Abstract
Poly-aluminium chloride (PAC) is often used to enhance phosphorus removal and control membrane fouling in membrane bioreactors (MBRs). However, the influence of aluminium accumulation on the biological nitrification and phosphorus removal of MBRs has not been well assessed. In the present study, the effects of accumulated aluminium on sludge activity and morphology were investigated in a lab-scale anoxic–oxic membrane bioreactor. The reasonably high removal efficiencies of NH4+-N, TN, and COD, i.e. 94.9%, 84.8%, and 92.8%, respectively, were achieved in the reactor when the percentage of atomic aluminium on sludge surface increased to 14.2%. However, the decreases in the ammonia oxidation rate, nitrite oxidation rate, and specific oxygen uptake rate of sludge by 82.1%, 79.8%, and 46.4%, respectively, were observed. Meanwhile, the activity of phosphate-accumulating organisms was completely inhibited. Furthermore, the protein content in the extracellular polymeric substances of sludge decreased substantially, and the sludge became more dispersed due to the alum accumulation, compared with that of the initial phase. Therefore, long-term dosing of PAC in the MBR should be managed to avoid excessive aluminium accumulation in the sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Membrane bioreactor (MBR) technology is a promising alternative for the treatment and reuse of industrial and municipal wastewater due to its small footprint and high effluent quality (Judd 2016; Wang et al. 2006). To enhance the removal efficiencies of nitrogen and chemical oxygen demand (COD), the sludge retention time (SRT) of MBR is generally prolonged (El-Fadel et al. 2018; Liu et al. 2018). Nevertheless, the MBR with long SRT is usually characterised by a reduction of biological phosphorus removal. Therefore, poly-aluminium chloride (PAC) is often dosed to enhance phosphorus removal and control membrane fouling in the MBR (Ingildsen et al. 2006; Feng et al. 2015). However, the impacts of aluminium accumulation on the microorganisms, such as ammonia-oxidising bacteria (AOB), nitrite-oxidising bacteria (NOB), and phosphate-accumulating bacteria (PAO), remained unclear in MBRs.
Previous studies mainly focused on achieving a high removal efficiency of phosphorus in the reactors (Li et al. 2018, 2019), but the attention to the influences of accumulated chemical flocculants on sludge activities and the structure of microbial communities was often not sufficient. A competition between biological and chemical phosphorus removal for available phosphorus and an inhibitory effect of chemical flocculants on biological phosphorus removal in the activated sludge processes were observed in the studies (De Haas et al. 2001; Liu et al. 2011; Zheng et al. 2017). Zheng et al. (2017) found that the dosing of PAC, using for membrane fouling control, indirectly influenced the bacterial diversity and structure of microbial community in the anaerobic membrane bioreactors. Liu et al. (2011) found that the inhibitory effect of Fe salt on the nitrification process, especially for AOB, was slightly noticeable when the accumulated amount of Fe salt was lower than 40 mg/L. However, the inhibition degree of aluminium salt was substantial with larger amounts of aluminium dosing in a sequencing batch reactor (Liu et al. 2011). Chen et al. (2018) assessed the effect of PAC on short-chain fatty acid production during the anaerobic fermentation of waste activated sludge. The dosing of PAC benefited the aggregates of sludge and provided a better protection of extracellular polymeric substances (EPS) against harmful environments, thereby inhibiting the solubilisation process. To date, the studies on the effect of aluminium salt on activated sludge system mainly remained at the level of short time and low load. The potential impact of high-strength aluminium on the performance of MBRs was not systematically studied thus far.
Therefore, the aims of this study were to systematically investigate the influence of accumulated aluminium on the performance of an anoxic–oxic membrane bioreactor (A/O-MBR) treating actual campus sewage. Additionally, the impacts of accumulated aluminium on the sludge activity and morphology were evaluated. This present study should provide novel insights into the potential impacts of accumulated aluminium on the performance of A/O-MBR.
Material and methods
Experimental setup and operating conditions
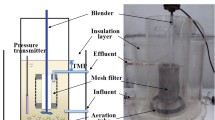
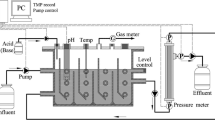
The reactor was composed of an A/O reactor and a submerged microfiltration membrane module of hollow fibre polyvinylidene fluoride (PVDF), as shown in Fig. 1. The A/O reactor was operated under ambient conditions and with a working volume of 6.1 L and a hydraulic retention time (HRT) of 15 h. A blower was employed to provide air to the aerobic zone in the MBR. The DO concentration of the aerobic zone was controlled at 2–4 mg/L during the whole experiment. When the DO was lower than 2 mg/L, the blower would be adjusted. At phase I, the effluent pH was in the range of 7.6–8.2 and the pH slightly decreased to 7.5–8.0 after the dosing of PAC. The pump 1 was employed to provide and guarantee a recirculation ratio of 300%, and the pump 2 was used for sludge mixing with a flow rate of 3.6 L/min. The membrane module, with a pore size of 0.2 μm and a membrane flux of 15 L/(m2 h), was installed in the aerobic zone. The MBR was operated at a cycle of 8 min of filtration and 2 min of relaxation to control the membrane fouling. The transmembrane press (TMP) was continuously measured using the pressure sensor. With long-term operation of membrane filtration, the TMP values gradually increased at phase II and the increasing rate of TMP decreased after washing by 0.5% NaClO at phase III (Fig. S1). To realize the continuous accumulation of aluminium salt on the sludge surface, therefore, no excess sludge was discharged during the whole period of the experiment. The experiment could be divided into three phases. At phase I (0–30 days), the reactor was started-up to achieve a stable performance for removal of nitrogen and COD. At phases II (30–70 days) and III (70–106 days), 28.3 mg/L of PAC was continuously pumped from the stock solution into the aerobic zone of the reactor to enhance phosphorus removal.
Wastewater characteristics and inoculum
The reactor was fed with campus sewage (10 L/day). The sewage was characterised with COD 107.5 ± 89.9 mg/L, NH4+-N 25 ± 15 mg/L, NO2−-N 0.34 ± 0.38 mg/L, NO3−-N 0.86 ± 1.13 mg/L, total phosphorus (TP) 2.2 ± 1.5 mg/L, and pH 7.0–7.8. The ratio of COD to total nitrogen (TN) was about 2.4, which was not adequate for denitrification (Jin et al. 2013). Therefore, sodium acetate (130 mg/L COD) was dosed into the anoxic zone of the reactor to ensure a sufficient carbon source (COD/TN > 6) for denitrification during the whole experiment. The period between days 80 and 100 was the rainy season in China, and the campus sewage was often diluted by rainwater, and thus, the concentration of ammonia and phosphorus abruptly decreased. The mixed liquid suspended solid (MLSS) and mixed liquid volatile suspended solid (MLVSS) concentrations of the inoculum were 3 and 1.77 g/L, respectively.
Activity tests of sludge
The activities of sludge, including specific oxygen uptake rate (SOUR), ammonia oxidation rate (AOR), nitrite oxidation rate (NOR), nitrate utilisation rate (NUR), phosphorus release rate (PRR), and phosphorus uptake rate (PUR), were determined by the batch tests in duplicate. The SOUR, AOR, NOR, and NUR were performed according to the studies (Zubrowskasudol and Walczak 2014; Wang et al. 2017; Yang et al. 2018). The PPR and PUR tests were conducted as follows. The sludge was aerated in a sodium phosphate solution to saturate the phosphate-accumulating organisms (PAOs) with phosphorus. The sludge was prepared as for the NUR tests and was dosed into the beakers with 120 mg COD/L at the beginning of the PRR tests. The PUR tests were carried out after the PRR tests. At the beginning of the PUR tests, the sludge in the beakers was continuously aerated with a phosphate concentration of 6 mg/L. The PRR and PUR of sludge were calculated as the phosphorus release and phosphorus depletion rate with time divided by the VSS concentration, respectively.
EPS analyses
The sludge obtained from the reactor was centrifuged at 10324×g for 15 min at 4 °C. The EPS of sludge was extracted using the cation exchange resin (CER) technique (Gummadi et al. 2012). The concentrations of carbohydrates and proteins were measured according to a previous study (Wang et al. 2017a).
Analytical methods
The concentrations of COD, NH4+-N, NO2−-N, NO3−-N, TN, TP, MLSS, and MLVSS were measured according to standard methods (APHA 2005). The aluminium concentration of effluent was detected by spectrophotometry chrome azurol S. A laser granularity distribution analyser (Malvern Instruments, MS-2000, Britain) with a detection range of 0.02–2000 μm was used to analyse the particle size distribution (PSD) of sludge. A zeta potentiometer (Nano-ZS90, Malvern, Britain) was employed to analyse the zeta potential of sludge. The surface morphology of sludge was scanned with a field emission scanning electron microscope (FESEM, SU8020, Hitachi Co., Japan). Prior to an SEM analysis, sludge samples were fixed with 3% (w/v) glutaric aldehyde and dehydrated with ethanol. Subsequently, the dehydrated samples were dried in a vacuum freezing drying oven and sputtered by gold for SEM observation. The percentage of atomic aluminium on sludge surface was measured using an energy dispersive spectrometer (EDS) simultaneously.
Results and discussion
Impact of aluminium accumulation on treatment performance of A/O-MBR
Figure 2 shows the treatment performance of TN, TP, and COD at three phases. At phase I (0–30 days), the sludge was acclimated to the campus sewage, and the average removal efficiencies of NH4+-N, TN, and COD were 97.5%, 80.9%, and 94.0%, respectively. The corresponding effluent concentrations were 0.84 ± 0.55, 3.24 ± 2.09, and 18.74 ± 5.54 mg/L, respectively. The concentrations were all lower than the sewage discharge standards of the first class, Class A, for municipal wastewater treatment plants (WWTPs) in China (Wang et al. 2015). However, the effluent phosphorus concentration (1.88 ± 0.93 mg/L) could not meet the discharge standard (0.5 mg/L). Subsequently, 28.3 mg/L of PAC was dosed into the reactor to enhance phosphorus removal in phases II (30–70 days) and III (70–106 days). The effluent phosphorus concentration decreased to less than 0.3 mg/L because of the coagulation precipitates of phosphorus in the A/O-MBR. Additionally, the average removal efficiencies of NH4+-N, TN, and COD were 97.0%, 87.9%, and 92.7% at phase II as well as 94.9%, 84.8%, and 92.8% at phase III, despite the continuously accumulated aluminium salt in the reactor. Liu et al. (2011) reported that the inhibition of aluminium on the AOB was dominant in the nitrification process and the inhibitory effect increased gradually with the increase of alum dosage. With the accumulation of aluminium in the reactor, the aluminium salt-tolerant microorganisms might have become the main populations in the sludge, allowing the reactor to maintain a stable treatment performance during the whole experiment. The result indicated that dosing of PAC could effectively enhance phosphorus removal without destroying treatment performance for nitrogen and COD removal. Additionally, the concentration of aluminium in the effluent was basically below the detection, resulting in the theoretical concentration of aluminium up to 0.107 (phase II) and 0.142 (phase III) g Al/g SS in the reactor. To further clarify the potential impact of aluminium accumulation on the performance of A/O-MBR, the changes of sludge activities and morphology during the three phases were evaluated.
Impact of aluminium accumulation on biological nitrification of A/O-MBR
The activities of AOB, NOB, and SOUR of sludge during the three phases are depicted in Fig. 3a. With the accumulation of aluminium in the reactor, the activity of AOB decreased continually from 56.6 ± 3.0 (phase I) to 27.8 ± 4.3 in phase II and further to 10.3 ± 0.3 mg NH4+-N/(g VSS·day) in phase III. The activity of NOB declined from 121.4 ± 0 (phase I) to 24.9 ± 0.3 mg NO2−-N/(g VSS·day) in phase II and remained relatively stable in phase III. A similar tendency was observed for the SOUR of sludge, which decreased from 0.25 ± 0.1 mg O2/(g VSS·min) (phase I) to 0.09 ± 0 mg O2/(g VSS·min) in phase II and then increased slightly to 0.13 ± 0.1 mg O2/(g VSS·min) in phase III. The results indicate that the accumulated aluminium substantially impaired the activity of sludge, although the reactor operated stably. The inhibition of accumulated aluminium was more severe to AOB than NOB. The result coincided with the result of Liu et al. (2011), who found that AOB was more easily inhibited by aluminium in the activated sludge processes. Because AOB could co-metabolise the organic pollutants via a non-specific enzyme namely ammonia monooxygenase (Wu et al. 2019), the dosing of PAC might flocculate this enzyme that is inhibiting the activity of AOB. The SOUR of sludge increased from phase II to III, which might be attributable to the increase in aluminium salt-tolerant microorganisms. Lin et al. (2017) reported that the growth of two aquatic organisms Vallisneria natans and Hydrilla verticillata was suppressed by residual aluminium in West Lake, Hangzhou, China. Matsumoto (2000) revealed the toxicity of aluminium to aquatic biota, algal community, higher plants, and fish from the perspective of biological effects. The hydrolysed products of aluminium might be the main factor for the inhibition of microbial metabolism. Figure 3b shows the nitrate utilisation rate of sludge during the three phases. At phase I, about half of NOX− (NO3− + NO2−) concentration remained in the effluent, but during phases II and III, only 3.7 and 0 mg/L of NOX− remained, respectively. The NUR of sludge increased substantially with the aluminium accumulation in the reactor. Li et al. (2012) found that the surrounding small flocs and organics could bind tightly with aluminium salt. Therefore, the aluminium-containing sludge affected the oxygen transfer and provided better niche for the metabolism of denitrifying bacteria.
Impact of aluminium accumulation on biological phosphorus removal of A/O-MBR
The profiles of the phosphorus release and uptake rates during the three phases are shown in Fig. 4. A substantial decrease in phosphorus concentration from 3.6 (phase I) to 0.2 (phase II) and then further to 0 mg/L (phase III) was observed, indicating that the phosphate accumulating organisms (PAOs) were severely inhibited under the anaerobic conditions. Nevertheless, the PAOs maintained a little activity of phosphorus uptaking under the aerobic condition at phases II and III, but the uptake rates were lower than that of phase I. The result was presumably mainly attributable to the hydrolysed products of aluminium absorbed on the surface of sludge. The accumulated aluminium presented an apparent inhibitory effect on phosphorus releasing and uptaking in the presence of phosphate limitation, which agreed well with the results obtained in the previous studies (Liu et al. 2011; Röske and Schönborn 1994; Xie et al. 2010). De Haas et al. (2001) reported that the inhibition degree of the biological phosphorus removal increased slightly in the presence of phosphate limitation. Xie et al. (2010) found that Al3+ stimulated the activity of alkaline phosphatases to 120% in the concentration range of 0.5–2.5 mM Al3+, but the activity was reduced dramatically to 20.6% at 5.0 mM Al3+. Röske and Schönborn (1994) reported that biological phosphorus removal was substantially inhibited if adequate aluminium salt was dosed into the process. In this present study, the adequate aluminium salt precipitated with phosphorus preferentially in the aerobic zone of the reactor, which inhibited biological phosphorus uptaking and thereby restrained the metabolism of PAOs.
Impact of aluminium accumulation on the morphology of sludge
Figure 5 shows the SEM-EDS images of the sludge during the three phases. At the end of phase I, the sludge flocs presented large particle size with irregular and loose shape, and the sludge existed in the floc structure with the skeleton built by the filamentous bacteria. However, as the percentage of atomic aluminium on sludge surface increased from 2% (phase I) to 9.4% (phase II), the surface morphology of sludge evidently changed. Figure 5 (II) reveals that the size of sludge flocs became smaller and that the floc structure of sludge was disrupted, being replaced by a closed and compact structure. When the percentage of atomic aluminium on sludge surface increased to 14.2% (phase III), the sludge presented a much denser structure. Additionally, the filamentous bacteria almost disappeared, and thus, the skeleton of sludge flocs was replaced by aluminium salt. A similar phenomenon was reported by He et al. (2016), in which the authors also stated that the activated sludge became small and extremely close when salt (> 1 wt% salinity) intruded activated sludge processes. The result indicated that high aluminium salt suppressed the growth of filamentous bacteria and substantially affected the floc structure of sludge.
To characterise the change of sludge morphology in depth, the profiles of PSD, EPS, and zeta potential are depicted in Fig. 6. As shown in Fig. 6a, the mean particle size of sludge flocs decreased from 25.01 (phase I) to 9.94 (phase II) and further to 9.32 μm (phase III), respectively, which matched well with the result obtained from the SEM images. However, the result conflicted with the flocculation mechanism. As a chemical flocculant, PAC could stimulate the growth of sludge flocs to larger and stronger flocs through charge neutralisation and polymer bridging effects (Lee et al. 2014), corresponding with the zeta potential decrease from − 16 ± 0.66 (phase I) to − 1.43 ± 0.09 (phase II), and further to − 4.86 ± 0.02 mV (phase III), respectively. The concentration of EPS was 68.17 ± 1.65, 55.80 ± 1.32, and 44.32 ± 0.46 mg/g VSS at the end of phases I, II, and III, respectively. The variation trend of EPS concentration was consistent with the size of sludge flocs. Additionally, the proportion of proteins and carbohydrates in the EPS of sludge at phase III declined by 49.6% and 27.4% compared with those of phase I, which indicated that the aluminium salt affected the morphology of sludge mainly by influencing the protein of EPS. Cao et al. (2016) found that a polymeric aluminium salt coagulant, especially middle polymer state aluminium (Alb) or high polymer state aluminium (Alc) coagulant, could compress the structure of EPS and remove protein-like substances from soluble EPS. With the dosing of inorganic flocculants, the positive charges generated from Al3+ salts hydrolysis neutralised the negative charges on the surface of cell wall and EPS (Wu et al. 2006). Li et al. (2012) pointed out that since Al3+ possessed a higher binding affinity with activated sludge, the small flocs and EPS could also bind tightly with trivalent cations. Therefore, the decrease of EPS in the sludge was caused by the flocculation of PAC. With the accumulation of aluminium on sludge surface, the hydrolysed products of aluminium salt could act as skeleton builders to support the cake structure instead of filamentous bacteria. Durban et al. (2015) found that the possible growth limitation of filamentous bacteria in addition to the flocculation effect at the high dosage of aluminium salts. As shown in Fig. 7, it would induce the decrease of MLVSS/MLSS from 55.1% (phase I) to 47.9% (phase II) and further to 41.9% (phase III). The sludge flocs with many inorganic constituents might be broken easily by the high shear stress resulting from the recycle pump. Furthermore, the accumulation of aluminium inhibited the activities of sludge by affecting the metabolism of nitrifying bacteria and phosphorus-accumulating bacteria. On the contrary, the NUR of sludge was not inhibited by the accumulated aluminium, which indicated that the better denitrifying condition was formed in the aluminium-containing sludge.
Conclusion
Based on the experimental results, the main conclusions in this study were as follows:
-
1.
The A/O-MBR could achieve excellent treatment performance for nutrient removal despite the percentage of atomic aluminium on the sludge surface increasing to 14.2%.
-
2.
The activities of AOB, NOB, PAOs, and SOUR of sludge were substantially inhibited by the accumulated aluminium in the sludge, while the NUR of sludge was enhanced.
-
3.
The sludge flocs became more dispersed with the accumulation of aluminium compared with that of the initial phase.
References
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. Washington, DC, American Water Works Association
Cao B, Zhang W, Wang Q, Huang Y, Meng C, Wang D (2016) Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: role of aluminum speciation. Water Res 105:615–624. https://doi.org/10.1016/j.watres.2016.09.016
Chen Y, Wu Y, Wang D, Li H, Wang Q, Liu Y, Peng L, Yang Q, Li X, Zeng G, Chen Y (2018) Understanding the mechanisms of how poly aluminium chloride inhibits short-chain fatty acids production from anaerobic fermentation of waste activated sludge. Chem Eng J 334:1351–1360. https://doi.org/10.1016/j.cej.2017.11.064
De Haas DW, Wentzel MC, Ekama GA (2001) The use of simultaneous chemical precipitation in modified activated sludge systems exhibiting biological excess phosphate removal: part 5: experimental periods using a ferrous-ferric chloride blend. Water S A 27:117–134. https://doi.org/10.4314/wsa.v27i2.4987
Durban N, Juzan L, Krier J, Gillot S (2015) Control of Microthrix parvicella by aluminium salts addition. Water Sci Technol 73:414–422. https://doi.org/10.2166/wst.2015.456
El-Fadel M, Sleem F, Hashisho J, Saikaly PE, Alameddine I, Ghanimeh S (2018) Impact of SRT on the performance of MBRs for the treatment of high strength landfill leachate. Waste Manag 73:165–180. https://doi.org/10.1016/j.wasman.2017.12.003
Feng L, Shuang Z, Sun S, Wang W, Gao B, Yue Q (2015) Effect of pH with different purified aluminum species on coagulation performance and membrane fouling in coagulation/ultrafiltration process. J Hazard Mater 300:67–74. https://doi.org/10.1016/j.jhazmat.2015.06.034
Gummadi SN, Bhavya B, Ashok N (2012) Physiology, biochemistry and possible applications of microbial caffeine degradation. Appl Microbiol Biotechnol 93:545–554. https://doi.org/10.1007/s00253-011-3737-x
He H, Chen Y, Li X, Cheng Y, Yang C, Zeng G (2016) Influence of salinity on microorganisms in activated sludge processes: a review. Int Biodeterior Biodegradation 119:520–527. https://doi.org/10.1016/j.ibiod.2016.10.007
Ingildsen P, Rosen C, Gernaey KV, Nielsen MK, Guildal T, Jacobsen BN (2006) Modelling and control strategy testing of biological and chemical phosphorus removal at Avedøre WWTP. Water Sci Technol 53:105–113. https://doi.org/10.2166/wst.2006.115
Jin R, Liu G, Li C, Xu R, Li H, Zhang L, Zhou J (2013) Effects of carbon–nitrogen ratio on nitrogen removal in a sequencing batch reactor enhanced with low-intensity ultrasound. Bioresour Technol 148:128–134. https://doi.org/10.1016/j.biortech.2013.08.141
Judd SJ (2016) The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem Eng J 305:37–45. https://doi.org/10.1016/j.cej.2015.08.141
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ 92:489–508. https://doi.org/10.1016/j.psep.2014.04.010
Li H, Wen Y, Cao A, Huang J, Zhou Q, Somasundaran P (2012) The influence of additives (Ca2+, Al3+, and Fe3+) on the interaction energy and loosely bound extracellular polymeric substances (EPS) of activated sludge and their flocculation mechanisms. Bioresour Technol 114:188–194. https://doi.org/10.1016/j.biortech.2012.03.043
Li RH, Wang XM, Li XY (2018) A membrane bioreactor with iron dosing and acidogenic co-fermentation for enhanced phosphorus removal and recovery in wastewater treatment. Water Res 129:402–412. https://doi.org/10.1016/j.watres.2017.11.035
Li RH, Wang WJ, Li B, Zhang JY, Liu J, Zhang GY, Guo XC, Zhang XH, Li XY (2019) Acidogenic phosphorus recovery from the wastewater sludge of the membrane bioreactor systems with different iron-dosing modes. Bioresour Technol 280:360–370. https://doi.org/10.1016/j.biortech.2019.02.060
Lin QW, He F, Ma JM, Zhang Y, Liu BY, Min FL, Dai ZG, Zhou QH, Wu ZB (2017) Impacts of residual aluminum from aluminate flocculant on the morphological and physiological characteristics of Vallisneria natans and Hydrilla verticillata. Ecotoxicol Environ Saf 145:266–273. https://doi.org/10.1016/j.ecoenv.2017.07.037
Liu Y, Shi H, Li W, Hou Y, He M (2011) Inhibition of chemical dose in biological phosphorus and nitrogen removal in simultaneous chemical precipitation for phosphorus removal. Bioresour Technol 102:4008–4012. https://doi.org/10.1016/j.biortech.2010.11.107
Liu J, Tian Z, Zhang P, Qiu G, Wu Y, Zhang H, Xu R, Fang W, Ye J, Song Y, Zeng G (2018) Influence of reflux ratio on two-stage anoxic/oxic with MBR for leachate treatment: performance and microbial community structure. Bioresour Technol 256:69–76. https://doi.org/10.1016/j.biortech.2018.01.146
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1. https://doi.org/10.1016/S0074-7696(00)00001-2
Röske I, Schönborn C (1994) Interactions between chemical and advanced biological phosphorus elimination. Water Res 28:1103–1109. https://doi.org/10.1016/0043-1354(94)90196-1
Wang Z, Wu Z, Yu G, Liu J, Zhou Z (2006) Relationship between sludge characteristics and membrane flux determination in submerged membrane bioreactors. J Membr Sci 284:87–94. https://doi.org/10.1016/j.memsci.2006.07.006
Wang XH, Wang X, Huppes G, Heijungs R, Ren NQ (2015) Environmental implications of increasingly stringent sewage discharge standards in municipal wastewater treatment plants: case study of a cool area of China. J Clean Prod 94:278–283. https://doi.org/10.1016/j.jclepro.2015.02.007
Wang D, Fu Q, Xu Q, Liu Y, Hao Ngo H, Yang Q, Zeng G, Li X, Ni B-J (2017) Free nitrous acid-based nitrifying sludge treatment in a two-sludge system enhances nutrient removal from low-carbon wastewater. Bioresour Technol 244:920–928. https://doi.org/10.1016/j.biortech.2017.08.045
Wang W, Wang S, Ren X, Hu Z, Yuan S (2017a) Rapid establishment of phenol- and quinoline-degrading consortia driven by the scoured cake layer in an anaerobic baffled ceramic membrane bioreactor. Environ Sci Pollut Res 24:26125–26135. https://doi.org/10.1007/s11356-017-0284-8
Wu JL, Chen FT, Huang X, Geng WY, Wen XH (2006) Using inorganic coagulants to control membrane fouling in a submerged membrane bioreactor. Desalination 197:124–136. https://doi.org/10.1016/j.desal.2005.11.026
Wu H, Sun Q, Sun Y, Zhou Y, Wang J, Hou C, Jiang X, Liu X, Shen J (2019) Co-metabolic enhancement of 1H-1,2,4-triazole biodegradation through nitrification. Bioresour Technol 271:236–243. https://doi.org/10.1016/j.biortech.2018.09.112
Xie C, Lu R, Huang Y, Wang Q, Xu X (2010) Effects of ions and phosphates on alkaline phosphatase activity in aerobic activated sludge system. Bioresour Technol 101:3394–3399. https://doi.org/10.1016/j.biortech.2009.12.047
Yang G, Xu Q, Wang D, Tang L, Xia J, Wang Q, Zeng G, Yang Q, Li X (2018) Free ammonia-based sludge treatment reduces sludge production in the wastewater treatment process. Chemosphere 205:484–492. https://doi.org/10.1016/j.chemosphere.2018.04.140
Zheng W, Yu Z, Xia Y, Wen X (2017) Influence of polyaluminum chloride on microbial characteristics in anaerobic membrane bioreactors for sludge digestion. Appl Microbiol Biotechnol 102:1–13. https://doi.org/10.1007/s00253-017-8613-x
Zubrowskasudol M, Walczak J (2014) Effects of mechanical disintegration of activated sludge on the activity of nitrifying and denitrifying bacteria and phosphorus accumulating organisms. Water Res 61:200–209. https://doi.org/10.1016/j.watres.2014.05.029
Funding
This work was supported by the National Natural Science Foundation of China (51878232), the Science and Technology Foundation of Anhui Provincial Housing, and Urban Rural Development Office (2017YF-05), and the CAS Key Laboratory of Urban Pollutant Conversion, University of Science and Technology of China (KF201702).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerald Thouand
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
The TMP variation of A/O-MBR during the whole experiment. (DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Yang, C., Qiu, C., He, C. et al. Influence of aluminium accumulation on biological nitrification and phosphorus removal in an anoxic–oxic membrane bioreactor. Environ Sci Pollut Res 26, 28127–28134 (2019). https://doi.org/10.1007/s11356-019-06004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06004-1