Abstract
Recently, due to increased drought risks, need for crops with higher water stress tolerance has increased strongly. Those crops have a wide range of uses such as supplying food as well as land restoration. Medicago scutellata (L.) Mill. is a Fabaceae widely cultivated for its capacity to produce high-quality forage. This study was designed as a factorial experiment based on a completely randomized design with three replications. Different cadmium levels were the first factor and included 0, 5, 25, 50, 100, and 125 mg kg−1. Second factor was the drought stress, which had three levels (100, 75, and 50% feed consumption). According to the results, Cd uptake in different organs increased with increasing Cd levels up to 100 mg kg−1 while the water stress had a negative effect on Cd uptake by M. scutellata. Average concentration of Cd in the leaves, stems, and roots were 63.16, 30.12, and 20.45 mg kg−1, respectively. The high value of translocation factor (TF) confirms the high ability of M. scutellata in translocation Cd from root to shoot. Fe, Zn, and K concentration of different organs significantly decreased with increasing Cd level. Fe and Zn concentration increased by increasing water stress levels in all organs and K concentration of roots decreased while in leaves and shoots increased by increasing water stress level. These results indicate that M. scutellata has a good ability for eliminating Cd from contaminated soil attribute to its powerful absorption and accumulation for Cd. It also showed a good performance under the co exposure of water stress and Cd indicated by accumulating proline and K in leaves.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From a global view after climate, soil crust is considered as one of the important parts of the human environment. Soil is not only considered as the living environment for terrestrial organism, especially human, but also a unique environment for the other living organisms such as plants. Soil contamination by inorganic pollutants such as heavy metals is one of environmental hazards that threaten human life today. Soil pollution causes loss of vegetation, reducing the plants growth and development, rangeland degradation, soil erosion, and finally desertification. Excessive accumulation of heavy metals in soil reduces biological activity and fertility resulting in reduced yield and quality loss of products that can be dangerous to human health or animal consumption (Stingu et al. 2012).
Cadmium (Cd) is the most prevalent pollution heavy metal in soil which is toxic to human and animal. Cadmium is used in some kinds of batteries, plastic byproducts, fertilizers, and pesticides (Amini et al. 2005). The total amount of Cd in agricultural soils should not exceed 0.4–0.5 mg kg−1 and higher values reflect the excessive use of phosphatic fertilizers as well as sewage sludge (Bjelkova et al. 2011; Stingu et al. 2012). During the past 30 to 40 years, using of fertilizers containing phosphoric acid (superphosphate and ammonium phosphate) to agricultural soil in the Iran has increased. Most of agricultural lands in Iran received 100–300 kg superphosphate per hectare annually for more than four decades (Jalali and Khanlari 2008). Amini et al. (2005) assessed 255 top soil samples from central Iran; they reported the total Cd concentration in most of tested samples were more than recommended thresholds of 0.8 mg kg−1. Therefore, ways to remove or degrade this pollutant has been a great concern. Phytoremediation is a green, simple, eco-friendly, sustainable, and cost-effective remediation technology that uses plants to remove and degrade contaminants from the soil. In this regard, Medicago species has been widely adopted for the phytoremediation of polluted soils with heavy metals (Lopez et al. 2005; Fan et al. 2008; Wei and Pan 2010). Medicago sativa L. has shown the ability to hyperaccumulate heavy metals in its tissues (Angle and Linacre 2005) and to grow well in highly contaminated soils (Amer et al. 2013).
Arid and semi-arid regions comprise more than 60% of Iran (Fakhari and Sadeghi 2016). The average annual rainfall in Iran is about 251 mm and recently, due to increased drought risks, need for crops with higher water stress tolerance has increased strongly (Sadeghi and Robati 2015). Medicago scutellata (L.) Mill. is a Fabaceae widely cultivated for it capacity to produce high-quality forage (Fakhari and Sadeghi 2016). Annual Medicago is found in almost all regions of Iran. More than 556,000 ha of Medicago species are grown as continuous cropping in Iran. This suggests that these plants are appropriate for Iranian pastures. Medicago has several annual species, such as Medicago rigidula L., Medicago polymorpha L., and Medicago scutellata L., which can produce high numbers of seeds. It establishes relatively easily but its early growth in autumn is rapid and erect, making it susceptible to overgrazing (Sadeghi and Fakhari 2016). The barrel medic is adapted to different soil texture types, from sandy to clay, and particularly to well-drained neutral-to-alkaline soils with a pH of 6 to 8; it is suited to warm temperate conditions, especially Mediterranean-type climates, has 250–600 mm annual rainfall with dry hot summers and mild moist winters, and is intolerant of winter frosts. It has poor regrowth for a second harvest, but has a good ability to self-regenerate from seeds present in the soil seed bank (Fakhari and Sadeghi 2016). Legumes are a good choice for phytoremediation since they have the capability of N2 fixation (Amer et al. 2013). A review of current literature indicated that no research, to best of our knowledge, has yet tested the co-exposure of Cd and water stress on the phytoremediation ability of M. scutellata. Therefore, the present study was designed with the intention to evaluate the biochemical response of M. scutellata to Cd–water stress co-exposures and its utilization potential for phytoremediation.
Materials and methods
Study site and plant material
This research was conducted as a factorial experiment based on completely randomized design in a greenhouse at the College of Agriculture, Shiraz University (29° 43′ N and 52° 35′ W), Shiraz, Iran, during 2014. The temperatures of day and night in the greenhouse were set at 28 and 22 °C. The seeds of Medicago scutellata were prepared from Pakan Bazr Co. and stored at 5 °C.
Experimental design
Silty clay loam soil collected from the surface layer (0–30 cm) of soils (fine mixed, mesic Typic Calcixerpets soil) in the College of Agriculture was selected for the pot experiments. Some of soil characteristics are presented in Table 1 and the heavy metals of original soil were as follows: absorbable Cd, 0.2 mg kg−1; absorbable Pb, 0.4 mg kg−1; absorbable Cu, 1.31 mg kg−1; and absorbable Ni, 0.15 mg kg−1.
Silty clay loam soil gathered from the top layer (0–30 cm) of soils in the College of Agriculture was selected for the pot experiments. Some of soil characteristics are presented in Table 1. Soil samples were air dried and grounded to pass 2-mm nylon sieve prior to use. The soils were spiked with sulfuric acid cadmium salt at the rate of 0, 5, 25, 50, 100, and 125 mg kg−1 and incubated for 2 weeks. After incubation, each plastic pot was filled with 4 kg of the treated soil. Ten seeds were planted in each pot. After emergence, six plants per pot were kept. Pots were watered at field capacity. Water stress was imposed on the plants 20 days after planting by applying different irrigation regimes (100% as control, 75% and 50% field capacity (FC)) for 3 months. At the end of the trial, different plant organs (roots, shoot, leaves) were separated and sampled for analysis.
Analysis and measurement
The harvested plant parts were washed completely with distilled water, dried at 70 °C, and weighed. For the measurement of mineral elements, plant samples were dried at 550 °C for 4 h to obtain ashes, then extracted with 2 M HCl, filtered through Whatman No. 42 filter paper, and analyzed for Cd, Fe, and Zn by an atomic absorption spectrophotometer (model Perkin Elmer 4110) (Azizian et al. 2011). Potassium concentrations were determined by flame photometry (INESA, FP6410, Shanghai, China). Available metals were determined using diethylene-triamine-pentaacetic acid (DTPA) buffered at pH 7.3. Cadmium was determined by atomic absorption spectrophotometry (AAS).
For the proline measurements, plant’s part samples were grounded in liquid nitrogen. The concentration of proline was estimated based on the acid-ninhydrin method (Bates et al. 1973) by spectrophotometer with minor modifications (Fakhari and Sadeghi 2016).
Crude protein was estimated from nitrogen by multiplying total N by 6.25, according to the Kjeldahl method described in the Association of Official Analytical Chemists (2002). The translocation factor (TF) from root to shoot was calculated as follows (Su et al. 2014):
Statistical analysis
This experiment was conducted as a factorial experiment based on a completely randomized design with three replications. Analysis was performed with SAS Program Version 9.1.3 (2004). Different cadmium levels were the first factor and included 0, 5, 25, 50, 100, and 125 mg kg−1. Second factor was the water stress, which had three levels (100, 75, and 50% FC).
Results
Some physiochemical properties of the selected soil for the pot experiment are presented in Table 1. The tested soil has a medium texture (silty clay loam) with no acidity and salinity problem and its cadmium (Cd) level was very low.
ANOVA (Table 2) showed that the interaction effect of the cadmium application and water stress ([CD] × water stress) was significant on all the measured factors of Medicago scutellata, with the exception of potassium concentration in root and proline content, at 1% probability level. However, the main effect of cadmium application and water stress was significant at 1% probability level about potassium concentration in root and proline content (Table 2).
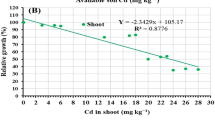
According to Table 3, with increasing the level of cadmium of up to 100 mg kg−1, Cd concentration in roots, stems, and leaves increased but then decreased at the level of 125 mg kg−1 and the Cd concentration of different organs (roots, stems, and leaves) reduced with increasing water stress level from 100 to 50% FC (Table 3). The highest Cd average concentration of root was observed at 100 mg kg−1 and 100% FC which increased 54-fold compared with the control (0 mg kg−1 cadmium and 100% FC). Cadmium concentration of roots from 100 to 125 mg kg−1 decreased 7% (Table 3). The highest Cd average of shoot and leaves belonged to 100 mg kg−1 and 100% FC which increased 35-fold and 58-fold compared with the control, respectively (Table 3). The average concentrations of Cd in leaves, stems, and roots were 63.16, 30.12, and 20.45 mg kg−1, respectively. In other words, the average concentrations of Cd in leaves and shoots were 3.08 and 1.47 times higher than roots, respectively. The highest amount of Cd in soil was observed at 125 mg kg−1 Cd under 50% FC which was 298 times higher than control (0 mg kg−1 cadmium and 100% FC) (Table 3).
The highest translocation factor (TF) was observed at the concentration of 50 mg kg−1 under 75% FC while the lowest one, with 50% reduction, was observed at 125 mg kg−1 under 100% FC (Table 4). In all treatments, the TF was more than 1 which reflected the high ability of M. scutellata in translocation Cd from root to shoot (Table 4).
According to Table 5, the average of iron (Fe) concentration in different organs (root, shoot, and leaves) decreased with the increasing Cd level from 0 to 125 mg kg−1. Under water stress, the Fe concentration of different organs significantly increased. In all tested organs, the highest Fe concentration belonged to the 0 mg kg−1 Cd and 50% FC while the lowest one was observed at 100 mg kg−1 and 100% FC (Table 5).
Table 6 showed that Zinc (Zn) concentration in different organs increased significantly with the increasing water stress level from 100 to 50% FC. Similar to Fe concentration, in all organs (roots, shoots, and leaves), the highest Zn concentration belonged to the 0 mg kg−1 Cd under 50% FC while the lowest one was observed at 100 mg kg−1 and 100% FC. Also, the average of Zn concentration in different organs decreased with increasing Cd level from 0 to 125 mg kg−1 (Table 6).
Potassium (K) concentration of different organs significantly decreased with the increasing Cd level up to 100 mg kg−1 but increased at 125 mg kg−1 compared with 100 (Table 7). K concentration of roots decreased while in leaves and shoots increased by increasing water stress level. The highest amount of K concentration in all organs belonged to the 5 mg kg−1 Cd under 50% FC while the lowest one was observed at 100 mg kg−1 Cd and 100% FC in the case of shoots and leaves and 100 mg kg−1 and 50% FC in the case of roots (Table 7).
According to Table 8, proline content increased significantly with the increasing Cd level and water stress. The highest amount of proline content was observed at 125 mg kg−1 Cd under 50% FC while the lowest one belonged to the control treatment. Unlike proline, the percentage of crude protein decreased significantly with the increasing Cd level and water stress. The highest percentage of crude protein was observed in control treatment while the lowest belonged to 125 mg kg−1 Cd and 50% FC. By consuming Cd, protein content decreased significantly (Table 8).
The result of correlation analysis (Table 9) showed that the highest correlation coefficient was observed between Zn concentrations in roots with Zn concentration in shoot (0.991**). Protein had negative and significant correlation with proline and Cd concentration in different organs; however, it showed a positive and significant correlation with other measured elements (Fe, Zn, and K). K showed a negative and significant correlation with Cd and positive and significant correlation with Zn and Fe concentration in different organs. Fe and Cd showed a negative and significant correlation within different organs (Table 9).
Discussion
In this study, the effect of different Cd levels of soil on the biochemical response of Medicago scutellata was investigated under water stress. According to the results, Cd uptake in different organs increased with increasing Cd levels up to 100 mg kg−1 while the water stress had a negative effect on Cd uptake by M. scutellata. These results are supported by Shen et al. (2002) who founded that Cd uptake of corn (Zea mays L.) increased with the increasing Cd level of soil. Azizian et al. (2011) also found the same results about Cd concentration of corn (Zea mays L.) and oat (Avena sativa L.) under different irrigation treatments. Gardea-Torresdey et al. (2000) reported that nickel uptake of (Medicago sativa L.) increased with increasing soil nickel and shoots showed to have more nickel concentration than the roots. In contrast, Bjelkova et al. (2011) observed that the most Cd was accumulated by roots in linseed (Linum usitatissimum L.) cultivars. These phenomena may be due to a difference in chemical functional groups in the plants species roots and shoots.
Suitable plants for phytoremediation should be able to prevent the transportation of heavy metals to the aerial parts and keep them at the root level thus preventing them from entering to the food chain (Stingu et al. 2012), or they are grown for industrial, non-food purpose such as using in textile or furniture industry. In our study, the average concentrations of Cd in leaves and shoots were 3.08 and 1.47 times higher than roots, respectively. The high value of translocation factor (TF) confirms the high ability of M. scutellata in translocation Cd from root to shoot. Since the main application of M. scutellata is for forage production for feeding animals, this factor should be taken into consideration. Studies have shown that cattle which graze on metal contaminated plants will accumulate the toxic metals in their bodies which could then be passed to humans (Chamberlain and Miller 1982). The standard limit of Cd is 0.1 mg kg−1 of the weight of the plant for human consumption and 10 mg kg−1 for animal consumption, while in our study, it was 63.16 mg kg−1 just in leaves.
In our study, Cd concentration of different organs (roots, stems, and leaves) reduced with increasing water stress level; similar findings were reported by Angle et al. (2003) who observed higher metal uptake as well as biomass production at higher soil moisture.
Fe, Zn, and K concentration of different organs significantly decreased with the increasing Cd level. Wong et al. (1986) observed a significant reduction in Zn and Fe by increasing Cd level and stated it can be due to the antagonistic effects of accumulation of Cd in plant tissues on the uptake of these essential metals. Yildiz (2005) reported that Zn concentration in tomato (Solanum lycopersicum L.) and corn (Zea mays L.) plant decreased by increasing Cd level. Also, similar findings were reported by Veselov et al. (2003) and Sandalio et al. (2001) in wheat (Triticum aestivum L.) and pea (Pisum sativum L.) plants. Cd toxicity in plants can be due to the interaction with essential nutrients for the plant which cab affect the balance of nutrients and reduced plants fertility. Cadmium and zinc are chemically similar; hence, they are competing with each other for absorbing by plants (Nazar et al. 2012).
Fe and Zn concentration increased by increasing water stress level in all organs and K concentration of roots decreased while in leaves and shoots increased by increasing water stress level. These results are in line with the findings of Alizadeh (2010) in corn (Zea mays L.). Osmotic advantage of K in improving cell water saturation under water stress is well documented which can explain the reason of its translocation from roots to the aerial parts under water stress (Cakmak 2005; Zhao 2000).
According to the result, proline content increased significantly with increasing Cd level and water stress. Proline acts as an osmolyte as well as an osmoprotectant under stressed condition which helps in protecting of enzymes, biological membranes, and photosynthetic apparatus from oxidative damages (Batish et al. 2006). Since proline supplies energy for growth and survival, its accumulation during stress helps the plant to tolerate the stressed condition better and easier (Gill and Toteja 2010). The observations made in this study are parallel with that in earlier studies (Shah et al. 2001; Mehta and Gaur 1999; Parida et al. 2008). According to the results, protein content decreased significantly by increasing Cd level. Cd alters the conformation of proteins such as enzymes and transporter proteins due to its strong affinity as ligands to sulfhydryl and carboxylic group (Nazar et al. 2012). Studies have shown the activity of reactive oxygen species during stress can damage biological molecules such as proteins and lipids (Molassiotis et al. 2006).
Conclusion
The forage crop M. scutellata can be considered as a Cd accumulator plant species. It also showed a good performance under the co-exposure of water stress and Cd indicated by accumulating proline and K in leaves. Its translocation factor was more than 1 which reflected the high ability of M. scutellata in translocation Cd from root to shoot. The amount of Cd accumulation in leaves is about 63 mg kg−1 which is 6 times more than standard limit for animal consumption. Since the main application of M. scutellata is for forage production for feeding animals and it does not have any remarkable non-food uses, it cannot be a good choice, due to the further transfer into the food chain. However, the ability enhancement of Cd translocation to M. scutellata shoot in higher concentrations of soil Cd indicates a great performance of the plant for Cd phytoextraction and could be introduced as Cd hyperaccumulator plant.
References
Alizadeh O (2010) Evaluation effect of water stress and nitrogen rates on amount of absorbtion some macro and microelement in corn plant mycorrhiza and nonmycorrhiza. Adv Nat Appl Sci 4:153–158. https://doi.org/10.3923/rjbsci.2010.350.355
Amer N, Al Chami Z, Al Bitar L, Mondelli D, Dumontet S (2013) Evaluation of Atriplex halimus, Medicago lupulina and Portulaca oleracea for phytoremediation of Ni, Pb and Zn. Int J Phytoremediation 15:498–512. https://doi.org/10.1080/15226514.2012.716102
Amini M, Khademi H, Afyuni M, Abbaspour KC (2005) Variability of available cadmium in relation to soil properties and land use in an arid region in central Iran. Water Air Soil Pollut 162:205–218. https://doi.org/10.1007/s11270-005-6273-4
Angle JS, Linacre NA (2005) Metal phytoextraction — a survey of potential risks. Int J Phytoremediation 7:241–254. https://doi.org/10.1080/16226510500215779
Angle JS, Baker AJM, Whiting NS, Chaney RL (2003) Soil moisture effects on uptake of metals by Thlaspi, Alyssum, and Berkheya. Plant Soil 256:325–332. https://doi.org/10.1016/j.envpol.2004.04.001
Association of Official Analytical Chemists International (AOAC) (2002) Official methods of analysis, of AOAC international (17th ed.). Method, 950.46
Azizian A, Amin S, Noshadi M, Maftoon M, Emam Y (2011) Phytoremediation potential of corn and oat for increased levels of soil cadmium under different irrigation intervals. Iran Agric Res 30(1):47–60
Bates LS, Waldern RP, Teave ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–209
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol Biochem 44:819–827. https://doi.org/10.1016/j.plaphy.2006.10.014
Bjelkova M, Gencurova V, Griga M (2011) Accumulation of cadmium by flax and linseed cultivars in field-simulated conditions: a potential for phytoremediation of Cd-contaminated soils. Ind Crop Prod 33:761–774. https://doi.org/10.1016/j.indcrop.2011.01.020
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530. https://doi.org/10.1002/jpln.200420485
Chamberlain WF, Miller JA (1982) Barium in forage plants and the manure of cattle treated with barium. J Agric Food Chem 30:463–465
Fakhari F, Sadeghi H (2016) The effect of pod elimination on water stress in relation to antioxidant enzymes activity and proline in three annual medics species. J Crop Sci Biotechnol 19:109–115. https://doi.org/10.1007/s12892-015-0097-6
Fan S, Li P, Gong Z, Ren W, He N (2008) Promotion of pyrene degradation in rhizosphere of alfalfa (Medicago sativa L.). Chemosphere 71:1593–1598. https://doi.org/10.1016/j.chemosphere.2007.10.068
Gardea-Torresdey JL, Tiemann KJ, Gonzalez JH, Cano-Aguilera I, Henning JA, Townsend MS (2000) Ability of Medicago sativa (alfalfa) to remove nickel ions from aqueous solution. Proceedings of the 10th Annual Conference on Hazardous Waste Research 239-248
Gill SS, Toteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Jalali M, Khanlari ZV (2008) Cadmium availability in calcareous soils of agricultural lands in Hamadan, Western Iran. Soil Sediment Contam 17:256–268. https://doi.org/10.1080/15320380802006970
Lopez ML, Peralta-Videa JR, Benitez T, Gardea-Torresdey JL (2005) Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 61:595–598. https://doi.org/10.1016/j.chemosphere.2005.02.028
Mehta SK, Gaur JP (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Cohlorella vulgaris. New Phytol 143:253–259
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62. https://doi.org/10.1016/j.envexpbot.2005.01.002
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489. https://doi.org/10.4236/ajps.2012.310178
Parida AK, Dagaonkar VS, Phalak MS, Aurangabadkar LP (2008) Differential response of the enzymes involved in proline biosynthesis and degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery. Acta Physiol Plant 30:619–627. https://doi.org/10.1007/s11738-008-0157-3
Sadeghi H, Fakhari F (2016) Response of growth and nitrogen fixation to pod elimination of three annual Medicago species submitted to drought and subsequent recovery. Can J Plant Sci 96:757–764
Sadeghi H, Robati Z (2015) Response of Cichorium intybus L. to eight seed priming methods under osmotic stress conditions. Biocatal Agric Biotechnol 4:443–448. https://doi.org/10.1016/j.bcab.2015.08.003
Sandalio LM, Dalurzo HC, Gomes M, Remero-Puertas MC, delRio LA (2001) Cadmiuminduced changes in the growth and oxidative metabolism of Pea plants. J Exp Bot 52:2115–2126
SAS Institute Inc (2004) SAS/STAT 9.1 User’s Guide. SAS Publishing, Cary, North Carolina: SAS Institute Inc., pp 5136
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shen ZG, Li XD, Wang CC, Chen HM, Chua H (2002) Lead phytoextraction from contaminated soil with high-biomass plant species. J Environ Qual 31:1893–1900
Stingu A, Volf I, Popa VI, Gostin I (2012) New approaches concerning the utilization of natural amendments in cadmium phytoremediation. Ind Crop Prod 35:53–60. https://doi.org/10.1016/j.indcrop.2011.06.005
Su Y, Liu J, Lu Z, Wang X, Zhang Z, Shi G (2014) Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ Exp Bot 97:40–48. https://doi.org/10.1016/j.envexpbot.2013.10.001
Veselov D, Kudoyarova G, Symonyan M, Veselov S (2003) Effect of Cadmium on ion uptake transpiration and cytokinin content in wheat seedlings. Bulg J Plant Physiol Special issue pp 353–359
Wei SQ, Pan SW (2010) Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J Soils Sediments 10:886–894
Wong MK, Chuah GK, Ang KP, Koh LL (1986) Interactive effects of lead, cadmium and copper combinations in the uptake of metals and growth of Brassica chinensis. Environ Exp Bot 26(4):331–339
Yildiz N (2005) Response of Tomato and Corn plants to increasing Cd levels in nutrient culture. Pak J Bot 37(3):593–599
Zhao HC (2000) Influence of water stress on the lipid physical membrance from P. betuloefolia. Bio Interfaces 19:181–185. https://doi.org/10.1016/S0927-7765(00)00153-3
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Average concentration of Cd in leaves was 63.16 mg kg−1, 6 times more than standard limits.
• Fe, Zn, and K concentration of different organs significantly decreased with increasing Cd level
• It also showed a good performance under the co-exposure of water stress and Cd.
• It cannot be a good choice for phytoremediation due to the further transfer into the food chain
Rights and permissions
About this article
Cite this article
Parsamanesh, S., Sadeghi, H. The phytoremediation effect of Medicago scutellata (L.) Mill. on soils under Cd–water stress: a good choice for contaminated dry lands. Environ Sci Pollut Res 26, 29065–29073 (2019). https://doi.org/10.1007/s11356-019-05989-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05989-z