At Spectrosc 86. Elsevier B.V:131–136. https://doi.org/10.1016/j.sab.2013.03.003
Messarah M, Klibet F, Boumendjel A, Abdennour C, Bouzerna N, Boulakoud MS, El Feki A (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol 64(3). Elsevier GmbH):167–174. https://doi.org/10.1016/j.etp.2010.08.002
Mirowsky JE, Jin L, Thurston G, Lighthall D, Tyner T, Horton L, Galdanes K et al (2015) In vitro and in vivo toxicity of urban and rural particulate matter from California. Atmospheric Environment 103. Elsevier Ltd:256–262. https://doi.org/10.1016/j.atmosenv.2014.12.051
Nebendahl K (2000) Routes of administration. In: Krinke GJ (ed) The laboratory rat, vol 16. Elsevier, London, pp 463–483. https://doi.org/10.1016/B978-012426400-7.50063-7
Oakes KD, Van Der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in White Sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63(4):447–463. https://doi.org/10.1016/S0166-445X(02)00204-7
Olawoyin R, Schweitzer L, Zhang K, Okareh O, Slates K (2018) Index analysis and human health risk model application for evaluating ambient air-heavy metal contamination in Chemical Valley Sarnia. Ecotoxicol Environ Saf 148(June 2017). Elsevier Inc):72–81. https://doi.org/10.1016/j.ecoenv.2017.09.069
Pascal M, Falq G, Wagner V, Chatignoux E, Corso M, Blanchard M, Host S, Pascal L, Larrieu S (2014) Short-term impacts of particulate matter (PM10, PM10-2.5, PM2.5) on mortality in nine French cities. Atmos Environ. https://doi.org/10.1016/j.atmosenv.2014.06.030
Paßlack N, Mainzer B, Lahrssen-Wiederholt M, Schafft H, Palavinskas R, Breithaupt A, Neumann K, Jürgen, Zentek (2014) Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium and lead in the equine liver and kidneys. SpringerPlus 3(1):343. https://doi.org/10.1186/2193-1801-3-343
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97. Elsevier Masson SAS:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Polichetti G, Cocco S, Spinali A, Trimarco V, Nunziata A (2009) Effects of particulate matter (PM10, PM2.5 and PM1) on the cardiovascular system. Toxicology 261(1–2):1–8. https://doi.org/10.1016/j.tox.2009.04.035
Pope CA III (2000) Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ Health Perspect 108:713–723. https://doi.org/10.1289/ehp.00108s4713
Pulido MD, Parrish AR (2003) Metal-induced apoptosis: mechanisms. Mutat Res Fundam Mol Mech Mutagen 533:227–241. https://doi.org/10.1016/j.mrfmmm.2003.07.015
Qin Z, Hou H, Feng F, Wu J, Han B, Yang W, Zhang L et al (2017) Fine particulate matter exposure induces cell cycle arrest and inhibits migration and invasion of human extravillous trophoblast, as determined by an ITRAQ-based quantitative proteomics strategy. Reprod Toxicol 74. Elsevier Inc:10–22. https://doi.org/10.1016/j.reprotox.2017.08.014
Reckziegel P, Dias VT, Benvegnú DM, Boufleur N, Barcelos RCS, Segat HJ, Pase CS, dos Santos CMM, Flores ÉMM, Bürger ME (2016) Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol Rep 3. Elsevier Ireland Ltd:351–356. https://doi.org/10.1016/j.toxrep.2016.02.005
Ribeiro J d P, Kalb AC, Campos PP, De La Cruz ARH, Martinez PE, Gioda A, de Souza MM, Gioda CR (2016) Toxicological effects of particulate matter (PM2.5) on rats: bioaccumulation, antioxidant alterations, lipid damage, and ABC transporter activity. Chemosphere 163:569–577. https://doi.org/10.1016/j.chemosphere.2016.07.094
Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, Valença SS, Saldiva PH, Faffe DS, Zin WA (2011) Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol 23(December 2010):257–267. https://doi.org/10.3109/08958378.2011.566290
Rodríguez-Cotto RI, Ortiz-Martínez MG, Rivera-Ramírez E, Mateus VL, Amaral BS, Jiménez-Vélez BD, Gioda A (2014) Particle pollution in Rio de Janeiro, Brazil: increase and decrease of pro-inflammatory cytokines IL-6 and IL-8 in human lung cells. Environ Pollut 194:112–120. https://doi.org/10.1016/j.envpol.2014.07.010
Sá RC, Zeman KL, Bennett WD, Kim Prisk G, Darquenne C (2017) Regional ventilation is the main determinant of alveolar deposition of coarse particles in the supine healthy human lung during tidal breathing. J Aerosol Med Pulm Drug Deliv 30(5):322–331. https://doi.org/10.1089/jamp.2016.1336
Shi H, Hudson LG, Liu KJ (2004) Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med 37(5):582–593. https://doi.org/10.1016/j.freeradbiomed.2004.03.012
Shrey K, Suchit A, Deepika D, Shruti K, Vibha R (2011) Air pollutants: the key stages in the pathway towards the development of cardiovascular disorders, Environmental Toxicology and Pharmacology. Elsevier B.V. https://doi.org/10.1016/j.etap.2010.09.002
Shridhar V, Khillare PS, Agarwal T, Ray S (2010) Metallic species in ambient particulate matter at rural and urban location of Delhi. J Hazard Mater 175(1–3):600–607. https://doi.org/10.1016/j.jhazmat.2009.10.047
Siedlinski M, Postma DS, Van Diemen CC, Blokstra A, Smit HA, Marike Boezen H (2008) Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am J Respir Crit Care Med 178(1):13–19. https://doi.org/10.1164/rccm.200711-1749OC
Simkhovich BZ, Kleinman MT, Kloner RA (2008) Air pollution and cardiovascular injury. J Am Coll Cardiol 52(9):719–726. https://doi.org/10.1016/j.jacc.2008.05.029
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2015) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 1(September):1–37. https://doi.org/10.1007/s00204-015-1579-5
Vreeland H, Weber R, Bergin M, Greenwald R, Golan R, Russell AG, Verma V, Sarnat JA (2017) Oxidative potential of PM2.5 during Atlanta rush hour: measurements of in-vehicle dithiothreitol (DTT) activity. Atmos Environ 165. Elsevier Ltd:169–178. https://doi.org/10.1016/j.atmosenv.2017.06.044
Wang Y, Xiong L, Tang M (2017) Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol 37(6):644–667. https://doi.org/10.1002/jat.3451
Weber SA, Insaf TZ, Hall ES, Talbot TO, Huff AK (2016) Assessing the impact of fine particulate matter (PM2.5) on respiratory-cardiovascular chronic diseases in the New York City Metropolitan Area using hierarchical Bayesian model estimates. Environ Res 151. Elsevier:399–409. https://doi.org/10.1016/j.envres.2016.07.012
Wei T, Tang M (2018) Biological effects of airborne fine particulate matter (PM 2.5) exposure on pulmonary immune system. Environ Toxicol Pharmacol 60(July). Elsevier):195–201. https://doi.org/10.1016/j.etap.2018.04.004
White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ (2003) Fluorescence-based microtiter plate assay for glutamate–cysteine ligase activity. Anal Biochem 318(2):175–180. https://doi.org/10.1016/S0003-2697(03)00143-X
Williams KM, Franzi LM, Last JA (2013) Cell-specific oxidative stress and cytotoxicity after wildfire coarse particulate matter instillation into mouse lung. Toxicol Appl Pharmacol 266(1). Elsevier Inc):48–55. https://doi.org/10.1016/j.taap.2012.10.017
Wu J-Z, Ge D-D, Zhou L-F, Hou L-Y, Zhou Y, Li Q-Y (2018) Effects of particulate matter on allergic respiratory diseases. Chronic Dis Transl Med 4(2). Elsevier Masson SAS):95–102. https://doi.org/10.1016/j.cdtm.2018.04.001
Yi S, Fang, Zhang F, Qu, Ding W (2011) Water-insoluble fraction of airborne particulate matter (PM 10) induces oxidative stress in human lung epithelial A549 cells. Environ Toxicol 29(2):1–8. https://doi.org/10.1002/tox.21750
Zar J (2010) Biostatistical analysis. In: Pearson (ed) Journal of Chemical Information and Modeling. Fifth. Prentice-Hall/Pearson, United States of America
Zou A-P, Li N, Cowley AW (2001) Production and actions of superoxide in the renal medulla. Hypertension 37(2):547–553. https://doi.org/10.1161/01.HYP.37.2.547
Acknowledgments
The authors thank Brazilian Agencies CNPq, CAPES, and FAPERJ for financial support and INEA-RJ for providing PM2.5 samples. J.M. Monserrat is a productivity research from CNPq (PQ 308539/2016-8). A. Gioda thanks CNPq for the Bolsa de Produtividade and FAPERJ for the Auxilio Cientista do Nosso Estado.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Paula Ribeiro, J., Kalb, A.C., de Bastos Maya, S. et al. The impact of polar fraction of the fine particulate matter on redox responses in different rat tissues. Environ Sci Pollut Res 26, 32476–32487 (2019). https://doi.org/10.1007/s11356-019-06452-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06452-9