Abstract
The Argentine giant tegu, a large lizard native to South America, was first discovered as established in the USA in scrub habitats of west-central Florida in 2006. Invasive populations potentially could occupy an extensive range of habitats and in much of the southern United States and Mexico and threaten many native species. The Argentine giant tegu was recently deemed as having a “highest impact concern” among the invasive reptile species most threatening to Florida ecology. Among the most rewarding research directions identified for this species was “having a reliable and practical method to detect/monitor” them. We address this need by evaluating five methods for monitoring Argentine giant tegus on how well each method detected the species and whether the observations were sufficient to quantitatively assess population abundance using a widely applicable framework for indexing animal populations. Passive tracking plots were the most efficient and effective means for detecting tegus and calculating abundance indices but were best suited for late winter to spring before summer rains compacted tracking substrates. Gopher tortoise burrows are often used by tegus and camera traps on their entrances proved able to obtain data suitable for indexing populations but required more labor and expense than tracking plots. Trapping either at gopher tortoise burrows or along drift fences was ineffective at capturing tegus. Similarly, visual encounter transects were not effective for observing tegus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Florida’s invasive species: the making of reptile dysfunction
Florida has more introduced animals than any other region of the USA, and it also ranks highly in this regard worldwide (Corn et al. 2002; SFWMD 2008; U.S. Congress 1993). Florida is exceptionally susceptible to the introduction and establishment of a vast array of species due to the state’s warm climate, major ports of entry to the USA for many wildlife species (both legal and illegal), a flourishing captive wildlife industry, and its location in a region vulnerable to destructive hurricanes that can release captive animals (e.g., Corn et al. 2002; Hardin 2007). Furthermore, potential invaders to Florida often contend with relatively fewer native species than in most tropical/subtropical areas, since Florida is relatively isolated from land with similar climates. Thus, the state’s vertebrates typically originate at the southern extremes of their ranges in the USA (Hardin 2007).
Considering Florida’s invitingly mild climate, it is not surprising that the largest proportion of its invasive vertebrate species are reptiles (Hardin 2007). For nearly a century and a half, non-native herpetofauna species have been documented as established in Florida (Cope 1875; Meshaka 2011; Meshaka et al. 2004), with new species establishment accelerating over the last half-century (Meshaka 2011; Meshaka et al. 2004). Invasive snakes, lizards, turtles, and crocodilians are all breeding in Florida (e.g., Engeman et al. 2011; Meshaka 2011; Meshaka et al. 2004), and some of the largest snakes and lizards in the world are now invasive species in Florida (e.g., Engeman et al. 2011; Hardin 2007; Meshaka 2011; Meshaka et al. 2004). The preponderance of invasive reptiles in Florida and their impacts on native fauna led to this situation being pronounced as “reptile dysfunction” (Engeman et al. 2011).
Invasive lizards are particularly prominent in terms of numbers of species, the variety of niches occupied, and the sizes of the largest species (e.g., Hardin 2007; Meshaka 2011; Meshaka et al. 2004). Florida has been swept by waves of non-native lizards, which subsequently were joined or superseded by a tide of additional non-native lizard species (e.g., Bartlett and Bartlett 1999; Meshaka 2011; Meshaka et al. 2004). The rapid expansion of some species has been closely documented (e.g., Meshaka et al. 2005), while others suddenly seem to appear. Now, the number of exotic lizard species breeding in Florida exceeds the number of native species, and over three times as many exotic lizard species as native species breed in south Florida (Hardin 2007). The largest lizard species breeding in North America are invasive species established in Florida, with native origins in Africa, Central America, and South America. The Argentine giant tegu (aka, Argentine black and white tegu, Salvator merianae), with which we are concerned in this paper, is one of these species.
Argentine giant tegu
The Argentine giant tegu is a large, stout, omnivorous lizard (Fig. 1) native to South America (Galetti et al. 2009; Luxmoore et al. 1988; Mercolli and Yanosky 1994), and it is relatively new to the established exotic reptile scene in Florida (Hardin 2007). Tegus, the largest lizards in the New World (weighing up to 8 kg, Lopes and Abe 1999), were first discovered in 2006 as established in Florida in Hillsborough and Polk counties of west-central Florida (Hardin 2007). The likely origin of this population was animals imported from Paraguay during 2000–2002 (Enge et al. 2006), with anecdotal reports suggesting these lizards may have been introduced into the wild by a reptile dealer (Hardin 2007). At the time of discovery, credible accounts indicated the population already occurred in an area over 100 km2 in size. All age classes, adults, juveniles, and hatchlings have been regularly observed, particularly in the vicinity of the Balm-Boyette Scrub Nature Preserve (Avery et al. 2016; Enge et al. 2006).
The Argentine giant tegu can occupy an extensive range of habitats (e.g., Vitt 1995), with invasive populations potentially occupying a large portion of the southern United States and Mexico (Jarnevich et al. 2018). It is a fecund species with annual clutches of 20–45 eggs and can rapidly populate an area (Enge et al. 2006; Engeman and Avery 2016). Tegus are omnivorous generalists that pose threats to many native species in Florida, including species of special management concern like gopher tortoise (Gopherus Polyphemus) and Florida burrowing owls (Athene cunicularia floridana) (Engeman et al. 2011). Being omnivorous, tegus could also disperse seeds of invasive plants in the same fashion as iguanas (Enge et al. 2006; Engeman et al. 2011; Jackson and Jackson 2007). This species has also been observed to exhibit agonistic killing behavior towards native snakes, thereby possibly posing a non-predatory threat to listed species like eastern indigo snakes (Drymarchon couperi) (Kaiser et al. 2013). Tegu usage of gopher tortoise burrows suggests similar burrow usurpation and juvenile predation impacts might take place, as observed in another large invasive lizard species in south Florida, the black spiny-tailed iguana (Ctenosaura similis) (Avery et al. 2009; Engeman et al. 2009b). In sum, tegus appear to present the entire spectrum of threats to the environment as Florida’s other largest invasive lizard species combined, including Nile monitors (Varanus niloticus), green iguanas (Iguana iguana), and black spiny-tailed iguana (Engeman et al. 2011).
The Argentine giant tegu population in Hillsborough and Polk counties was highlighted in a recent collaboration of scientists and managers that reviewed the invasive reptile and amphibian species posing the greatest threats for ecological harm in Florida (Engeman and Avery 2016). Key products from the collaboration included identification of the circumstances and geographic scales where threats from invasive reptiles are most likely to have practical and successful mitigation, and the identification of practical research directions most likely to rapidly produce useful control tools. The Argentine giant tegu was identified as having a “highest impact concern” among the 37 invasive reptile species evaluated. Reflecting an old adage that states “If you can’t monitor it, you can’t manage it” (Engeman et al. 2013), one of the actions identified for most rewarding research for this species included “having a reliable and practical method to detect/monitor Hillsborough and Polk County tegu populations…,” and “…allow managers to: detect and control incipient populations, identify where control is most needed, determine optimal timing of control, assess control efficacy, and recognize reinvasion” (Engeman and Avery 2016). Echoing this, the Florida Fish and Wildlife Conservation Commission also affirmed the necessity for monitoring tegu populations: “Monitoring these (tegu) populations and stopping the spread of this species is vital to maintaining Florida’s native wildlife” (FWC 2015).
Here, we address this need for methods to detect and monitor tegu populations. We considered five observation methods for monitoring Argentine giant tegus in the wild to determine how well the species was detected and whether the observations were sufficient to quantitatively monitor the population abundance using a widely applicable framework for sampling and indexing animal populations (Engeman 2005).
Methods
Study area
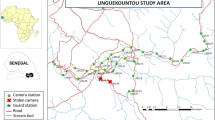
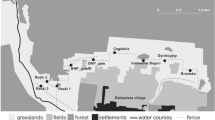
The study was conducted in the Balm-Boyette Scrub Preserve (BBSP), a 2316-ha preserve in Hillsborough County, Florida. BBSP holds a large area of undeveloped scrub habitat and is listed as an “Exemplary Site” for scrub habitat by the Florida Natural Areas Inventory (FNAI 2010). Natural habitats within BBSP include both sand pine (Pinus clausa) and xeric oak (Quercus spp) scrub, as well as pine flatwoods, hardwood hammock, wet prairie, freshwater marsh, cypress swamp, and hardwood swamp (FNAI 2010), as well as abandoned phosphate pits. BBSP is the apparent ground-zero area for establishment of Argentine giant tegus as an invasive species in Florida, with the initial observations recorded there in 2006 (Hardin 2007). Many protected plants and animal species have been verified in BBSP, a variety of which could be negatively impacted by Argentine giant tegus.
Observation methods
We tested 5 observation methods for detecting Argentine giant tegu presence and monitoring their abundance. Ideally, methods aimed at monitoring populations would (1) be practical to use in field conditions, (2) be sensitive enough to reflect changes in numbers or trends, (3) rely on few assumptions which could be relatively easily met, and (4) be associated with statistical methodology that allows valid comparisons (e.g., Engeman 2005). Substantial efficiencies in sampling might be gained if the method makes it possible to simultaneously monitor multiple species, which may indicate inter-species interactions or might allow assessment of management actions towards more than one species at the same time. Examination of trends through time or across geographical areas for potentially interacting species may provide insight into the influences between species (e.g., Allen et al. 2013, 2014; Engeman 2005). For example, in the scrub habitat where our study took place, valuable information potentially could be obtained on possible impacts from tegus on gopher tortoises (which could occur through predation of nests or hatchlings, or exclusion from burrows).
The observation methods we tested, when joined with proper field design and analytical procedures, as well as sufficient tegu observations, held promise to validly monitor tegu populations. The methods that we formally tested in a designed study had first shown promise in ad hoc trials to gauge whether formal testing was merited.
Visual encounter transects
This has been a common approach for surveying herpetofauna (e.g., Hare 2012; Lambert 2002), and it was the simplest technique to implement of the five we identified for field-testing. It also was the least costly in terms of labor and material, and the method was chosen for testing because it was the means by which tegus were first documented in BBSP.
Five visual encounter transects were established, three in scrub habitat restored by canopy reduction using mechanical means and fire (referred to here as restored habitat), and two in long-term fire-suppressed scrub habitat (referred to here as fire-suppressed habitat). Each transect began at the edge of a fire lane, extended across a patch of scrub to end at a fire lane on the opposite side. Each transect’s pathway was marked at 50-m intervals with an upright 1.2-m section of PVC pipe. Each pipe was mapped using a handheld GPS unit and labeled to identify specific locations. Transects ranged from 250 to 400 m in length. Surveys were conducted approximately monthly in June, July, and August 2011 and once in March 2012. Surveys were conducted midmorning and mid-afternoon except for the July survey, which was limited to the mid-morning session because of severe afternoon thunderstorms. All participants attempted to walk transects at approximately the same rate. The walking speed of each person was gauged by recording arrival times at each of the 50-m interval markers. Lizards observed along the 50-m intervals of each transect were identified to species, if possible, and recorded. If a lizard could not be identified to species the observation was recorded as “unknown lizard.”
Trapping at gopher tortoise burrows
Argentine giant tegus regularly invade gopher tortoise burrows and have consistently been observed to do so at BBSP (Hardin 2007; Johnson and McGarrity 2017). Being able to take advantage of animal behavior can be especially valuable for monitoring their populations (Allen and Engeman 2015; Engeman 2005; Engeman et al. 2017), and we sought to take advantage of tegu usage of gopher tortoise burrows (Fig. 2) to test its suitability for developing monitoring procedures. Following Enge (2007) for one such approach, gopher tortoise burrows were targeted for trapping tegus.
Box traps were constructed of hardware cloth (approx. 2.54 × 2.54 cm mesh) and measured approximately 60 × 60 × 8 cm (Fig. 3). The roof of the trap was one piece, as were each of the four vertical walls. The lower half of the four vertical walls was wrapped with plastic gutter screens to prevent small tegus from escaping through the 2.54-cm square mesh. A 20 × 20 cm opening was cut into the center of the trap floor and the interior was accessed by a one-way door placed over this opening. When in use, the box trap was secured to the ground with tent stakes after being placed over the burrow entrance to force animals leaving the burrow into the trap. A 60 × 30 × 2.54 cm sheet of foam insulation secured to the roof of the trap provided shade.
For another trap design, one-way funnel traps were constructed of nylon window screen material formed in the shape of a tube approximately 20 cm in diameter and 1 m long. A funnel made of the same screening material and facing inwardly was attached to one end of the tube, with the opposite end secured shut. The end of the trap with the funnel was placed a few centimeters into the entrance of a gopher tortoise burrow. Gaps between the trap and walls of the burrow were filled with sand. Cut palmetto fronds were placed over the trap to provide shade for any captive animals.
We first conducted surveys to locate gopher tortoise burrows suitable for trap placement. Each burrow encountered was assigned a status of active, inactive, or abandoned per Cox et al. (1987) and categorized as to whether the burrow was suitable for a trap. Two trapping sessions were conducted in April 2010 (5–8 Apr and 26–28 Apr). Ten burrows were selected for trapping during the first session, with 5 burrows having a box trap placed over the entrance, and a one-way funnel trap placed in the entrance of the other 5. Trapping was limited to five box traps during the second session.
Drift fence arrays
Two drift fence arrays, one each in restored and fire-suppressed scrub habitat, were constructed of four fences aligned to the magnetic cardinal compass directions. Each of the four fences within an array was constructed from a single piece of 7.6 m × 50.8 cm aluminum sheet metal placed lengthwise and held in place vertically by burying the lower 25 cm of the 50.8 cm into the ground (Fig. 4). An 18.9-L bucket was placed at both ends of each fence and buried deep enough that the opening was countersunk approximately two cm below the ground surface. The bottom and sides of each bucket were perforated with numerous holes (approx. 3.2-mm diameter) to allow for drainage. Cover for captured burrowing animals was provided by 2–3 cm of sand in the bottom of each bucket. Each bucket was shaded with a lid suspended approximately 5 cm above the bucket opening and held in place with wooden clothespins. We also used these lids to seal the buckets shut when the arrays were not in use.
Also, one box-shaped funnel trap was positioned on each side of each of the 4 fences in each array at the fence’s mid-point. The box-shaped funnel traps were constructed of 1.27-cm mesh hardware cloth, and were approximately 25 cm in height, 22 cm in width, and 60 cm in length. The funnel traps were held in place along the fence with four metal rods pushed through the corners of each trap and into the ground. A 60 × 60 cm piece of plywood provided shade for animals captured in the funnel traps. Trapping took place for five consecutive days on a monthly basis from May 2011 through April 2012, except for the months of October and December 2011, and January and February 2012. Traps were opened between 9:00–10:00 am on the first day and checked twice daily, first between 8:00–10:00 am and again between 15:00–17:00 pm. All captured vertebrates besides tegus were identified to at least the generic level, and to the species level if possible and then released. At the end of a trapping session, the funnel traps and plywood were removed; all materials were removed from each bucket and the array deactivated by securing the lid on each bucket.
Tracking plots
Tracking plots have been a valuable means to detect and monitor many species of wildlife in a quantitatively valid manner (often simultaneously), including reptiles (e.g., Allen and Engeman 2015; Engeman 2005; Allen and Engeman 2015; Allen et al. 2014). The sandy substrates found at BBSP formed generally excellent tracking surfaces, and the many fire lanes throughout the property allowed for general and thorough geographic placement of tracking plots with little constraint on the plot size.
Fourteen 100-m tracking plots were delineated on fire lanes in restored scrub habitat. Each sampling event for observing these plots took place over three consecutive days. The same plot locations were used on each sampling occasion throughout the course of the study (e.g., Ryan and Heywood 2003; Engeman 2005). On the first day, plot surfaces were smoothed for reading tracks by dragging a heavy metal mat pulled by a pickup truck (Fig. 5) between the hours of 3:00 and 5:00 pm (for descriptions of other applications see Engeman et al. 2000, 2007). Prior to any expected rainfall during the afternoon of the second day, every plot was examined for tracks made by tegus, gopher tortoises, snakes, feral swine, and other sympatric vertebrate species that could be identified. The numbers of track incursions (number of sets of tracks) into the plots were recorded for each species. One set of tracks counted as one incursion without regard to the distance that the animal traveled in the plot before exiting. The freshly smoothed, sandy soil allowed tracks to be easily detected. The process of preparing the tracking surfaces and recording the number of intrusions was repeated, with every plot again examined for tracks. Since only two days of observations were made, the tracking plots were not smoothed after this second reading. Sampling events took place in April 2011, twice in June 2011, July 2011, August 2011, September 2011, March 2012, and April 2012.
Camera traps
This was the second method tested aiming to take advantage of tegu usage of gopher tortoise burrows and it was the most technical and costly method tested in terms of time and material. Preliminary field trials demonstrated that adult and older juvenile tegus were large enough to trigger a typical trail camera, thereby establishing a viable foundation for testing camera traps to detect the presence of tegus, quantitatively monitor their population, investigate the frequency with which tegus visited tortoise burrows, and potentially document whether tegus depredate nests of gopher tortoise eggs (which often are located within the apron of an adult tortoise’s burrow). Additionally, the broad distribution of tortoise burrows in areas with different habitat management histories at BBSP potentially allowed investigation of whether tegus were more likely to exploit the more open, restored scrub habitat versus fire-suppressed scrub habitat with a dense canopy of hardwoods and sand pines.
Eight burrows in each of restored and fire-suppressed scrub habitats were randomly selected as camera trap stations. At each station, one Cuddeback No Flash camera was attached with a two-way universal-joint tree mount to a fence post within 2 m of the burrow entrance. Each fence post was located to avoid accidentally collapsing the burrow (i.e., directly above the roof of a burrow), not block obvious pathways used by gopher tortoises, and not require significant vegetation removal. Each post was placed at acute or oblique angles relative to the burrow entrance (Fig. 6), because preliminary field tests indicated tegus passing in front of a camera at a right angle often moved beyond the camera’s field of view before the motion sensor triggered the camera. Each camera was programed to capture one still image followed by 60 s of video and then to automatically reset 60 s after recording the video. The motion sensor of each camera was set to the highest level of sensitivity. All cameras were operational 24 h/day. Each image and video along with the date and time were recorded onto a 2.0-Gb SD data card. Cameras were placed and became operational at the 16 selected burrows between 4 and 10 May 2010 and remained so until 5 July 2011 (totaling 6,768 potential camera trapping days).
Cameras were checked roughly bi-weekly when data cards were replaced. Batteries were replaced if the status indicator showed ≤ 25% power remaining. Data cards were reviewed to confirm each camera had been functional for the previous 2 weeks and to search for evidence of tegus. Malfunctioning cameras were replaced.
General approaches for abundance metrics
Engeman (2005) outlined a widely applicable framework for sampling animal populations using common observation methods, such as tracking plots, camera traps, visual observations, trapping, and other means. This paradigm provided principles and analytical methods governing their proper use for making reliable inferences about species abundance. Our observation and data collection methods were designed to incorporate these concepts as a structural foundation for valid population monitoring.
Briefly, the paradigm is a straightforward procedure for data structure, making observations at each sampling location, and analyzing the resulting data according to statistically derived formulae reliant on minimal assumptions (Engeman 2005). This very general approach has been used for monitoring substantial numbers of wildlife species by applying a diversity of observation methods (e.g., Allen et al. 2013, 2014; Baldwin et al. 2014; Engeman 2005). The locations for making observations are referred to as stations. For example, each station might be a camera location, a plot for observing tracks, or other types of observation (see Engeman 2005). To account for variability over time, stations are best observed on more than one occasion during an assessment period. This typically denotes taking measurements at each station on each of multiple days. For simplicity, we refer to the time dimension as a day effect, representing the common situation where observations at each monitoring session are made on multiple, usually consecutive, days. Many measurement types can fit this structure, including the general categories of animal counts, measurement of animal sign, and catch per unit effort. Given this data structure and associated observational measurements, a versatile abundance index (AI) has been defined as the mean of the daily means and is represented by the following formula (e.g., Engeman 2005):
where xij represents the measurement at the ith observation station on the jth day, d is the number of days of observation, and sj is the number of stations contributing data on the jth day. Its associated variance formula was derived using minimal analytic assumptions whereby neither days nor stations are assumed independent (Engeman 2005). When traps form the observation stations, the response for each trap each day is binary, yes-no a species was caught. Thus, when entered as 0’s in cases of no captures or 1’s for captures, the calculation becomes captures per trap-day, as customarily reported for trapping data.
Thus, the specific observation stations and measurements for the 5 methods we tested included (1) visual encounter transects comprising the “stations,” with counts per transect per day as the measurements (2) gopher tortoise burrows with a box or funnel trap formed the stations and the number (0 or 1) of tegus captured per trap per day were the observations, (3) each drift fence array was a station and the number captured per array per day were the measurements, (4) the 100-m tracking plots were the stations and the number of track intrusions per plot per day were the measurements, and (5) gopher tortoise burrows with a camera trap were the stations and the number of photos of tegus per camera per day was the measurement. Considering that it is not the intent to monitor a geographical area, but rather the animal population inhabiting the geographical area, substantial sampling efficiencies may be possible if animal usage within the study area can be anticipated (e.g., Engeman 2005). In such cases, observation stations could be placed where animals would more likely be anticipated to be observed. Because of regular observed use of gopher tortoise burrows, two of our observation station placement strategies attempted to take advantage of this behavior whereby traps were placed over burrow entrances and camera stations were placed at burrow entrances.
Results
Four of the five methods detected the presence of tegus by yielding at least one tegu observation. Although placing box or funnel traps in the entrance of gopher tortoise burrows resulted in the capture of tegus during preliminary trials, this method did not capture any tegus during the formal field tests (e.g., Fig. 7). The other four methods resulted in varying degrees of efficacy and efficiency, relative to effort.
Visual encounter transects
Only one confirmed sighting of a tegu was made while walking ~ 24,000 m of transects combined, making this method very inefficient for detecting tegus. Also indicating the inefficiency of the method, the encounter rates for the two most common native lizards also were very low, approximately 1 six-lined race runner (Aspidoscelis sexlineata) for every 750 m of transect walked, and one fence lizard (Sceloporus undulatus) for every 923 m walked.
Drift fence arrays
Both arrays were operational for forty days. Only one tegu was captured by this method for a capture rate of 0.0125 tegus/array/day for the combined 80 array-days. Drift fence arrays, while sound conceptually, appear in practice to have very limited applicability for detecting the presence or monitoring the abundance of tegus. The 18.9-L buckets we used appeared only large enough to hold hatchling or young-of-the-year juvenile tegus, with larger individuals probably able to climb out. Similarly, no tegus of any size entered funnel traps positioned along the drift fences, although several times, tegu tracks were observed leading to and away from a bucket or funnel trap without entering the device.
Tracking plots
The 14 100-m long plots established in fire lanes throughout the study area proved to be the most efficient method for detecting the presence of tegus, and also allowed calculation of abundance indices (Table 1). With encounter rates ranging from 0.107 to 0.714 track intrusions /100-m plot/day, and an overall average of 0.333 track intrusions /100-m plot/day, this method provided roughly an order of magnitude greater number of observations than the overall rate from the camera trapping effort (next section), the second most effective means for obtaining tegu observations. The most distinctive evidence of a tegu entering a tracking plot was footprints straddling an undulating track left by the tail as the lizard walked (Fig. 8). The number of detectable and identifiable tracks in the plots declined across the seasons from April to August (Table 1), which we attributed to frequent summertime rains compacting the sand substrate such that tracks were not readily produced. For example, during July and August, even the footprints left by workers as they checked the plots were barely detectable.
As an example of the method’s potential for simultaneously monitoring multiple species we also include the observations of gopher tortoise tracking plot intrusions in Table 1. They too were readily detected, with the numerous observations allowing the calculation of abundance indices. This might be one example where over the course of a sufficient period of time (years) impacts to the tortoise population by tegus potentially could be indicated (e.g., Allen et al. 2014; Engeman et al. 2017). Also, while not the focus of our tegu monitoring method research, our tracking plots also recorded the track intrusions of feral swine (Sus scrofa), a highly destructive invasive species pervasive throughout Florida (e.g., Engeman et al. 2009a). Feral swine also are found in most areas of Florida where tegus have been reported and managers could therefore potentially monitor two important invasive species simultaneously, although there would be little expectation of interaction between them.
Camera traps at gopher tortoise burrows
Tegus are known to have a distinct active season likely influenced by temperature and photoperiod (Enge 2007), and we were able to define tegu active seasons post hoc for our study. The active season for 2010 began for our purposes the day cameras were deployed (4 May) and ended on 29 September, the last date for which a tegu was photographed prior to the onset of winter (21 December). The 2011 active season began 28 February, the first day a tegu was photographed since the 29 September 2010 observation. Sampling during the 2011 active season ended on 5 July when the camera trapping portion of our study concluded, and all cameras were retrieved (although this was during the time of prime activity period). There were 4,608 potential camera trapping days overall, sampled during two tegu active seasons, with 2384 and 2224 camera trapping days during the 2010 and 2011 active seasons, respectively. We calculated abundance indices for both restored and fire-suppressed habitats during 19 camera trapping sessions in the 2010 and 2011 active seasons (Table 2). Most often (68% of sessions), the number of tegu photos/camera station/day was higher in restored habitat than in fire-suppressed habitat. Overall sessions, the number of tegu photos/camera station/day averaged 0.044 and 0.020 in the restored and fire-suppressed habitats, respectively. Recall there was an overall average of 0.333 tegu track intrusions/100-m plot/day, a rate over 7.5 times as great as the higher rate of photos/camera station/day from the restored scrub habitat.
Discussion
General considerations
The method to select for detecting or monitoring a species depends in large part on what is needed to fulfill the surveillance objective (Engeman 2005; Engeman et al. 2017). Considerations would include whether the focus is to detect the presence of tegus or to monitor a tegu population, given that it has already been detected. Once an objective has been defined, the geographical scale of the effort and the resources available are probably the two most crucial elements for selecting the approach(es).
Throughout the west-central Florida area where our study took place, there are many square kilometers of very similar scrub habitat apparently suitable for occupation by Argentine giant tegus. (Such habitat is also extensively found in east-central Florida and other parts of the state.) Because much of the southern United States and Mexico appear suitable for invasion by the species, monitoring methods could potentially have extensive application should invasive populations become established elsewhere. At present, outside of anecdotal reports little is known about whether tegu introduction has taken place in this extensive landscape. Probably, the most efficient approach to survey these widespread lands would be through tracking plots. Since population monitoring would not be a consideration until a population is detected in an area, further efficiencies can be obtained by not having a need for a rigorous sampling design. Many of these areas have dirt roads and fire lanes with similarly sandy substrates that could be searched for tracks. A midday search conducted the day after an afternoon thundershower in late winter or spring would reduce the need to smooth the roads prior to searches for tracks. Argentine giant tegus are the only large lizard species in this area of the state, although it is conceivable that monitor lizards (Varanus spp.) could be introduced and become established in the area, with iguana species less likely. In any case, all other lizard species in Florida that could make tracks like adult tegus are all invasive and of concern, thereby making their detection important also.
Once a population has been located, a more rigorous observational approach can be instituted to assess its abundance. Given a tegu population has been documented in an area, there is little value in a single measure of abundance, but there is great comparative utility for abundance measures. Changes across time or differences between geographical locations can be evaluated, which is especially important for assessing the impacts of management actions such as controlling tegus or deciding how to prioritize areas for control. Well-constructed indices can fulfill monitoring objectives while optimizing resources and offering quantitative comparisons between populations or changes within a population (e.g., Caughley 1977; Engeman 2005; Krebs 1998). Furthermore, methods that are easily understood and practical to apply (low-labor, low-cost methods) naturally make larger sample sizes more feasible and reduce survey costs (Engeman 2005; Franzetti et al. 2012). The ability to statistically detect differences increases with the precision (decreasing variance), and a method that is easily applied in the field will likely encourage more observations, with a consequent improvement in precision.
The five methods evaluated in this study demonstrated varying degrees of success in obtaining tegu observations as well as varying degrees of resources and effort to collect observations. All methods were implemented based on prior experience from observing tegus in BBSP and/or based on tried and true methods for monitoring wildlife. Method utility is strongly related to monitoring objectives, geographic scale, and effort. We also identified means to potentially improve methods for tegu observability.
Visual encounter transects
Opportunities to intercept and visually observe tegus with visual encounter transects were clearly limited to the times during which the surveys were conducted, while the other four observation methods operated around the clock for multiple consecutive days without a human observer present. Observing only 1 tegu in designed surveys in an area with a history of plentiful tegu sightings is too insensitive to reliably detect tegus, let alone monitor their abundance. However, enlisting the public to volunteer tegu sightings through citizen science could be valuable for detecting tegus. Fliers identifying tegus similar to the one developed by the Florida Fish and Wildlife Conservation Commission (FWC 2015) might be made available to hikers or workers traversing areas most susceptible to tegu invasion, with sighting locations reported by form (as part of a flier), website, or phone recording.
Trapping at gopher tortoise burrows
This was one of the more labor- and resource-intensive methods tested. Also, that zero tegus were captured during our testing of passive (no attractants) traps placed on gopher tortoise burrow entrances in an area where tegus were seemingly plentiful suggests this method would be too insensitive for general detection or monitoring of tegu populations. Subsequent to our data collection, some success at trapping tegus has taken place by using baited traps in the vicinities of tegu sightings such as the styles described in Avery et al. (2016). This invites the notion that baited traps placed near gopher tortoise burrows or sightings would yield more promising results. We feel this would be a mistake and would likely not be permitted, because it very likely would inadvertently increase the frequency with which tegus and other predators visit gopher tortoise burrows, thereby elevating risks to gopher tortoises, their eggs, and burrow commensals.
Drift fence arrays
This method was the most labor-intensive tested and decidedly inefficient at producing tegu observations. While modifications to the construction and procedures (including baited traps) might improve the collection of tegu observations, reductions in labor or resources would not occur. Envisioning a practical application for this method is difficult except possibly in highly sensitive circumstances where it would be essential to determine tegu presence, and all feasible methods would need to be applied (such as for a limited-range endangered species protection).
Tracking plots
This method was by far the most sensitive to tegu presence and resulted in data well-suited for monitoring abundance. It also required little labor and minimal resources.
Thus, this method appeared most useful for monitoring tegu population changes, such as from control efforts. This method has been used in many places in the world to gauge population trends and management actions on many species (e.g., Allen and Engeman 2015; Engeman and Allen 2000; Engeman et al. 2001, 2017). However, this method also had limitations. First, distinguishing between tracks left by small- to mid-sized tegus from those left by an armadillo (Dasypus novemcinctus) was sometimes difficult. There are two distinctive components of tegu tracks, footprints, and an undulating tail track (Fig. 8). The lateral tail undulation may not always be distinguishable, and the tail track can appear straight, like armadillo tracks. Second, this method requires patches of bare ground, making fire lanes and other primitive roads ideal options. They are typically composed of fine, white “sugar sand” in scrub habitats. This technique may not be as useful for hard substrates where tracks are difficult to record or where bare patches are uncommon, such as when fire lanes are vegetated. Third, the condition of the sand affects the likelihood that an animal walking through the plot will leave detectable and identifiable tracks. This study identified the optimal timeframe during their active season when tegu track deposition and identification would be best. The noticeable decline in detectable and identifiable tracks as the sand substrate became compacted by summer rains contrasted with our camera trapping results which did not indicate a decline in activity. Lastly, it may not be possible to attribute a set of large lizard tracks specifically to tegus, if more than one large lizard species inhabits an area. Prime examples would be Nile monitors, black spiny-tailed iguanas, and green iguanas (Engeman et al. 2011). Crucially, all large lizards of the general size of tegus are invasive species in Florida. Thus, it would be important to detect and monitor any large invasive lizard species. Use of tracking plots is the only method tested where observations of hatchling tegus could not be readily distinguished from similar-sized (adult) native lizard species. However, hatchling tegus rapidly grow to become larger than native lizards in 2–3 months (Enge 2007), thus having only a negligible impact on monitoring capabilities. In all cases, follow-up surveys could identify or verify species suspected to be tegus (or other large invasive lizard species).
Tracking plots present a cost-effective method that potentially can be applied over very large areas for detecting large exotic lizards, monitoring population trends, and assessing management actions. Because its application must operate within the method’s limitations to obtain maximal utility, we recommend limiting its use to late winter through late spring (i.e., March–May or June) before tracking substrates become compacted from summer rains. Our field-testing was limited to scrub habitats where fire lanes were mostly bare sugar sand. Using this method in other habitats may require vegetation removal to create tracking surfaces. Furthermore, a viable substrate for tracking plots may not exist for some areas, such as the Florida Keys which are typified by coral substrates. In such situations, sand might be brought in to create tracking surfaces, only if it is 100% certain to not introduce exotic plant or invertebrate species. In fact, our personal experience includes an application of importing sand on Key Largo for monitoring the feral cats (Felis catus) impacting the highly endangered Key Largo woodrat (Neotoma floridana smalli) (Engeman et al. 2009a). While suitable data were obtained for indexing cat populations, the amount of labor and resources needed to bring in sand and create tracking plots would all but restrict the practicality of this approach to the direst of circumstances (Engeman, unpublished data). Similarly, to import a substrate material that would not overly compact in summer rains to areas like our field site would be minimally rewarding relative to effort considering that the existing substrate allows tegus to be well-monitored during the winter-spring seasons.
Camera traps at gopher tortoise burrows
Camera trapping at tortoise burrows proved functional for detecting tegu presence, assessing relative abundance, obtaining demographic information, and obtaining detailed temporal information on daily and seasonal activity. Moreover, cameras provided clear, well-focused images that allowed confirmation of species identity. Tegus did not spend substantial time at burrows. Individuals were rarely documented both entering a burrow and exiting it, either that same day or the next. Tegus exit from a burrow usually occurred during the 60-s video phase that immediately followed a still image, although the next still image after the 60-s camera reset sometimes captured an exiting lizard’s tail. Thus, tegus typically spent only 1–2 min at, or in, a burrow. Nevertheless, our camera observations were able to document tegu willingness to agonistically attack and kill snakes (Kaiser et al. 2013). The brief time tegus remain in burrows may explain why traps on tortoise burrows were not successful at capturing tegus, as the likelihood of placing a trap over a burrow holding a tegu would be minimized.
When tegus were first observed at gopher tortoise burrows prior to initiation of this study, we speculated that females might use burrows for nesting with an accompanying expectation that recently hatched tegus would be photographed at burrows. However, our camera trapping results did not support this with only three hatching tegus recorded. We know hatchling tegus were not too small to trigger the cameras, because adult six-lined race runners are approximately the same size as hatchling tegus and were frequently photographed. The seasonally earliest photographs of tegus adult specimens basking on the aprons of gopher tortoise burrows suggested tegus may use gopher tortoise burrows for brumation.
Further method considerations
Tracking plots and cameras stationed on gopher tortoise burrows were the two most efficient and effective means to obtain tegu observations. While camera traps on gopher tortoise burrows are an example of setting up observation stations based on species behavior that promotes intercepting its activities, it still was not surprising based on station size that the observation rate for 100-m tracking plots was many folds (7.5 times) higher than that for the camera stations. Based on track directions, tegus appeared to simply intercept tracking plots on roads somewhat randomly in their daily activities, rather than selectively using roads as travel ways as done by many other species that have been well-monitored using tracking plots (e.g., Allen et al. 2013, 2014; Engeman and Allen 2000; Engeman et al. 2017). If there had been 7.5 times as many camera stations used, then the total incursions to tracking plot and camera stations might have been about the same. However, increasing the number of cameras 7.5-fold would add substantial expense and maintenance to sampling. On the other hand, 100-m tracking plots require no more resources or effort than a large number of small plots would require. However, increasing tracking plot size much beyond 100 m on backcountry roads becomes impractical due to the tangled nature of the roads and occasional obstructions. In scrub areas where fire lanes or other primitive roads do not offer good geospatial coverage of the area, tortoise burrows are still likely to be well-distributed throughout the area of interest. In such situations cameras on tortoise burrows may provide a means to detect and monitor tegu abundance.
Because traps with baits have been applied since our study to target and remove tegus where they had been observed (Avery et al. 2016), baits might also be considered to attract tegus to camera stations. Baits would have to be protected and secured so they could not be removed by tegus or non-target species; otherwise, the ability to attract tegus would be limited by non-target take or take by the first tegu to visit the bait when others might also be attracted. Greater station visitation by tegus and non-target species likely would require greater memory card and battery maintenance. Also, the time-consuming post-processing of photos would likely increase substantially with more photos.
Another approach might be to sample inside gopher tortoise burrows during the time of year when tegus are not active. Tegus will brumate in burrows which could include gopher tortoise burrows (Ashton and Ashton Jr. 2004; Balsai 1998; Enge 2007; King et al. 1994; McEachern et al. 2015). A closed-circuit television (CCTV) camera system might allow observation and possibly removal of tegus in gopher tortoise burrows during brumation (McEachern et al. 2015). Carried out over multiple winters, population trends or control efficacy could be indicated but would also be resource- and labor-intensive. This method would require investment in specialized equipment and staff time in the field to conduct inspections. By contrast, each burrow would only need inspection once, as the lizards do not move when brumating. This could answer many questions about brumation behavior and demographics. It also could provide especially high-quality detection and population assessments if tegus are highly reliant on animal burrows for brumation. The method would allow observations to be collected during the time of year when the methods we tested are not applicable.
Conclusions
When an invasive species threat is discovered, it is incumbent to rapidly assess its population so that optimal management strategies can be developed and implemented before the situation worsens (e.g., Engeman et al. 2006). An integration of monitoring methods would likely provide the most reliable results. When comparing abundance metrics between years, it is essential that assessments are from similar times of year (e.g., Engeman 2005). For example, tracking plot results from early in the tegu active season would not be comparable with results later in the active season when tracking conditions are suboptimal. A smaller population would be indicated later even if there was no change simply because conditions were less conducive to recording tracks.
By far, the most efficient means to search a wide area for tegu presence (detection) and, if present, assess abundance would be to observe tracks. Initially, this could involve extensively driving backcountry roads in search of tracks. It would be ideal to smooth the roads first, but to save time on initial inspection, this step could be skipped, if necessary. Very large areas with fire lanes, farm roads, and other backcountry roads on sugar sands or other high-quality tracking substrates might be periodically surveyed in this manner at minimal cost. Much of Florida fits this description. In areas where suspicious tracks are located, tracking plots could be set up to verify tracks and assess abundance. Tracking plots established in fire lanes require a small investment of time and material to detect the presence of large, non-native lizards, but method sensitivity declines during the summer rainy season. Camera stations could be applied to animal burrows or on bait stations (if testing shows this to be a viable method). Although camera traps at burrows detected the presence of tegus throughout the active season, the low rate of visitation to individual burrows suggests many cameras may be required. Importantly, cameras can provide positive identifications of the species present, can be effectively applied throughout the tegu active season, and can indicate population demographics. Camera applications are most valuable if they can be installed with minimal fear of interference, theft, or vandalism. The more labor-intensive capture methods might have increased value if improved to enhance capture rates, especially because they would also be removing the lizards. For monitoring tegus during brumation, we recommend testing CCTS as a survey method and as a support for tegu control once discovered in a burrow.
References
Allen BL, Allen LR, Engeman RM, Leung, LK.-P. (2014) Sympatric prey responses to lethal predator control: predator manipulation experiments. Front Zool 11:56
Allen LR, Engeman RM (2015) Evaluating and validating abundance monitoring methods in absence of populations of known size. Environ Sci Pollut Res 22:2907–2915
Allen BL, Allen LR, Engeman RM, Leung LK-P (2013) Intraguild relationships between sympatric predators exposed to lethal control: predator manipulation experiments. Front Zool 10:39
Ashton PS, Ashton RE Jr (2004) The gopher tortoise: a life history. Pineapple Press, Sarasota
Avery ML, Tillman EA, Krysko KL (2009) Gopherus polyphemus (gopher tortoise), Ctenosaura similis (Gray’s spiny-tailed iguana). Predation Herpetol Rev 40:435
Avery ML, Humphrey J, Engeman RM (2016) Evaluating trap alternatives for removal of black and white tegus Salvator merianae. Southeast Nat 15:107–113
Baldwin RA, Quinn N, Davis DH, Engeman RM. (2014) Effectiveness of rodenticides for managing invasive roof rats and native deer mice control in orchards. Environ Sci Pollut Res 21:5795–5802
Balsai M (1998) Tegus: South America’s cunning teiids. Reptiles 6(9):52–69
Bartlett RD, Bartlett PP (1999) A field guide to Florida reptiles and amphibians. Gulf Publishing Co., Houston
Caughley G (1977) Analysis of vertebrate populations. Wiley, New York
Corn ML, Buck EH, Rawson J, Segarra A, Fischer E (2002) Invasive non-native species: background and issues for Congress. Congressional Research Service and The Library of Congress, Washington, DC
Cope ED. (1875) Check-list of North-American Batrachia and Reptilia. Bulletin of the United States National Museum 1:1–104
Enge KM (2007) FWC bioprofile for the Argentine black and white tegu (Tupinambis merianae). Florida Fish and Wildlife Conservation Commission report, Tallahassee
Enge KM, Kaiser BW, Dickerson RB. (2006) Another large exotic lizard in Florida, the Argentine black and white tegu. In: Proceedings of the 28th Annual Gopher Tortoise Council Meeting. Gopher Tortoise Council, Valdosta, Georgia Npn
Engeman RM (2005) A methodological and analytical paradigm for indexing animal populations applicable to many species and observation methods. Wildl Res 32:203–210
Engeman R, Allen LR (2000) Overview of a passive tracking index for monitoring wild canids and associated species. Integr Pest Manag Rev 5:197–203
Engeman RM, Avery ML (2016) Prioritizing management and research actions against invasive reptiles in Florida: a collaboration by an expert panel. Herp Alliance and USDA/WS/National Wildlife Research Center, Fort Collins
Engeman RM, Pipas MJ, Gruver KS, Allen L (2000) Monitoring coyote populations with a passive activity index. Wildl Res 27:553–557
Engeman RM, Massei G, Sage M, Gentle M. (2013) Monitoring wild pig populations: a review of methods. Environ Sci Pollut Res 20:8077–8091
Engeman RM, Constantin BU, Nelson M, Woolard J, Bourassa J (2001) Monitoring changes in feral swine population and spatial distribution of activity. Environ Conserv 28:235–240
Engeman RM, Woolard JW, Perry ND, Witmer G, Hardin S, Brashears L, Smith HT, Muiznieks B, Constantin BU (2006) Rapid assessment for a new invasive species threat: the case of the Gambian giant pouched rat in Florida. Wildl Res 33:439–448
Engeman RM, Stevens A, Allen J, Dunlap J, Daniel M, Teague D, Constantin BU (2007) Feral swine management for conservation of an imperiled wetland habitat: Florida’s vanishing seepage slopes. Biol Conserv 134:440–446
Engeman RM, Constantin BU, Hardin S, Smith HT, Meshaka WE Jr (2009a) “Species pollution” in Florida: a cross-section of invasive vertebrate issues and management responses. In: Wilcox CP, Turpin RB (eds) Invasive species: detection, impact and control. Nova Science Publishers, Hauppauge, pp 179–197
Engeman RM, Kennedy M, Constantin BU, Christie ML, Hall PT (2009b) Ctenosaura similis (black spinytail iguana), Gopherus polyphemus. (gopher tortoise) concurrent burrow use. Herpetol Rev 40:84
Engeman RM, Jacobson E, Avery M, Meshaka WE Jr (2011) The aggressive invasion of exotic reptiles in Florida with a focus on prominent species: a review. Curr Zool 57:599–612
Engeman RM, Allen BL, Allen LR (2017) Study design concepts for inferring functional roles of mammalian top predators. Special issue on “Strengths, weaknesses, opportunities and threats to the study and conservation of top-predators and their role in terrestrial food webs”. Food Webs 12:56–63
FNAI (Florida Natural Areas Inventory) (2010) Guide to the natural communities of Florida. Florida Natural Areas Inventory and Florida Department of Natural Resources, Tallahassee
Franzetti B, Ronchi B, Marini F, Scacco M, Calmanti R, Calabrese A, Paola A, Paolo M, Focardi S (2012) Nocturnal line transect sampling of wild boar (Sus scrofa) in a Mediterranean forest: long-term comparison with capture–mark–resight population estimates. Eur J Wildl Res 58:385–402
FWC (Florida Fish and Wildlife Conservation Commission) (2015) Tegus in Florida. In: Florida Fish and Wildlife Conservation Commission. FL, Tallahassee
Galetti M, Bovendorp RS, Fadin RF, Gussoni COA, Rodrigues M, Alvarez AD, Guimaraes PR, Alves K (2009) Hyper-abundant mesopredators and bird extinction in an Atlantic forest island. Zoolog 26:288–298
Hardin S (2007) Managing non-native wildlife in Florida: state perspective, policy and practice. In: Witmer G, Pitt W, Fagerstone K (eds) Managing Vertebrate Invasive Species: Proceedings of an International Symposium. USDA/APHIS/WS, National Wildlife Research Center, Fort Collins, pp 43–52
Hare KM (2012) Herpetofauna: systematic searches v1.0. Inventory and monitoring toolbox: herpetofauna. Department of Conservation, Wellington
Jackson JA, Jackson BJS (2007) An apparent mutualistic association between invasive exotics: Brazilian pepper (Schinus terebinthifolius) and black spiny-tailed iguanas (Ctenosaura similis). Nat Areas J 27:254–257
Jarnevich CS., Hayes MA, Fitzgerald LA, Yackel Adams AA, Falk BG, Collier MAM, Bonewell LR, Klug PE, Naretto S, Reed RN. (2018) Modeling the distributions of tegu lizards in native and potential invasive ranges. Scientific Reports. Online first
Johnson SA, McGarrity M (2017) Florida invader: tegu lizard. University of Florida/IFAS Extension, Gainesville
Kaiser BW, Osorio KJ, Enge KM, Engeman RM (2013) Tupinambis merianae (Argentine giant tegu), Pantherophis gutattatus guttatus (red rat snake). Nonpredatory killing SSAR. Herpetol Rev 44:329
King D, Green B, Herrera E (1994) Thermoregulation in a large teiid lizard, Tupinambis teguixin, in Venezuela. Copeia 1994:806–808
Krebs CJ (1998) Ecological methodology. Benjamin/Cummings, Menlo Park
Lambert MRK (2002) Amphibians and reptiles. In: Grant IF, Tingle CCD (eds) Ecological monitoring methods for the assessment of pesticide impact in the tropics. Natural Resources Institute, Chatham, pp 213–227
Lopes HR, Abe AS. (1999) Biologia reprodutivo e comportamento do teiú, Tupinambis merianae, em cativeiro (Reptilia, Teiidae). In: Fang TG, Montenegro OL, Bodme RE (Eds.). Manejo y Conservación de Fauna Silvestre en América Latina. Instituto de Ecología, La Paz, Bolivia. pp 259–272
Luxmoore R, Groombridge B, Broads S (eds) (1988) Significant Trade in wildlife: a review of selected species in CITES Appendix II. Volume 2: Reptiles and invertebrates. IUCN Conservation Monitoring Centre, Cambridge
McEachern MA, Yackel Adams AA, Klug PE, Fitzgerald LA, Reed RN (2015) Brumation of introduced black and white tegus, Tupinambis merianae (Squamata: Teiidae), in Southern Florida. Southeast Nat 14(2):319–328
Mercolli C, Yanosky A (1994) The diet of adult Tupinambis teguixin (Sauria: Teiidae) in the eastern chaco of Argentina. Herpetol J 4:15–19
Meshaka WE Jr, Butterfield BP, Hauge JB (2004) The exotic amphibians and reptiles of Florida. Krieger Publishing Company, Malabar
Meshaka WE Jr, Smith HT, Engeman RM, Dean CL, Moore JA, O’Brien WE (2005) The geographically contiguous and expanding range of the northern curlytail lizard (Leicephalus carinatus armouri) in Florida. Southeast Nat 4:521–526
Meshaka WE Jr (2011) Runaway train in the making: the exotic amphibians, reptiles, turtles, and crocodilians of Florida. Monograph 1. Herpetol Conserv Biol 6:1–101
Ryan DA, Heywood A (2003) Improving the precision of longitudinal ecological surveys using precisely defined observational units. Environmetrics 14:83–293
SFWMD (South Florida Water Management District) (2008) Executive Summary 2008 South Florida Environmental Report. South Florida Water Management District, West Palm Beach
U.S. Congress (1993) Harmful non indigenous species in the United States. Office of Technology Assessment, OTA F 565 Government Printing Office, Washington DC
Vitt LJ (1995) The ecology of tropical lizards in the Caatinga of Northeast Brazil. Occasional Papers of the Oklahoma Museum of. Nat Hist 1:1–29
Acknowledgments
This research was supported by the intramural research program of the U.S. Department of Agriculture; Hillsborough County Conservation and Environmental Lands Management Department; partial funding for this project was provided through Cooperative Agreement No. 40181AJ085 between Hillsborough County Conservation and Environmental Lands Management Department and the U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Engeman, R.M., Kaiser, B.W. & Osorio, K.J. Evaluating methods to detect and monitor populations of a large invasive lizard: the Argentine giant tegu. Environ Sci Pollut Res 26, 31717–31729 (2019). https://doi.org/10.1007/s11356-019-06324-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06324-2