Abstract
Exposure to PM10 generated by biomass burning may reduce lung function and induce cytogenetic effects, especially in chronic obstructive pulmonary disease patients. This study investigated the frequency of DNA-damaged cells, cells with cytokinetic defect, and different types of cell death using a buccal micronucleus cytome assay. The correlations between each biomarker and lung function were investigated. The changes in these biomarkers associated with high pollutant levels (PM10 > 50 μg/m3) and low pollutant levels (PM10 < 50 μg/m3) were evaluated to explore whether PM10 exposure induced genotoxic damages and cytokinetic defects in COPD patients when the daily average PM10 concentration reached above 50 μg/m3. Fifty-eight COPD patients and 26 healthy subjects living in Chiang Dao district, Chiang Mai, Thailand, were recruited in this study. The results revealed that buccal cells with micronuclei (high vs low 1.09 ± 1.95 vs 0.29 ± 0.64 in COPD patients) and binucleated cells (high vs low 11.43 ± 18.68 vs 1.60 ± 1.31 and 7.77 ± 12.76 vs 1.00 ± 1.17 in COPD and healthy subjects, respectively) observed during the high pollutant period were more frequent than in the low pollutant period. Moreover, exposure to PM10 increased the risk of micronucleus induction in COPD patients 295.23-fold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of chronic obstructive pulmonary disease (COPD) and its level of severity are complicated due to the interactions among several mechanisms, including genetic susceptibility, inflammation, oxidative stress, chromatin modifications, DNA damage, apoptosis, and defective DNA repair (Hu et al. 2015; Neofytou et al. 2012; Yang and Omaye 2009). The incidence of COPD patients in Thailand has become a matter of concern at the Ministry of Public Health, Thailand, during the past decade (Ministry of Public Health Thailand 2015). According to the Global Burden of Disease, COPD is now the third leading cause of death globally (Hu et al. 2015; Quaderi and Hurst 2018). Moreover, high prevalence of COPD is reported in several countries in which air pollution has become a major concern including China (prevalence of 3.7–17.5%) (Liu et al. 2011; Liu et al. 2005; Qiu et al. 2013; Zhong et al. 2007), Hong Kong (prevalence of 19.2–25.9%) (Ko et al. 2006), Japan (prevalence of 1.9–13.6%) (Fukahori et al. 2009; Nishimura et al. 2013; Takemura et al. 2005), Thailand (prevalence of 6.8–7.1%), and the Philippines (prevalence of 7.1–20.8%) (Adeloye et al. 2015; Idolor et al. 2011; Li et al. 2016; Lim et al. 2015; Pothirat et al. 2016a; Ran et al. 2019). Cigarette smoking is the major risk factor for COPD; however, air pollution has been recognized as well (Ceylan et al. 2006; Hu et al. 2015; Pothirat et al. 2016b; Yang and Omaye 2009). In Northern Thailand, air pollutants have been implicated as causes of serious health problems, especially during the high-pollution season, when open burning occurs from December to April every year (Pothirat et al. 2016b; Sillapapiromsuk et al. 2013; Wiwatanadate and Liwsrisakun 2011). The most significant sources of air pollution in this area are biomass or agricultural debris burning for land clearing, wildfires, and vehicle emissions (Pengchai et al. 2009; Tsai et al. 2013; Wiriya et al. 2013; Wiwatanadate and Liwsrisakun 2011). The resulting haze has been recognized as a major health risk in Northern Thailand as well as in neighboring countries (Chantara et al. 2012; Wiriya et al. 2013; Wiwatanadate and Liwsrisakun 2011).
According to the Pollution Health Department of Thailand (PCD), 89% of the pollutants found in Chiang Mai originate from forest fires, 5.4% from solid waste burning, and 2.3% from burning of agricultural waste (Kim Oanh and Leelasakultum 2011). Diesel combustion and industry sources contributed less than 4% (Kim Oanh and Leelasakultum 2011; Sirimongkonlertkul and Phonekeo 2012; Tsai et al. 2013). The dust and ash materials from the burning or airborne particulate matter (PM) with a diameter less than 10 μm (PM10) have become significant threats to local and nearby populations (Sirimongkonlertkul and Phonekeo 2012). The PM10 measured in Chiang Mai ambient air contained several inorganic and organic species, including nitrogen oxides (NOx), sulfur oxides (SOx), PM10-bound ions, and polycyclic aromatic hydrocarbons (PAHs) (Chantara et al. 2012; Gadde et al. 2009; Simoneit 2002; Wiriya et al. 2013). These components, especially PAHs and metals, are carcinogens that can induce DNA damage and respiratory distress (Danielsen et al. 2011; Morales et al. 2016; Pengchai et al. 2009).
The PM10 concentration in Chiang Mai, Thailand, has followed a similar pattern every year since 2010. The annual monthly PM10 concentration is at a high level at the beginning of December and reaches a peak in March, subsequently decreasing from May until November (Chantara et al. 2012; Punsompong and Chantara 2018). Chiang Dao, Mae Rim, and Mae Chaem districts are the dominant areas where rice and maize are produced and consequently experience greater agricultural waste burning (Sillapapiromsuk et al. 2013; Wiriya et al. 2016). Mae Chaem district was reported to be the area with the highest rate of agricultural residual burning during the harvest season, while the biomass burning from Chiang Dao district apparently emits more agricultural chemicals into the air (Sillapapiromsuk et al. 2013).
This study examined whether DNA damage and buccal cell death in COPD patients and healthy subjects were higher during the burning season due to excessive pollutant exposure. Increases in DNA damage, cytokinesi inhibition, and different types of cell death may be correlated with the severity of COPD. Therefore, the buccal micronucleus cytome (BMCyt) assay was employed to investigate the effects of PM10 exposure during the dry season or highly polluted period (daily average PM10 > 50 μg/m3) in comparison with the rainy season or low pollution period (daily average PM10 < 50 μg/m3) as per WHO air quality guidelines for particulate matter (WHO 2006). In addition, the relationship between abnormal buccal cell types and the severity of COPD status was investigated.
Materials and methods
Subjects and sampling site
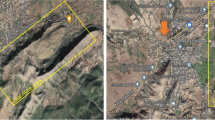
This study included 58 patients with mild to very severe COPD stages and 29 healthy subjects living in Chiang Dao (19.3668°N, 98.9648°E), Chiang Mai Province, Thailand (Fig. 1).
COPD was diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease guidelines (GOLD) (Pothirat et al. 2007). The inclusion criteria were as follows: (1) subjects aged over 40 years who had been living in Chiang Dao for more than 1 year, and (2) the post bronchodilator FEV1/FVC ratio was less than 0.7 for the COPD group and more than 0.7 for the healthy group. The COPD patients were grouped according to COPD stage as mild, moderate, severe, or very severe as per the GOLD standard (Rabe et al. 2007; Vestbo et al. 2013). The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand (study codes: FOR-2559-03852, FOR-2558-03434, and MED-2558-0303). The subjects were informed of the protocol of this study and signed a consent form according to the guidelines of the Faculty of Medicine ethical committee. All individuals were interviewed face-to-face using a demographic data questionnaire and a COPD Assessment Test (CAT) to evaluate health-related quality of life as well as a detailed personal questionnaire containing individual characteristics; medical records; potential confounders such as smoking status, alcohol-drinking habits, diet, medication, and X-rays; and occupation related to pesticides. A high CAT score indicates that COPD is worsening and causing airflow limitation in stable COPD patients (Ghobadi et al. 2012). The spirometry test for assessment of pulmonary function was performed in both COPD and healthy subjects approximately 1 h before buccal cell collection. All subjects were evaluated for FVC, FEV1, ratio of FEV1/FVC, and forced expiratory flow at 25–75% (FEF25–75%) using a spirometer (Spiromaster PC-10, CHEST M.I., Inc. Tokyo, Japan) following the standard guidelines recently published by the American Thoracic Society (ATS)/European Respiratory Society (ERS) (Stanojevic 2018). Predicted values were calculated using the Knudson reference equation (Knudson et al. 1983).
COPD GOLD stage
This study classified the severity of COPD according to the GOLD 2017 Guidelines (GOLD 2017) as follows: stage 1 mild FEV1 greater than or equal to 80% predicted; stage 2 moderate FEV1 between 50 and 80% predicted; stage 3 severe FEV1 between 30 and 50% predicted; stage 4 very severe FEV1 less than or equal to 30% predicted or FEV1 less than 50% predicted plus chronic respiratory failure.
Buccal sample collection
The buccal cells from each subject were collected in March 2016 (PM10 > 50 μg/m3) and August 2016 (PM10 < 50 μg/m3) as shown in Fig. 2. The collection times were selected based on PM10 peak levels in March and the PM10 reduction in August (Chantara et al. 2012). The stage and severity of COPD at the sampling times were verified according to the GOLD 2017 Guidelines to evaluate the relationships between DNA damage and COPD status. The buccal samples or oral mucosa were collected and processed in accordance with Thomas et al. (2009). Briefly, buccal cells were collected in a circular motion from inside of the left and right cheek wall using a sterile wood spatula. Then, the collected cells were placed into fixative containers containing Saccomanno’s fixative solution. Afterward, the cell suspension was centrifuged at 4000 rpm for 15 min, and the supernatant was removed. The cell suspension was washed twice using 500 μl of the fixative solution and centrifuged at 13,000 rpm for 10 min. Next, the supernatant was removed and replaced with 500 μl of the fixative solution. The final suspension was vortexed, and then 100 μl of the cell suspension was placed directly onto a glass microscope slide. The slides were air-dried overnight and then placed in methanol/glacial acetic acid (3:1) for 10 min before being stained with Feulgen reaction solution before counterstaining with Fast-green reagent.
Buccal micronucleus cytome assay
The slides were coded before double-blinded scoring by two investigators and were examined at × 1000 magnification using a good-quality bright field. Two thousand buccal cells were observed, and the frequency of various cell types found in these 2000 buccal cells were recorded, including the frequency of buccal cells with micronuclei (BCMN), micronuclei (MNi), binucleated cells (BN), nuclear bud cells (NBUD), pyknotic cells (PY), condensed chromatin cells (CC), karyorrhectic cells (KR), and karyolytic cells (KL). Criteria for identifying and scoring cell types and nuclear abnormalities in the BMCyt assay were performed following Thomas et al. (Thomas et al. 2009) and Bolognesi and Fenech (Bolognesi and Fenech 2013).
PM10 data
The PM10 data used in this study were retrieved from the Thailand air quality and situation reports of the Pollution Health Department (PCD) Ministry of National Resources and Environment database (http://air4thai.pcd.go.th/webV2/) from January to December 2016. Pollution levels were measured at the Chiang Mai City Hall station, Thailand. The PM10 concentrations were performed using beta-ray attenuation operated by the PCD. The data were reported as daily average concentrations of PM10.
Statistical analysis
Results with numerical values were expressed as mean ± SD. Categorical data were represented as absolute frequencies and percentages. The statistical analysis was performed on GraphPad Prism software version 8.01 and IBM® SPSS® Statistics version 22. Differences were considered statistically significant when p ≤ 0.05. As the data were not normally distributed, the non-parametric tests were employed. The Wilcoxon matched-pairs signed rank test was used to compare the mean variables of the same subjects between low- and high-pollution seasons. Chi-square tests or Fisher’s exact test were used to compare the proportional categorical data. Statistical differences between the COPD and healthy groups were tested using a Mann-Whitney U test. The correlations between different variables were determined using Spearman’s rank correlation test. A Poisson log-linear model was applied to the data to estimate the association between BCMN, MNi, and factors involving micronucleus induction, including PM10 levels, disease status, host factors, smoking habit, drinking habit, diet, X-ray history, and occupational pesticide exposure. Adjustments with dependent covariates were made for age, gender, FEV1/FVC ratio, FEV1, COPD severity, and CAT score.
Results
PM10 data
The 24-h mean-PM10 concentration from January to December 2016 (Fig. 2) indicated that the PM10 levels exceeded the WHO Air quality guidelines (50 μg/m3) from January to April, with a peak level of 187.33 μg/m3 in March.
Subjects
Fifty-eight COPD patients and 26 healthy subjects living in an agricultural area in Chiang Dao, Chiang Mai, Thailand, were enrolled in this study. General characteristics of the study population (COPD patients and healthy subjects) including gender, age, smoking and alcohol-drinking status, occupational exposure to pesticides, and X-ray exposure are shown in Table 1. The subjects from the healthy group were on average 10 years younger than the COPD group. The majority of the participants had not been exposed to X-rays before sample collection and had not been working with pesticides. More than 50% of the subjects were former smokers, and most of the subjects did not consume alcohol.
The healthy group had normal lung function according to the GOLD standard. The post bronchodilator FEV1/FVC ratio during the polluted season was significantly lower than that measured during the low pollution months in COPD patients. A similar pattern was seen in the percentage predicted FEV1 (Table 2).
Buccal micronucleus cytome assay
The photomicrographs of scored abnormal cell types for the BMCyt assay are shown in Fig. 3. The mean frequencies of dead cells (PY, CC, KR, and KL) and different types of DNA-damaged and cytokinetic-defected cells (BCMN, MNi, BN, NBUD) are shown in Fig. 4. The numbers of BCMN and MNi cells were not significantly different between the COPD and healthy groups during both high and low PM10 levels. The MNi detected in the COPD and healthy groups during low pollution were used as a baseline for MNi in this study.
Images of the different cell types stained using Feulgen and Light Green viewed by the light microscope. a Normal differentiated cell. b Buccal cell with micronuclei. c Nuclear bud cell. d Binucleated cell. e Karyolitic cell. f Condensed chromatin cell. g Karyorrhectic cell. h Pyknotic cell. All images were taken at x 1000 magnification and the scale bar = 20 μm
DNA damage and cell death markers. The frequencies of a buccal cells with micronuclei, b micronuclei, c nuclear bud cells, d binucleated cells, e condensed chromatin cells, f Karyorrhectic cells, g pyknotic cells, h karyolitic cells. Groups showing the same letter are significantly different from each other
A significant increase in BCMN and BN from their baseline values was detected (Fig. 4). The mean frequency of BCMN observed in the COPD group during the high-pollution period was significantly higher than that found during the low pollution period (Fig. 4a), with the mean frequencies of 1.09 ± 1.95 vs 0.29 ± 0.64 (p = 0.027), respectively. However, there was no difference in BCMN frequencies between the two periods in the healthy population (0.77 ± 0.95 vs 0.65 ± 0.61, respectively; p = 0.449). The mean frequency of MNi (Fig. 4b) during the high-pollution period was slightly higher but not significant compared to during the low pollution period (1.39 ± 2.12 vs 0.88 ± 1.05, p value = 0.681, 1.59 ± 2.47 vs 1.05 ± 2.95, p = 0.162) in the healthy and COPD groups, respectively.
The frequency of the BN cells (Fig. 4d) was dramatically increased during high pollution compared to during low pollution in both COPD (11.43 ± 18.68 vs 1.60 ± 1.3, p = 0.004) and healthy groups (7.77 ± 12.76 vs 1.00 ± 1.17, p = 0.012). In contrast, the frequency of CC cells was lower during the high-pollution period in the COPD group (132.40 ± 104.30 vs 230.20 ± 87.35, p = 0.006) and healthy group (168.10 ± 119.90 vs 267.78 ± 97.66, p = 0.012).
Only the BCMN, MNi, and CC cells were significantly different among COPD severity groups (Fig. 5). The frequencies of BCMN and MNi (Fig. 5a and b) in patients with mild symptoms (stage I) were higher than in the patients with moderate, severe, and very severe stages. In contrast, the frequencies of CC cells were greater in patients with stages II to IV (Fig. 5c).
Spearman’s rank correlation was performed to evaluate the relationship between DNA-damaged cells, cell death, and COPD severity. Pulmonary function indicators (FEV1 and FVC) were not correlated with any of the DNA damage or cell death markers. The severity defined by GOLD classes and CAT score was negatively correlated with the BCMN and MNi frequencies; however, only weak correlations were observed, with the Spearman rank correlations below 0.3 (Fig. 6a–c).
Association between DNA-damaged cells and confounding factors
The confounding factors that may be involved in micronucleus induction were taken into account to remove all bias from the analysis. After adjustment, COPD patients had a higher risk of PM10-induced BCMN and MNi formation, with the respective frequency ratios of 295.23 and 64.51 when the PM10 levels were higher than 50 μg/m3 (Table 3). This implies that COPD patients were more susceptible to PM10 toxicity when compared to the corresponding healthy group. In addition, high levels of pollutants enhanced the genotoxic effect of PM10, as the frequency of DNA damage during PM10 levels above 50 μg/m3 was higher than that in the low pollution period. Moreover, the DNA-damaged cells (BCMN and MNi) detected in subjects exposed to genotoxic chemicals such as pesticides were not different from those who were not exposed; the means were 0.89 ± 1.27 vs 1.12 ± 20.6 and 1.11 ± 1.54 vs 1.67 ± 2.61 for BCMN and MNi in COPD patients, respectively.
The role of confounding factors was also important for micronucleus induction. The associations between BCMN and life-style parameters in the COPD group are listed in Table 4. The factors most associated with BCMN cells were smoking and food consumption. The results indicated that former smokers had BCMN frequencies 12.75 times higher than those who never smoked. Subjects who had a high of intake fruits and vegetables in their daily diet had a lower BCMN frequency (FR = 0.036) than those who did not consume such a diet; the high fruit and vegetable diet was associated with over 96.4% DNA damage reduction.
Discussion
The PM10 concentrations presented in this study were higher than Thailand Air Quality Guideline acceptable safety value (daily average PM10 < 120 μg/m3) and WHO air quality guidelines (daily average PM10 < 50 μg/m3) (Pothirat et al. 2016b). This pattern was consistent with the previous results (Punsompong and Chantara 2018) stating that the monthly PM10 concentrations from 2010 to 2015 displayed the highest levels of PM10 during the dry season (January to April) and lower concentrations during the rainy season (May to October). Lung function tests indicated significant impact of air pollution in dry season on COPD patients. During polluted period (PM10 > 50 μg/m3), reductions of FEV1 and FVC parameters were observed reflecting more severity of lung disease induced by the air pollution. This finding confirms that PM10 levels involved in lung function status and disease progression. This result was consistent with the previous studies showing a strong correlation between PM10 exposure and exacerbations or COPD disease progression (MacNee and Donaldson 2003; Pope 2000; Pothirat et al. 2016b).
The BCMN and MNi frequencies are the markers of unrepaired or misrepaired DNA breaks, chromosome loss or fragmentation, hypomethylation of satellite centromeric/paracentromeric sequences, kinetochore defects, dysfunctional spindle, and mutations in anaphase checkpoint genes due to the exposure to genotoxic compounds (Holland et al. 2008; Luzhna et al. 2013). Micronucleus in exfoliated cells emerges during mitosis of the basal layers of the epithelium. The turnover rate for micronucleus induction in exfoliated buccal cells after exposure to an acute genotoxic event is approximately a minimum of 5–7 days (Holland et al. 2008). However, interindividual variation of the MN expression time-course has been reported to be up to 21 days. As a consequence, hypothetically, it is possible to see the genotoxic effects between 7 and 21 days after genotoxic exposure when the cells are brought to the stratum corneum layer (Majer et al. 2001; Thomas et al. 2009). As illustrated in Fig. 2, the PM10 concentrations were high above the safety limit throughout the dry season. The higher frequencies of BCMN and MNi detected in March could be a result of PM10 exposure between 1 and 3 weeks prior buccal cell collection depending on interindividual variation. Previous study also revealed that MN frequency correlated with concentration of air pollutions in the week before the buccal cell collection but not on the same day (Ceretti et al. 2014).
The BCMN and BN frequencies were remarkably higher in dry season. Increases in these two markers may be linked to an excessive exposure to airborne PM10 dust from the open burning during the dry season. Several studies have indicated that air pollution, especially from biomass burning, causes severe genotoxic effects in COPD patients at a level equal to cigarette smoking (Caramori et al. 2011; Ceylan et al. 2006; Danielsen et al. 2011; Hu et al. 2015; Yang and Omaye 2009). The PM10 collected from Chiang Mai area contains a mixture of inorganic and organic substances including metals, polycyclic aromatic hydrocarbons, carboxylic acids, anhydrosugars, and sugar alcohol (Tsai et al. 2013). High levels of levoglucosan, glycerol, and erythritol during the dry season in Chiang Mai indicate that biomass and agricultural burning contributed to the episodic air pollution (Tsai et al. 2013). Additionally, the PM10-bound carcinogenic polycyclic aromatic hydrocarbons (cPAHs) in Chiang Mai were relatively higher in the dry season than in the rainy season (Wiriya et al. 2016; Wiriya et al. 2013). These detected cPAHs included benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, and benzo[a]pyrene (Wiriya et al. 2013). In our study area, there were three main types of the biomass (rice straw, maize residue, and leaf litter) that emitted high concentrations of PM10-bound PAHs after burning (Wiriya et al. 2016). These PAH species are involved in DNA and oxidative damage (Xue and Warshawsky 2005); consequently, they may initiate and promote cancer (Armstrong et al. 2004).
As mentioned earlier, an increased frequency of micronuclei reflect exposure to agents with clastogenic (chromosome breaking; DNA as target) or aneugenic (aneuploidogenic; effect on chromosome number; mostly non-DNA target) mode of actions. Quinones, diol-epoxides, o-quinones, and radical cations are PAH metabolites that can covalently bind to nucleic acids in DNA, form an DNA adducts, and subsequently causing DNA deletions, fusions, translocations, aneuploidy, cell cycle alteration, DNA misrepair, DNA instability, and apoptosis (Bai et al. 2017). PAH metabolites can cause P53 suppression and P21 upregulation leading to apoptosis inhibition and S phase arrest. These expression changes and inappropriate S phase could promote DNA fragment accumulation during replication and consecutively micronucleus formation in mitosis (Bai et al. 2017). Previous research demonstrated that PM10-bound PAHs collected from different sites of São Paulo State, Brazil correlated with an elevation of micronucleus induction in human lung epithelial A549 cells (Palacio et al. 2016). Furthermore, Ceretti et al. (2014) stated that an increase in 10 μg/m3 unit of PM10 was significantly related to the elevation of MNi, CC cells, and KR cells. In our study, the COPD patients had susceptibility to PM10-induced DNA damage 96.51 times higher than in healthy subjects in rainy season. However, the risk went up to 295.23-fold in dry season. This high risk of BCMN induction during an episode of air pollution in Chiang Dao could be a result of oxidative stress-induced DNA damage and cytokinetic defects from genotoxic compounds that bounded with PM10 such as PAHs, as previously reported by Wiriya et al., indicating that the equivalent toxic concentration of carcinogenic PAHs in the dry season was higher than in the rainy season (Wiriya et al. 2013).
There was no significant difference in DNA-damaged cells in the healthy subjects between the two seasons. The healthy subjects were less susceptible to air pollution due to better xenobiotic clearance and immune system responses to the PM distribution and more effective elimination, as demonstrated by Ling et al. (2011). Those researchers reported that a relatively small amount of PM accumulated in the alveolar walls and blood vessel walls of the control subjects in comparison with the COPD group. A short retention time of PM10-bound carcinogenic agents in healthy subjects can result in less DNA damage, as shown in our present study.
High BN frequency was observed during polluted period in this present study could indicate the failure of cell division. Binucleated cells are an indicator of defective cytokinesis, cell cycle arrest due to aneuploidy (Bonassi et al. 2011). Aneuploidy reflects gain or loss of whole chromosomes as well as unbalanced rearrangements of chromosomes, including deletions, amplifications, or translocations of large regions of the genome (Orr et al. 2015). Moreover, aneuploid cells or cells with chromosomal instability have an elevated rate of DNA mutations and chromosome missegregation, which can lead to micronucleus formation (Varetti et al. 2014). In addition, the elevation of the binucleate/mononucleate cell ratio can be used to identify a failure of cytokinesis caused by higher than normal rates of aneuploidy, which is related with cancer risk (da Silva et al. 2013; Thomas et al. 2009). Therefore, the high frequency of BN observed in our study during the open burning season, and the subsequent return to the baseline rate in the rainy season, could be a result of failure of cytokinesis during cell division induced by exposure to biomass burning. Our finding is in agreement with Mondal et al. (2010), who stated that chronic exposure to biomass fuel not only causes chromosomal and DNA damage resulting in MNi induction but also induced cytokinesis defects leading to a higher frequency of binucleated cells. The frequency of BN was also slightly associated with air pollution, as reported by Ceretti et al. (2014). They reported that an increase in the PM10 concentration every 10 μg/m3 unit resulting in the elevation of BN for 0.01 unit (Ceretti et al. 2014).
The relatively high variability in the frequencies of DNA damage and other abnormal cell types is observed in this study (Figs.4 and 5). The large variability in MNi and other cell types within the individual data sets has been reported in several previous studies, even after adjusting with confounding factors such as life-style, health status, occupational and diet, or after removing the outliners. For example, the spontaneous micronucleus frequency has been reported ranging from 0.30 to 1.70%. One possible explanation would be the heterogeneity of the study subjects (Bolognesi et al. 2015b; Bonassi et al. 2011). Furthermore, the interindividual variance in chromosomal and cytogenetic responses has been reported to be an actual phenomenon and the genetic responses induced by different types of mutagens (clastogen or aneugen) showed the same significant interindividual variations (Odagiri et al. 1997). In addition, the participants in our study might expose to other unknown genotoxic agents or confounding factors that might alter the numbers of DNA damage and abnormal cells. On the other hand, the fluctuation of the abnormal cell frequencies might be caused by the different doses of exposure and individual responses to genotoxic compounds.
It is well established that DNA damage can stimulate apoptotic processes (Upadhyay et al. 2003). Several studies have reported that particulate matter can induce apoptosis (Bayram et al. 2013; Gualtieri et al. 2010; MacNee and Donaldson 2003; Upadhyay et al. 2003). In general, the airway surface epithelium responds to any injury by losing its surface epithelium integrity or by partial shedding of the epithelium. The epithelium then has to be repaired and regenerated to restore its functions. This process occurs through several mechanisms including cellular extrusion, in which dying cells are removed from epithelial tissues to prevent compromising their barrier function, and the epithelium subsequently undergoes renovation via apoptosis (Andrade and Rosenblatt 2011; Puchelle et al. 2006). An increase in the CC cells during the rainy season may indicate the repair and elimination processes in response to DNA damage in buccal cells that occurred earlier in the dry season (Fig. 4e) (Thomas et al. 2009). In addition, the lower frequency of CC cells found in the dry season may suggest the PM10-induced reduction of the regenerative capacity of epithelial tissue (Bolognesi et al. 2015a). Alternatively, the composition of PM10 in different seasons and different years may account for the different effects on buccal epithelial cells (Manzano-Leon et al. 2016).
Interestingly, our results showed the higher BCMN frequencies in stage I COPD patients. This finding may correspond to PM10-induced DNA damage, disturbed S phase, and finally inhibited apoptosis resulting in promoting micronucleus formation in stage I patients (Bai et al. 2017). Alternatively, less micronucleus frequencies observed in stage II–IV COPD patients possibly due to the greater rate of apoptotic process associated with COPD pathogenesis (Plataki et al. 2006). Apoptosis or a “programmed cell death” is an important process for the elimination of unwanted, damaged, or infected cells (Demedts et al. 2006). Numerous studies reported that increases in apoptosis of epithelial cells and decreases in cell proliferation in COPD cases led to oxidative stress induction and COPD severity progression (Demedts et al. 2006; Gogebakan et al. 2014; Plataki et al. 2006). Additionally, Ling et al. (2011) demonstrated that the PM retained in lung tissues was significantly lower in severe and very severe COPD patients compared to stages I and II (Ling et al. 2011). They suggested that lung tissue with a high PM burden in severe patients may be selectively eliminated with the progression of the disease. Moreover, the decline in PM accumulation in cells combined with short-term toxic exposure may result in less genotoxicity in patients with severe symptoms.
The increase in the apoptosis morphological feature (CC cells) in stage II–IV patients indicates that the host defense mechanism is active in regulating cell death and the disease progression as mentioned before (Plataki et al. 2006; Upadhyay et al. 2003). In addition, high CC frequencies in severe COPD patients may be a consequence of an increase in cytokine-related apoptosis in response to PM10 toxicity or pathogenic progression of COPD symptoms (MacNee and Donaldson 2003). Rohr et al. (2013) determined the relationship between genetic damage and cell death in open pit coal mine workers using the BMCyt assay. The results showed that subjects with high MNi frequency had a lower frequency of CC cells (Rohr et al. 2013). In our study, the cells with genetic damage occurring during PM10 exposure may be eliminated via apoptosis, resulting in a high frequency of CC cells and less DNA-damaged cells in the severe COPD groups.
Our results illustrate the impact of smoking and dietary habits on micronucleus induction. It has been well established that smoking and fruit and vegetable consumption affect micronucleus formation (Bonassi et al. 2011). Heavy cigarette smoking (≥ 40 cigarettes per day) was related with an increase in MNi frequency (Bonassi et al. 2011). Our study demonstrated that COPD patients who once smoked had a higher BCMN frequency than those who were still smoking or had never smoked. Furthermore, current smoking behavior had no impact on the BCMN frequency as shown in Table 4. All current smokers have been smoking for less than 2 ;, to this extent, the effect of this behavior on DNA damage is probably unnoticeable. However, the number of current smokers enrolled in our study (n = 9) may be too small to see substantial change in BCMN frequency. Subjects who consumed fruits or vegetables daily had a lower BCMN frequency than those who reported no consumption at all. This result is consistent with several studies demonstrating that regular consumption of fruits and vegetables, due to their antioxidant properties, significantly reduce micronucleus levels (Bonassi et al. 2011).
Some limitation of this study should be noted. First, PM10 was used as an indicator of air pollution rather than PM2.5 due to the limited capacity of the air quality monitor at the Chiang Mai City Hall Station. Nonetheless, the ratios of PM2.5/PM10 (ratio of 0.9) reported by Oanh et al. (2011) showed that the smoke contained both fine particles (PM2.5) and course particle (PM10–2.5) correspondingly. The contribution of PM10 was even higher when the fire suppression activities, such as beating at the edge of the fire with green tree branches, were done to stop the fire from spreading to surrounding paddies (Oanh et al. 2011). Second, there was a lack of individual pollutant exposure data, as real-time particulate matter measurements for each subject were not performed. The pollution levels measured at fixed site monitor might not sufficiently reflect the individual exposure levels. However, this limitation was compromised by the nature of the burning in our study area. The forest fire, open burning of agriculture residue field occurred everywhere across our research area, as a result, the pollutant concentrations were transported, dispersed, and deposited evenly. This burning characteristic could possibly reduce the potential for inconsistencies in the results as the exposure measurement error may be less of a problem for fine and course particles than for gases due to their more homogeneous geographical distribution (Atkinson et al. 2001; Monn 2001). Moreover, this study was focused on the overall effects of inter-season differences; thus, the actual individual doses of exposure may be insignificant.
Conclusions
The PM10 concentrations presented in our study exceeded the WHO guidelines starting from January (dry season), reaching a peak in March and subsequently decreasing until May. Frequencies of buccal cells with micronuclei and binucleated cells were higher during months of high pollution (March), particularly in the COPD patients, indicating DNA damage and instability. The micronucleus frequency in COPD patients with a mild condition was higher than in those with severe conditions. However, cells with condensed chromatin detected in the moderate to very severe COPD groups were significantly higher than in the mild group. These effects may be part of the pathogenesis of COPD as apoptotic rates in severe COPD groups are faster than in the mild group, resulting in higher elimination rates of DNA damage and cytokinetic-defected cells. Exposure to PM10 during the high pollutant period increased the frequency risk of DNA damage 295-fold in COPD patients in comparison with the healthy group. It can be concluded that the COPD patients were more sensitive to air pollution, thus experiencing more DNA damage and cytokinetic defect than that observed in healthy subjects.
References
Adeloye D et al (2015) Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 5:186–202. https://doi.org/10.7189/jogh.05.020415 UNSP 020415
Andrade D, Rosenblatt J (2011) Apoptotic regulation of epithelial cellular extrusion. Apoptosis 16:491–501
Armstrong B, Hutchinson E, Unwin J, Fletcher T (2004) Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect 112:970–978. https://doi.org/10.1289/ehp.6895
Atkinson RW et al (2001) Acute effects of particulate air pollution on respiratory admissions - results from APHEA 2 project. Am J Respir Crit Care Med 164:1860–1866. https://doi.org/10.1164/ajrccm.164.10.2010138
Bai HZ, Wu M, Zhang HJ, Tang GP (2017) Chronic polycyclic aromatic hydrocarbon exposure causes DNA damage and genomic instability in lung epithelial cells. Oncotarget 8:79034–79045. https://doi.org/10.18632/oncotarget.20891
Bayram H, Fakili F, Gegebakan B, Bayraktar R, Oztuzcu S, Dikensoy O, Chung KF (2013) Effect of serum on diesel exhaust particles (DEP)-induced apoptosis of airway epithelial cells in vitro. Toxicol Lett 218:215–223. https://doi.org/10.1016/j.toxlet.2013.02.006
Bolognesi C, Fenech M (2013) Micronucleus assay in human cells: lymphocytes and buccal cells. Methods Mol Biol 1044:191–207. https://doi.org/10.1007/978-1-62703-529-3_10
Bolognesi C, Bonassi S, Knasmueller S, Fenech M, Bruzzone M, Lando C, Ceppi M (2015a) Clinical application of micronucleus test in exfoliated buccal cells: a systematic review and metanalysis. Mutat Res Rev Mutat Res 766:20–31. https://doi.org/10.1016/j.mrrev.2015.07.002
Bolognesi C, Roggieri P, Ropolo M, Thomas P, Hor M, Fenech M, Nersesyan A, Knasmueller S (2015b) Buccal micronucleus cytome assay: results of an intra- and inter-laboratory scoring comparison. Mutagenesis 30:545–555. https://doi.org/10.1093/mutage/gev017
Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, Holland N, Kirsh-Volders M, Knasmueller S, Zeiger E, Carnesoltas D, Cavallo D, da Silva J, de Andrade VM, Demircigil GC, Odio AD, Donmez-Altuntas H, Gattas G, Giri A, Giri S, Gómez-Meda B, Gómez-Arroyo S, Hadjidekova V, Haveric A, Kamboj M, Kurteshi K, Martino-Roth MG, Montoya RM, Nersesyan A, Pastor-Benito S, Salvadori DMF, Shaposhnikova A, Stopper H, Thomas P, Torres-Bugarín O, Yadav AS, González GZ, Fenech M (2011) The human micronucleus project on exfoliated buccal cells (HUMNXL): the role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res 728:88–97. https://doi.org/10.1016/j.mrrev.2011.06.005
Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, Barnes PJ, Papi A (2011) Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax 66:521–527. https://doi.org/10.1136/thx.2010.156448
Ceretti E et al (2014) DNA damage in buccal mucosa cells of pre-school children exposed to high levels of urban air pollutants. PLoS One 9:e96524. https://doi.org/10.1371/journal.pone.0096524
Ceylan E, Kocyigit A, Gencer M, Aksoy N, Selek S (2006) Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir Med 100:1270–1276. https://doi.org/10.1016/j.rmed.2005.10.011
Chantara S, Sillapapiromsuk S, Wiriya W (2012) Atmospheric pollutants in Chiang Mai (Thailand) over a five-year period (2005-2009), their possible sources and relation to air mass movement. Atmos Environ 60:88–98
da Silva ALG, da Rosa HT, Karnopp TE, Charlier CF, Ellwanger JH, Moura DJ, Possuelo LG, Valim ARM, Guecheva TN, Henriques JAP (2013) Evaluation of DNA damage in COPD patients and its correlation with polymorphisms in repair genes. BMC Med Genet 14:93. https://doi.org/10.1186/1471-2350-14-93
Danielsen PH, Møller P, Jensen KA, Sharma AK, Wallin H̊, Bossi R, Autrup H, Mølhave L, Ravanat JL, Briedé JJ, de Kok TM, Loft S (2011) Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem Res Toxicol 24:168–184. https://doi.org/10.1021/tx100407m
Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG (2006) Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 7:53. https://doi.org/10.1186/1465-9921-7-53
Fukahori S, Matsuse H, Takamura N, Hirose H, Tsuchida T, Kawano T, Fukushima C, Mizuta Y, Kohno S (2009) Prevalence of chronic obstructive pulmonary diseases in general clinics in terms of FEV1/FVC. Int J Clin Pract 63:269–274. https://doi.org/10.1111/j.1742-1241.2008.01873.x
Gadde B, Bonnet S, Menke C, Garivait S (2009) Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ Pollut 157:1554–1558. https://doi.org/10.1016/j.envpol.2009.01.004
Ghobadi H, Ahari SS, Kameli A, Lari SM (2012) The relationship between COPD assessment test (CAT) scores and severity of airflow obstruction in Stable COPD Patients. Tanaffos 11:22–26
Gogebakan B, Bayraktar R, Ulasli M, Oztuzcu S, Tasdemir D, Bayram H (2014) The role of bronchial epithelial cell apoptosis in the pathogenesis of COPD. Mol Biol Rep 41:5321–5327. https://doi.org/10.1007/s11033-014-3403-3
GOLD (2017) GOLD (Global Initiative for Chronic Obstructive Lung Disease) 2017. Pneumologie 71:9–12
Gualtieri M, Ovrevik J, Holme JA, Perrone MG, Bolzacchini E, Schwarze PE, Camatini M (2010) Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol in Vitro 24:29–39
Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, Fenech M (2008) The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res 659:93–108. https://doi.org/10.1016/j.mrrev.2008.03.007
Hu G, Zhong N, Ran P (2015) Air pollution and COPD in China. J Thorac Dis 7:59–66. https://doi.org/10.3978/j.issn.2072-1439.2014.12.47
Idolor LF et al (2011) Burden of obstructive lung disease in a rural setting in the Philippines. Respirology 16:1111–1118. https://doi.org/10.1111/j.1440-1843.2011.02027.x
Kim Oanh NT, Leelasakultum K (2011) Analysis of meteorology and emission in haze episode prevalence over mountain-bounded region for early warning. Sci Total Environ 409:2261–2271. https://doi.org/10.1016/j.scitotenv.2011.02.022
Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B (1983) Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127:725–734. https://doi.org/10.1164/arrd.1983.127.6.725
Ko FWS, Lai CKW, Woo J, Ho SC, Ho CWM, Goggins W, Hui DSC (2006) 12-year change in prevalence of respiratory symptoms in elderly Chinese living in Hong Kong. Respir Med 100:1598–1607. https://doi.org/10.1016/j.rmed.2005.12.007
Li J, Sun S, Tang R, Qiu H, Huang Q, Mason TG, Tian L (2016) Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 11:3079–3091. https://doi.org/10.2147/COPD.S122282
Lim S, Lam DCL, Muttalif AR, Yunus F, Wongtim S, Lan LTT, Shetty V, Chu R, Zheng J, Perng DW, de Guia T (2015) Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: the EPIC Asia population-based survey. Asia Pac Fam Med 14:4. https://doi.org/10.1186/s12930-015-0020-9
Ling SH, McDonough JE, Gosselink JV, Elliott WM, Hayashi S, Hogg JC, van Eeden SE (2011) Patterns of retention of particulate matter in lung tissues of patients with COPD potential role in disease progression. Chest 140:1540–1549. https://doi.org/10.1378/chest.10-2281
Liu S, Wen DL, Li LY, Li ZH, Chronic pulmonary disease group of greenhouse farmers in Liaoning P (2011) an epidemiological study of chronic obstructive pulmonary disease in greenhouse farmers in Liaoning Province from 2006 to 2009. Zhonghua Jie He He Hu Xi Za Zhi 34:753–756
Liu SM, Wang XP, Wang DL, Zhou YM, Lü JC, Zheng JP, Zhong NS, Ran PX (2005) Epidemiologic analysis of COPD in Guangdong province. Zhonghua Yi Xue Za Zhi 85:747–752
Luzhna L, Kathiria P, Kovalchuk O (2013) Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front Genet 4:131. https://doi.org/10.3389/fgene.2013.00131
MacNee W, Donaldson K (2003) Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J Suppl 40:47s–51s
Majer BJ, Laky B, Knasmuller S, Kassie F (2001) Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res 489:147–172
Manzano-Leon N et al (2016) TNF alpha and IL-6 responses to particulate matter in vitro: variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environ Health Perspect 124:406–412
Ministry of Public Health Thailand (2015) The incidence of chronic obstructive pulmonary disease in northern Thailand. http://cmi.hdc.moph.go.th/hdc/reports/report.php?source=formated/format_1.php&cat_id=6a1fdf282fd28180eed7d1cfe0155e11&id=62cdb786f231afbaaaaaac1d5ff844b0. Accessed August 6th 2015
Mondal NK, Mukherjee B, Das D, Ray MR (2010) Micronucleus formation, DNA damage and repair in premenopausal women chronically exposed to high level of indoor air pollution from biomass fuel use in rural India. Mutat Res 697:47–54. https://doi.org/10.1016/j.mrgentox.2010.02.006
Monn C (2001) Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ 35:1–32. https://doi.org/10.1016/S1352-2310(00)00330-7
Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, Roy-Engel AM (2016) Heavy metal exposure influences double strand break DNA repair outcomes. PLoS One 11:e0151367. https://doi.org/10.1371/journal.pone.0151367
Neofytou E, Tzortzaki EG, Chatziantoniou A, Siafakas NM (2012) DNA damage due to oxidative stress in chronic obstructive pulmonary disease (COPD). Int J Mol Sci 13:16853–16864. https://doi.org/10.3390/ijms131216853
Nishimura K, Mitsuma S, Kobayashi A, Yanagida M, Nakayasu K, Hasegawa Y, Jones PW (2013) COPD and disease-specific health status in a working population. Respir Res 14. https://doi.org/10.1186/1465-9921-14-61
Oanh NTK, Ly BT, Tipayarom D, Manandhar BR, Prapat P, Simpson CD, Liu LJS (2011) Characterization of particulate matter emission from open burning of rice straw. Atmos Environ 45:493–502. https://doi.org/10.1016/j.atmosenv.2010.09.023
Odagiri Y, Uchida H, Shibazaki S (1997) Interindividual variation in cytogenetic response to X-ray and colchicine measured with the cytokinesis-block micronucleus assay. Mutat Res 381:1–13
Orr B, Godek KM, Compton D (2015) Aneuploidy. Curr Biol 25:R538–R542. https://doi.org/10.1016/j.cub.2015.05.010
Palacio IC, Barros SBM, Roubicek DA (2016) Water-soluble and organic extracts of airborne particulate matter induce micronuclei in human lung epithelial A549 cells. Mutat Res 812:1–11. https://doi.org/10.1016/j.mrgentox.2016.11.003
Pengchai P, Chantara S, Sopajaree K, Wangkarn S, Tengcharoenkul U, Rayanakorn M (2009) Seasonal variation, risk assessment and source estimation of PM 10 and PM10-bound PAHs in the ambient air of Chiang Mai and Lamphun, Thailand. Environ Monit Assess 154:197–218. https://doi.org/10.1007/s10661-008-0389-0
Plataki M, Tzortzaki E, Rytila P, Demosthenes M, Koutsopoulos A, Siafakas NM (2006) Apoptotic mechanisms in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis 1:161–171
Pope CA 3rd (2000) Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect 108(Suppl 4):713–723
Pothirat C, Phetsuk N, Deesomchok A, Theerakittikul T, Bumroongkit C, Liwsrisakun C, Inchai J (2007) Clinical characteristics, management in real world practice and long-term survival among COPD patients of Northern Thailand COPD club members. J Med Assoc Thail 90:653–662
Pothirat C, Phetsuk N, Liwsrisakun C, Bumroongkit C, Deesomchok A, Theerakittikul T (2016a) Major chronic respiratory diseases in Chiang Mai: prevalence, clinical characteristics, and their correlations. J Med Assoc Thail 99:1005–1013
Pothirat C, Tosukhowong A, Chaiwong W, Liwsrisakun C, Inchai J (2016b) Effects of seasonal smog on asthma and COPD exacerbations requiring emergency visits in Chiang Mai. Thailand Asian Pac J Allergy Immunol 34:284–289. https://doi.org/10.12932/Ap0668
Puchelle E, Zahm JM, Tournier JM, Coraux C (2006) Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3:726–733. https://doi.org/10.1513/pats.200605-126SF
Punsompong P, Chantara S (2018) Identification of potential sources of PM10 pollution from biomass burning in northern Thailand using statistical analysis of trajectories Atmospheric Pollution Research 9(6):1038–1051 https://doi.org/10.1016/j.apr.2018.04.003
Qiu J, Zhang YN, Chen J, Luo T, Yu XH, Wang JC, Tan H, Lu XL, Zhang J (2013) Prevalence of chronic obstructive pulmonary disease in Ningxia Hui autonomous region of China. Zhonghua Jie He He Hu Xi Za Zhi 36:265–268
Quaderi SA, Hurst JR (2018) The unmet global burden of COPD. Glob Health Epidemiol Genom 3:e4. https://doi.org/10.1017/gheg.2018.1
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, Global Initiative for Chronic Obstructive Lung Disease (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease - GOLD executive summary. Am J Respir Crit Care Med 176:532–555. https://doi.org/10.1164/rccm.200703-456SO
Ran J, Sun S, Yang A, Yang L, Han L, Mason TG, Chan KP, Li J, Tian L (2019) Effects of ambient benzene and toluene on emergency COPD hospitalizations: a time series study in Hong Kong. Sci Total Environ 657:28–35. https://doi.org/10.1016/j.scitotenv.2018.12.015
Rohr P, da Silva J, da Silva FR, Sarmento M, Porto C, Debastiani R, dos Santos CEI, Dias JF, Kvitko K (2013) Evaluation of genetic damage in open-cast coal mine workers using the buccal micronucleus cytome assay. Environ Mol Mutagen 54:65–71. https://doi.org/10.1002/em.21744
Sillapapiromsuk S, Chantara S, Tengjaroenkul U, Prasitwattanaseree S, Prapamontol T (2013) Determination of PM10 and its ion composition emitted from biomass burning in the chamber for estimation of open burning emissions. Chemosphere 93:1912–1919. https://doi.org/10.1016/j.chemosphere.2013.06.071
Simoneit BRT (2002) Biomass burning — a review of organic tracers for smoke from incomplete combustion. Appl Geochem 17:129–162. https://doi.org/10.1016/S0883-2927(01)00061-0
Sirimongkonlertkul N, Phonekeo V (2012) Remote sensing and GIS application analysis of active fire, aerosol optical thickness and estimated PM10 in the north of Thailand and Chiang Rai Province. APCBEE Proc 1:304–308. https://doi.org/10.1016/j.apcbee.2012.03.050
Stanojevic S (2018) Standardisation of lung function test interpretation: global lung function initiative. Lancet Respir Med 6:10–12. https://doi.org/10.1016/S2213-2600(17)30472-1
Takemura H, Hida W, Sasaki T, Sugawara T, Sen T (2005) Prevalence of chronic obstructive pulmonary disease in Japanese people on medical check-up. Tohoku J Exp Med 207:41–50. https://doi.org/10.1620/tjem.207.41
Thomas P, Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, Fenech M (2009) Buccal micronucleus cytome assay. Nat Protoc 4:825–837. https://doi.org/10.1038/nprot.2009.53
Tsai YI, Sopajaree K, Chotruksa A, Wu HC, Kuo SC (2013) Source indicators of biomass burning associated with inorganic salts and carboxylates in dry season ambient aerosol in Chiang Mai Basin, Thailand. Atmos Environ 78:93–104
Upadhyay D, Panduri V, Ghio A, Kamp DW (2003) Particulate matter induces alveolar epithelial cell DNA damage and apoptosis - role of free radicals and the mitochondria. Am J Respir Cell Mol Biol 29:180–187. https://doi.org/10.1165/rcmb.2002-0269OC
Varetti G, Pellman D, Gordon DJ (2014) Aurea Mediocritas: The importance of a Balanced Genome. Cold Spring Harb Perspect Biol 6:a015842. https://doi.org/10.1101/cshperspect.a015842
Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med 187:347–365. https://doi.org/10.1164/rccm.201204-0596PP
Wiriya W, Prapamontol T, Chantara S (2013) PM10-bound polycyclic aromatic hydrocarbons in Chiang Mai (Thailand): seasonal variations, source identification, health risk assessment and their relationship to air-mass movement. Atmos Res 124:109–122
Wiriya W, Chantara S, Sillapapiromsuk S, Lin NH (2016) Emission profiles of PM10-bound polycyclic aromatic hydrocarbons from biomass burning determined in chamber for assessment of air pollutants from open burning. Aerosol Air Qual Res 16:2716–2727
Wiwatanadate P, Liwsrisakun C (2011) Acute effects of air pollution on peak expiratory flow rates and symptoms among asthmatic patients in Chiang Mai, Thailand. Int J Hyg Environ Health 214:251–257. https://doi.org/10.1016/j.ijheh.2011.03.003
World Health Organization (2006) WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005 : summary of risk assessment. World Health Organization. http://www.who.int/iris/handle/10665/69477
Xue WL, Warshawsky D (2005) Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 206:73–93. https://doi.org/10.1016/j.taap.2004.11.006
Yang W, Omaye ST (2009) Air pollutants, oxidative stress and human health. Mutat Res 674:45–54. https://doi.org/10.1016/j.mrgentox.2008.10.005
Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P (2007) Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 176:753–760. https://doi.org/10.1164/rccm.200612-1749OC
Acknowledgments
The authors are grateful to Dr. Dale E. Taneyhill for proofreading the English.
Funding
This research was supported by the Thailand Research Fund (TRF), Grant No. MRG5980190 and Faculty of Medicine, Chiang Mai University Research Fund, Grant No. 013/2560 and Grant No. 067/2559. The funding body had no role in the design and execution of this study or interpretation of the data.
Author information
Authors and Affiliations
Contributions
KW, CP, and TP conceived and planned the study. KW, SM, NP and WC contributed to sample collection and lung function measurement. KW and SM carried out the BMCyt assay. KW interpreted the results and performed data analysis. KW and RP performed statistical analysis. KW wrote the manuscript in consultation with CP, TP and WC.
Corresponding author
Ethics declarations
This study was carried out in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wunnapuk, K., Pothirat, C., Manokeaw, S. et al. PM10-related DNA damage, cytokinetic defects, and cell death in COPD patients from Chiang Dao district, Chiang Mai, Thailand. Environ Sci Pollut Res 26, 25326–25340 (2019). https://doi.org/10.1007/s11356-019-05641-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05641-w