Abstract
Glucagon-like peptide-1 (GLP-1) is involved in postprandial glucose homeostasis. Secretion of which involves a cholinergic pathway. Anticholinergic agent like atropine could act as a competitive antagonist of acetylcholine at muscarinic receptors. This review explores studies that assess the role of atropine in GLP-1 secretion. We selected published original articles from PubMed, Science Direct, The Cochrane Library, Trip, Google and the reference lists of the selected articles. Reporting was done according to the PRISMA statement. Relevant standard and previously published tools were used to assess the risk of bias of the selected articles. Twelve articles out of 185 search results fulfilled the review criteria. Eight were in vivo studies (six animal and two human studies), three were ex vivo studies and one was an in vitro study. Animal studies had rats, mice, pigs and monkeys as the subjects. Human studies involved healthy men and women. Majority of the studies reported an atropine-mediated attenuation of GLP-1 secretion and postprandial secretion of GLP-1 was mainly affected. However, atropine failed to significantly affect GLP-1 secretion when dipeptidyl peptidase-4 (DPP-4) enzyme was inhibited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
An oral glucose load achieves a 40 to 60% increase in insulin secretion in comparison with iso-glycemic glucose infusion (Elrick et al. 1964; Perley and Kipnis 1967). The above is known as the incretin effect. Glucagon-like peptide-1 (GLP-1) is one of the two main incretin hormones (Prins 2008; Seino et al. 2010) which regulates glucose homeostasis mainly by increasing insulin release from pancreatic beta cells (UpToDate 2018a). Attenuation of GLP-1 secretion can result in hyperglycaemia. Thus, type 2 diabetes mellitus patients show a low GLP-1 response (3.113 pmol min L–1) in comparison with controls (3.599 pmol min L–1) (Vilsbøll et al. 2001). Therefore, dipeptidyl peptidase-4 enzyme (DPP-4) inhibitors are used in type 2 diabetes mellitus to enhance the incretin effect by inhibiting the DPP-4-mediated rapid inactivation of incretin hormones (Kuhre et al. 2015).
Secretion of GLP-1 at the distal ileum and colon occur in two phases (Prins 2008; Seino et al. 2010). Nutrients directly stimulate the late secretory phase (Anini et al. 2002). However, in the early secretory phase, glucose-dependent insulinotropic peptide (GIP) indirectly stimulates the secretion of GLP-1 via the vagus nerve. Acetylcholine action at muscarinic receptors is involved in the abovementioned indirect secretion (Herrmann-Rinke et al. 1996; Balks et al. 1997; Ahrén and Holst 2001; Anini et al. 2002). A recent hypothesis on a possible mechanism for organophosphate-related disruption of glucose homeostasis (Montgomery et al. 2008; Hectors et al. 2011; Karami-Mohajeri and Abdollahi 2011; Joshi and Rajini 2012; Thayer et al. 2012; Lasram et al. 2014) proposed an attenuation of GLP-1 secretion by atropine (Rathish et al. 2016). A subsequent study alluded towards an attenuated GLP-1 response among patients treated for organophosphate or carbamate poisoning (Rathish et al. 2019).
Atropine is an anticholinergic agent which reverses the muscarinic effects by acting as a competitive antagonist of acetylcholine at muscarinic receptors (UpToDate 2018b). Atropine is the therapeutic agent in many clinical indications. Bradycardia (symptomatic sinus bradycardia, bradycardia during surgery, bradycardia due to myocardial infarction and beta blockers induced bradycardia) and atrioventricular nodal block need atropine (Towers 2015; UpToDate 2018b). Pre-anaesthetic inhibition of salivation and secretions, pre-medication in rapid sequence intubation and post-operative reversal of neuromuscular blockade (adjuvant use with neostigmine) also need atropine (Towers 2015; UpToDate 2018b). Moreover, atropine is used in symptomatic relief of gastrointestinal disorders characterised by smooth muscle spasm, stress echocardiography (as an adjunct chronotropic agent), healthy eye penalisation (in amblyopia) and achievement of mydriasis and cycloplegia (Towers 2015; UpToDate 2018b). Atropine is the antidote for organophosphate, carbamate and muscarine-containing mushroom poisoning (Towers 2015; UpToDate 2018b).
The objective of this review was to summarise the evidence related to the role of atropine administration in GLP-1 secretion. Findings of the review would lead to future clinical studies and preventive measures.
Main text
Methods
The review is reported based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Moher et al. 2009).
Eligibility criteria
All published original research (in vivo, ex vivo and in vitro) related to the role of atropine administration on GLP-1 secretion was included. We disregarded the year of publication or the type of subjects in inclusion. We excluded articles published in languages other than English.
Information sources and search strategy
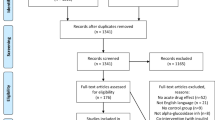
We searched electronic databases and grey literature in April 2018 using strings of keywords (Fig. 1). PubMed (advanced search) (PubMed 2018), Science Direct (advanced search) (Science Direct 2018), The Cochrane Library (advanced search) (Cochrane Library 2018), Trip (search) (Trip Database 2018) and Google (search) (Google 2018) were used for the search. We checked the reference lists of the selected studies for relevant articles. Medical Subject Headings (MeSH) and other relevant terms related to each search engine were used to produce a maximum yield (Fig. 1).
Study selection
The first author (DR) screened the titles and abstracts of all identified studies for eligibility. Authors examined full-text article when abstracts were unclear. Both SA and CJ independently reviewed the selected studies to confirm the eligibility.
Data collection process, data items and data analysis
We extracted year of publication, country of origin, primary objective, study type, subjects, methods and results related to the review objective from the selected studies. The population was human, animal and cell lines; intervention—atropine therapy; comparison—controls with alternatives or devoid of atropine therapy; outcome—GLP-1 secretion and study design—in vivo, ex vivo and in vitro controlled interventional studies. The first author (DR) extracted the relevant data, and SA and CJ independently reviewed the extracted data to confirm consistency. Data were analysed using Microsoft Excel. Descriptive statistics were used to describe data.
Risk of bias assessment
Risk of bias in selected studies was assessed using standard tools. Quality assessment tool for controlled intervention studies of the National Institutes of Health was used to assess the risk of bias in selected human studies (Quality assessment tool for controlled Intervention studies 2018). Quality assessment of the selected in vivo animal studies was done using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE’s) risk of bias tool for animal studies (Hooijmans et al. 2014). Due to the unavailability of standard tools, alternatives from previously published articles were used to assess the risk of bias in selected ex vivo (Smit et al. 2018) and in vitro studies (Khan et al. 2014). The first author (DR) performed the risk of bias assessment for each selected study, and SA and CJ independently reviewed the assessment to confirm consistency.
Results
Selected studies
The databases revealed a total of 184 results (Fig. 1). Further, the reference lists of the selected articles revealed another article. After removal of duplicates, 143 articles underwent title and abstract screening. Exclusion of 78 articles occurred due to irrelevance to the study objective, 32 articles due to non-research articles and another article due to non-English language. Exclusion of 20 out of the 32 full texts occurred as they had no data on the role of atropine administration on GLP-1 secretion. Following the above screening steps, the review had 12 articles (Abello et al. 1994; Herrmann-Rinke et al. 1995, 1996; Balks et al. 1997; Ahrén and Holst 2001; D’Alessio et al. 2001; Anini et al. 2002; Hansen et al. 2004; Sandoval et al. 2013; Lee et al. 2015; Ahlkvist et al. 2016; Lebrun et al. 2017) (Fig. 2).
Demographic data
Out of the selected articles, three articles were from Germany, two each from France, Sweden, the USA and one each from Canada, Denmark and Japan (Table 1). The first study related to the influence of atropine on GLP-1 secretion was reported in 1994 by Abello et al. from France (Abello et al. 1994). The latest was reported in 2017 by Lebrun et al. from France (Lebrun et al. 2017). There were eight in vivo studies out of which six were animal studies, and two were human studies. The six in vivo animal studies involved mice (3/6), rats (2/6) and monkeys (1/6). Also, there were three ex vivo studies and an in vitro study (Table 1). The three ex vivo studies used rat (2/3) and pig (1/3) ileum. The in vitro study used endocrine cell line STC-1 from mice (Table 1). Thirty-three percent (4/12) of the selected article had main objectives directly related to the review objective. Both human studies obtained ethical clearance and written consent from the participants. And, all in vivo animal studies have obtained ethical clearance for animal studies. Table 1 summarises the range of dose and methods of atropine administration in the selected articles. The control groups either received 0.9% sodium chloride or were devoid of a substitute for atropine therapy (Table 1).
Risk of bias assessment
Online Resource 1 summarises the results of the risk of bias assessment. Assessment of human studies revealed a lack of reporting on randomisation, blinding (subject, investigator, assessor) and power analysis. Assessment of animal studies revealed a lack of reporting on randomisation and blinding (investigator, assessor). Assessment of ex vivo studies revealed a lack of reporting on blinding (investigator, assessor), power analysis and conflict of interest. Assessment of in vitro studies revealed a lack of reporting on controls and power analysis.
Evidence for atropine-mediated attenuation of glucagon-like peptide-1 secretion
Human studies
Two human studies reported atropine-mediated attenuation of GLP-1 secretion. Ahrén and Holst (2001) explored the significance of pre-absorptive insulin response to meal ingestion among healthy postmenopausal women. Baseline GLP-1 was not affected by atropine. However, atropine significantly attenuated the post-absorptive GLP-1 response 15–25 min after meal ingestion (Ahrén and Holst 2001). Balks et al. (1997) investigated the GLP-1 secretion following glucose ingestion among healthy young men. Atropine did not affect basal GLP-1 levels. However, it significantly attenuated the increase in GLP-1 levels during the post-absorptive period (Balks et al. 1997).
In vivo animal studies
Four in vivo animal studies reported atropine-mediated attenuation of GLP-1 secretion. Anini et al. (2002) investigated the role of muscarinic receptors in GLP-1 secretion among rat models. Atropine completely inhibited postprandial GLP-1 secretion (Anini et al. 2002). Sandoval et al. (2013) studied the role of the autonomic nervous system in electrical stimulation-induced GLP-1 secretion among rats. Atropine significantly lowered postprandial GLP-1 levels regardless of time and electrical stimulation (Sandoval et al. 2013). Lee et al. (2015) compared two alpha-glucosidase inhibitors (miglitol and acarbose), by examining their effects on GLP1 secretion in mice. Atropine significantly attenuated GLP-1 secretion by maltose with miglitol (Lee et al. 2015). Lebrun et al. (2017) aimed at linking GLP-1 secretion in relation to gut inflammation among mice. There was substantial attenuation of basal GLP-1 secretion by atropine. However, it failed to significantly attenuate the stimulatory effect of lipopolysaccharide on GLP-1 secretion (Lebrun et al. 2017).
Ex vivo and in vitro studies
Three ex vivo animal studies and an in vitro cell line study reported atropine-mediated attenuation of GLP-1 secretion. Hansen et al. (2004) investigated the neural regulation of GLP-1 secretion in the porcine ileum. Acetylcholine stimulated GLP-1 secretion which was abolished by atropine (Hansen et al. 2004). Herrmann-Rinke et al. focused on the role of galanin in GLP-1 secretion in 1996 using rat ileum (Herrmann-Rinke et al. 1996). The stable release of GLP-1 induced by methacholine, a non-selective muscarinic receptor agonist, was abolished by atropine (Herrmann-Rinke et al. 1996). Similarly, in 1995, Herrmann-Rinke et al. administered carbachol and methacholine via the arterial route to induce a release of GLP-1 which was abolished by atropine (Herrmann-Rinke et al. 1995). All ex vivo studies showed that GLP-1 secretion was abolished by atropine. Abello et al. examined the effects of cholinergic stimulation on GLP-1 secretion using endocrine cell line STC-1, originated from an intestinal tumour in transgenic mice carrying insulin-promoted oncogenes (Abello et al. 1994). Atropine completely inhibited GLP-1 release induced by carbachol but did not affect basal GLP-1 secretion (Abello et al. 1994).
Evidence against atropine-mediated attenuation of glucagon-like peptide-1 secretion
Two in vivo animal studies opposed atropine-mediated attenuation of GLP-1 secretion. D’Alessio et al. (2001) aimed at finding a direct role of the parasympathetic nervous system on insulin secretion during the absorptive phase of the meal by measuring the effects of cholinergic blockade among rhesus monkeys. Atropine did not significantly affect the basal or postprandial concentration of GLP-1 (D’Alessio et al. 2001). Ahlkvist et al. (2016) examined the effects of muscarinic receptor blockade on the insulinotropic actions of DPP-4 inhibition among mice. Atropine failed to significantly affect GLP-1 levels during DPP-4 inhibition (Ahlkvist et al. 2016).
Discussion
All human studies (100%—2/2), majority of animal in vivo studies (66.7%—4/6), all ex vivo studies (100%—3/3) and the only in vitro study provided evidence for atropine-mediated attenuation of GLP-1 secretion. Both human studies showed atropine-mediated attenuation of post-absorptive GLP-1 secretion. Out of the animal studies with evidence for the above attenuation, 50% (2/4) had evidence for attenuation of the post-absorptive GLP-1 secretion. Out of the two animal in vivo studies without the above attenuation, one study revealed DPP-4 inhibitor mediated prevention of the above attenuation.
Atropine has a wide range of therapeutic indication including organophosphate poisoning, bradycardia, ophthalmology and pre- and post-anaesthetic management (Towers 2015; UpToDate 2018b). Moreover, organophosphate poisoning with severe symptoms requires a high dose of atropine as a continuous intravenous infusion (UpToDate 2018b). Therefore, this group of patients would have the highest risk of atropine-mediated attenuation of GLP-1 secretion. A recent hypothesis implicated atropine-mediated attenuation of GLP-1 among organophosphate poisoning patients (Rathish et al. 2016). Thus, research among patients receiving atropine therapy is essential to test the clinical implication of GLP-1 attenuation. The clinical study should find if the GLP-1 attenuation is good enough to disrupt glucose homeostasis among these patients. According to the findings of this review, the use of DPP-4 inhibitors could play a defensive role in atropine-mediated attenuation of GLP-1 secretion (Ahlkvist et al. 2016). Therefore, it is important to evaluate the place of DPP-4 inhibitors in preventing the atropine-mediated GLP-1 attenuation.
The risk of bias assessment shows an acceptable level of quality of the selected articles. However, there was a lack of reporting on randomisation, blinding and power analysis. Moreover, differences in reporting on atropine doses and details of controls were noted. These limited the review in producing a comprehensive summary and led to a descriptive nature of the review. Strict adherence to reporting guidelines like the Animal Research: Reporting of In Vivo Experiments (ARRIVE) (ARRIVE guidelines 2018) and the Consolidated Standards of Reporting Trials (CONSORT) 2010 for randomised trials (CONSORT 2010) would have minimised reporting errors. Also, negative studies are less likely to be published leading to publication bias. Nevertheless, the review has produced useful information on atropine-mediated attenuation of GLP-1 secretion.
Conclusions
In vivo, ex vivo and in vitro studies establish an atropine-mediated attenuation of post-absorptive GLP-1 secretion. Studies among patients receiving atropine therapy are essential in finding the clinical importance of the above phenomenon. Also, the role of DPP-4 inhibitors needs to be tested in preventing atropine-mediated attenuation of GLP-1 secretion.
Abbreviations
- ARRIVE:
-
Animal Research: Reporting of In Vivo Experiments
- CONSORT:
-
Consolidated Standards of Reporting Trials
- DPP-4:
-
dipeptidyl peptidase-4
- GIP:
-
glucose-dependent insulinotropic peptide
- GLP-1:
-
glucagon-like peptide-1
- MeSH:
-
Medical Subject Headings
- OGL:
-
oral glucose load
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- STC:
-
secretin tumour cell
- SYRCLE:
-
Systematic Review Centre for Laboratory Animal Experimentation
References
Abello J, Ye F, Bosshard A, Bernard C, Cuber JC, Chayvialle JA (1994) Stimulation of glucagon-like peptide-1 secretion by muscarinic agonist in a murine intestinal endocrine cell line. Endocrinology 134(5):2011–2017
Ahlkvist L, Omar B, Pacini G, Ahrén B (2016) Evidence for neural contribution to islet effects of DPP-4 inhibition in mice. Eur J Pharmacol 780:46–52
Ahrén B, Holst JJ (2001) The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50(5):1030–1038
Anini Y, Hansotia T, Brubaker PL (2002) Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 143(6):2420–2426
ARRIVE guidelines [online] (2018) National Centre for the Replacement, Refinement and Reduction of Animals in Research. Available from: https://www.nc3rs.org.uk/arrive-guidelines [Accessed 12 Apr 2018]
Balks HJ, Holst JJ, von zur Mühlen A, Brabant G (1997) Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab 82(3):786–790
Cochrane Library [online] (2018) Available from: http://www.cochranelibrary.com/ [Accessed 12 Apr 2018]
CONSORT [online] (2010) The CONSORT group, Ottawa Hospital Research Institute. Available from: http://www.consort-statement.org/consort-2010 [Accessed 12 Apr 2018]
D’Alessio DA, Kieffer TJ, Taborsky GJ, Havel PJ (2001) Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques 1. J Clin Endocrinol Metab 86(3):1253–1259
Elrick H, Stimmler L, Hlad CJ, Arai Y (1964) Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24:1076–1082
Google [online] (2018) Available from: https://www.google.com/ [Accessed 12 Apr 2018]
Hansen L, Lampert S, Mineo H, Holst JJ (2004) Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab 287(5):E939–E947
Hectors TLM, Vanparys C, Van Der Ven K, Martens GA, Jorens PG, Van Gaal LF, Covaci A, De Coen W, Blust R (2011) Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 54(6):1273–1290
Herrmann-Rinke C, Vöge A, Hess M, Göke B (1995) Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol 147(1):25–31
Herrmann-Rinke C, Hörsch D, McGregor GP, Göke B (1996) Galanin is a potent inhibitor of glucagon-like peptide-1 secretion from rat ileum. Peptides 17(4):571–576
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14(1):43
Joshi AKR, Rajini PS (2012) Organophosphorus insecticides and glucose homeostasis. In: Perveen F (ed) Insecticides-pest engineering. InTech. Available from: http://www.intechopen.com/books/insecticides-pest-engineering/organophosphorus-insecticides-andglucosehomeostasis
Karami-Mohajeri S, Abdollahi M (2011) Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum Exp Toxicol 30(9):1119–1140
Khan M, Rothrauff BB, Merali F, Musahl V, Peterson D, Ayeni OR (2014) Management of the contaminated anterior cruciate ligament graft. Arthroscopy 30(2):236–244
Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, Deacon CF, Holst JJ (2015) Measurement of the incretin hormones: Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complicat 29(3):445–450
Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N (2014) A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 322:1–13
Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros J-P, Le Guern N, Plesnik J, Thomas C, Bourgeois T, Dejong CHC, Kox M, Hundscheid IHR, Khan NA, Mandard S, Deckert V, Pickkers P, Drucker DJ, Lagrost L, Grober J (2017) Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep 21(5):1160–1168
Lee EY, Kaneko S, Jutabha P, Zhang X, Seino S, Jomori T, Anzai N, Miki T (2015) Distinct action of the α-glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J Endocrinol 224(3):205–214
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP (2008) Incident diabetes and pesticide exposure among licensed pesticide applicators: agricultural health study, 1993-2003. Am J Epidemiol 167(10):1235–1246
Perley MJ, Kipnis DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest 46(12):1954–1962
Prins JB (2008) Incretin mimetics and enhancers: mechanisms of action. Aust Prescr 31:102–104
PubMed [online] (2018) NCBI. Available from: https://www.ncbi.nlm.nih.gov/pubmed [Accessed 12 Apr 2018]
Quality assessment tool for controlled Intervention studies [online], 2018. National Institutes of Health. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Accessed 12 Apr 2018].
Rathish D, Agampodi SB, Jayasumana MACS, Siribaddana SH (2016) From organophosphate poisoning to diabetes mellitus: the incretin effect. Med Hypotheses 91:53–55
Rathish D, Senavirathna I, Jayasumana C, Agampodi S, Siribaddana S (2019) A low GLP-1 response among patients treated for acute organophosphate and carbamate poisoning: a comparative cross-sectional study from an agrarian region of Sri Lanka. Environ Sci Pollut Res 26(3):2864–2872
Sandoval D, Dunki-Jacobs A, Sorrell J, Seeley RJ, D’Alessio DD (2013) Impact of intestinal electrical stimulation on nutrient-induced GLP-1 secretion in vivo. Neurogastroenterol Motil 25(8):700–705
Science Direct [online] (2018) Available from: http://www.sciencedirect.com/ [Accessed 12 Apr 2018].
Seino Y, Fukushima M, Yabe D (2010) GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabet Investig 1(1–2):8–23
Smit B, Smulders YM, Eringa EC, Oudemans - van Straaten HM, Girbes ARJ, Wever KE, Hooijmans CR, Spoelstra - de Man AME (2018) Effects of hyperoxia on vascular tone in animal models: systematic review and meta-analysis. Crit Care 22(1):189
Thayer KA, Heindel JJ, Bucher JR, Gallo MA (2012) Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ Health Perspect 120(6):779–789
Towers K (2015) Atropine sulfate. In: British National Formulary. London: BMJ group and Pharmaceutical press, 1099–1100.
Trip Database [online] (2018) Available from: https://www.tripdatabase.com/ [Accessed 12 Apr 2018]
UpToDate [online] (2018a) Wolters Kluwer. Available from: https://www.uptodate.com/contents/search [Accessed 1 Aug 2018]
UpToDate [online] (2018b) Wolters Kluwer. Available from: https://www.uptodate.com/home [Accessed 12 Apr 2018]
Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001) Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50(3):609–613
Availability of data and materials
All data generated or analysed during this review were included in this published article (and its online resource).
Author information
Authors and Affiliations
Contributions
DR conceived the idea, and all authors designed the review. DR performed a comprehensive literature search. DR screened the titles and abstracts of all identified studies for eligibility. The selected study was independently reviewed by SA and CJ to confirm the eligibility. DR performed the risk of bias assessment for each selected study and SA, CJ independently reviewed the assessment to confirm consistency. DR extracted the relevant data and SA, CJ independently reviewed the extracted data to confirm reliability. DR was involved in data extraction and analysis. DR drafted the manuscript and SA, CJ critically revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
Not applicable
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
The title of data - Risk of bias assessment in individual studies of the systematic review on atropine mediated attenuation of glucagon-like peptide-1 secretion, 2018. Description of data - This provides the results for risk of bias assessment in individual studies of the systematic review (DOCX 20.8 kb)
ESM 2
Title of data - PRISMA 2009 checklist. Description of data - This provides the PRISMA 2009 checklist related to the systematic review (DOC 31.7 kb)
Rights and permissions
About this article
Cite this article
Rathish, D., Agampodi, S. & Jayasumana, C. In vivo, ex vivo and in vitro evidence for atropine-mediated attenuation of glucagon-like peptide-1 secretion: findings from a systematic review. Environ Sci Pollut Res 26, 29597–29605 (2019). https://doi.org/10.1007/s11356-019-06227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06227-2