Abstract
A re-circulating horizontal flow constructed wetland (RHFCW) system was developed in a greenhouse. This system was operated with Typha domingensis to study the phytoremediation capacity of this macrophyte species in different developing stages for synthetic textile wastewater with the pollutant type, the amaranth (AM) azo dye. Experiments were applied with a fixed flow rate Q = 10 L/h corresponding to a theoretical residence time of 3 h. The synthetic feeding to the RHFCW container was re-circulated back until the required water quality was achieved. The performance of this pilot-scale system was compared to an unplanted RHFCW. The effect of the initial dye concentration was studied using four dye concentrations (10, 15, 20, and 25 mg/L). The following parameters pH, color, COD, BOD5, NO3−, NO2−, and NH4+ were monitored during treatment. The maximum efficiencies obtained for discoloration, COD, NO3−, and NH4+ were 92 ± 0.14%, 56 ± 1.12%, 92 ± 0.34%, and 97 ± 0.17% respectively. Experiences demonstrate a decrease of removal efficiencies of studied parameters with the increase of dye concentrations, leading to an increase of the duration of treatment. Changes in activities of antioxidant enzymes (superoxide dismutase (SOD), guaiacol peroxidase (GPX), catalase (CAT), ascorbic peroxidase (APX), and glutathione reductase (GR)) and their relation to plant defense system against stress were studied. Enzymes were evaluated in leaves of T. domingensis during the remediation of the azo dye (amaranth). During treatment, an increase of enzymes activities was observed in accordance with the high removal efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic dyes can highly impact in environment and health. Due to their recalcitrant aspect, partial degradation was obtained in industrial pretreatment systems. Consequently, residual and toxic dye concentrations can be discharged in the natural system. As example, the dye amaranth, widely used in the food and cosmetic industries, is considered as synthetically prepared carcinogenic azo dye (Xu et al. 2018; Severo et al. 2016; Hadibrata and Nor 2014). Hence, the treatment of effluents containing dyes has been a challenging problem for environmental technologies development (Parsons 2004).
The physical and chemical methods can have numerous disadvantages, such as high cost, low efficiency, and formation of toxic sludge and by-products (Khandare and Govindwar 2015). It is therefore important to look for low cost, eco-friendly, less sludge forming, more sustainable, and in situ administrable methods for textile wastewater treatment. In the last two decades, phytoremediation that uses plants as viable alternative methods at low cost and environmentally friendly has been explored for the treatment of various industrial wastewaters (Sarioglu et al. 2007). Macrophytes plants are successfully explored in various pilot scales of constructed wetlands (CWs). The large biomass and complex metabolism are key tools behind superior dye removal potential of these plants (Juwarkar et al. 2010).
Previous studies proved that CWs are considered to be a sustainable technology and nature-based system to treat wastewaters (Meng et al. 2014; Khandare and Govindwar 2015; Chandanshive et al. 2017). They are designed to take advantage of many processes that occur in natural wetlands, with more controlled environment. Due to processes complexity, the design of these systems used to be based on experimental pilot studies (Stottmeister et al. 2003; Khandare and Govindwar 2015).
The classification of CWs is based on the type of macrophytic growth (emergent, submerged, free floating, and rooted with floating leaves). Further classification is also based on the water flow regime (surface flow, subsurface vertical, or horizontal flow) (Reddy et al. 2014; Sudha and Vasudevan 2012; El Hamouri et al. 2007). In HFCWs, wastewater from the inlet zone passes horizontally through the bed matrix until it reaches the outlet zone (Vymazal and Kröpfelová 2009). During the passage of wastewater through the reed bed, the wastewater makes contact with a network of aerobic, anoxic, and anaerobic zones. Many studies have shown that the oxygen release from roots of different macrophytes is far less than the amount needed for aerobic degradation of the oxygen consuming substances delivered with wastewater and that anoxic and anaerobic decomposition play an important role in horizontal surface flow constructed wetlands (Brix 1990; Vymazal 2005). As a result, organic compounds are degraded aerobically as well as anaerobically by bacteria attached to plant underground organs (i.e., roots and rhizomes) and media surface (Brix and Schierup 1990). This is why several studies prove the effectiveness of these anaerobic conditions that predominate in HFCW bed matrices in the removal of a majority of pollutants (Ong et al. 2009; Ding et al. 2012).
As an operational modification to improve the effluent quality of HFCWs, a re-circulating horizontal flow constructed wetland (RHFCW) where the water is re-injected was developed in an attempt to reduce the release of nutrients and toxic compounds into the environment (Sklarz et al. 2009). Therefore, effluent re-circulation has been proposed as an operational modification to improve the effluent quality of CWs (Wu et al. 2014). As demonstrated by Wu et al. 2014, when the effluent is being re-circulated, additional oxygen for aerobic microbial activities can be transferred into the wastewater and to the system. This operation will also bring benefit to the treatment by enhancing interactions between pollutants and microorganisms attached on the roots of macrophytes and surfaces of gravel.
Plant species and filtration material type are important in determining the treatment efficiency of constructed wetlands. Several recent studies have tested and evaluated the ability of some plants and especially their antioxidant enzymes for decontamination of water such as the textile dyes (Nilratnisakorn et al. 2007; Ong et al. 2009; Patil et al. 2009; Kagalkar et al. 2010; Khandare et al. 2011; Haddaji et al. 2015). The growth of roots and rhizomes allows maintenance and regulation of the initial hydraulic conductivity and contain air-filled channels connected to the atmosphere for the purpose of transporting oxygen to the root system (Mitsch and Gosselink 2000).
In CWs, macrophytes are important component of the system for the removal of pollutants. Some emergent macrophytes are efficiently used to treat wastewater, such as Typha domingensis. The use of T. domingensis to detoxify polluted environments has attracted a lot of interest (Martin et al. 2003). It has been identified in possessing the high potential to degrade textile dyes (Nilratnisakorn et al. 2007; Shehzadi et al. 2014; Masi et al. 2019). Typha angustifolia has been also employed for remediation experiments and lead to an efficient removal of azo dyes from textile effluent (Chandanshive et al. 2017). Previous study proved that macrophytes when irrigated with azo dye solutions possess an antioxidative defense system that minimizes the adverse effects of reactive oxygen species, and no toxicity has been identified for these plants during the degradation of textile dyes with different applied concentrations (Shehzadi et al. 2014). The physicochemical and biological processes of treatment take place in the rhizosphere which is induced by the interaction of pollutants with plants, microorganisms, and the soil. Pollutants cause the formation of reactive oxygen species (ROS) by plants. As a response to these ROS, antioxidant enzymes are released by plants as a protection mechanism against these toxic compounds. These enzymes play a pivotal role in organic matter decomposition by converting macromolecules into low molecular moieties (Kang et al. 1998). T. domingensis peroxidases have been tested and proved to be able for degradation of textile dyes such as amaranth and Amido black (Haddaji et al. 2015).
The main objectives of this study were (i) to explore the phytoremediation potential of an aquatic macrophyte named T. domingensis to degrade an azo dye Amaranth; (ii) to assess the efficacy of the re-circulated horizontal flow constructed wetland system (RHFCW) as technical process; and (iii) to investigate the role of plant in the degradation of the dye by the monitoring of the response of T. domingensis defense system through its antioxidant enzymes activities measurement (SOD, GPX, CAT, APX, and GR) during the treatment and in relation with the carbon oxygen demand (COD) removal.
Materials and methods
Dye
Amaranth dye (E123, Sigma-Aldrich), formula C20H11N2Na3O10S3 and molar mass 606.48 g mol−1, was chosen as organic dye pollutant. The UV-Vis spectrum was presented in Fig. 1. A wavelength of maximum absorbance at 520 nm was used to monitor the dye solution discoloration. Dye solutions with concentrations of 10, 15, 20, and 25 mg/L were prepared using tap water. The initial pH of dye solutions was 7 with maximum increase to 7.7 during treatment.
UV-Vis spectrum of amaranth dye (Haddaji et al. 2015). All other chemicals used for analysis were of the highest purity available and of analytical grade
Plant material
T. domingensis is a macrophyte species collected from nature environment (river; Oued Maizet, city Ezzahra, Governorate of Ben Arous; Tunisia (Fig. S1) and cultured to produce new shoots in pilot reactors (Fig. S2). The experiments were conducted from January to August 2017. Plants were selected and cultured under greenhouse conditions of temperature, moisture level, and light (temperature of 30 ± 2 °C, with 12 h of light, light intensity of 910 μmol m−1 s−2, and humidity from 50 to75%). Six shoots are planted in each tank. Cultured plants are selected at the same stage of growth: 0.4 to 0.6 m plant height that reaches about 1.5 to 1.9 m at the end of the treatment experimentation. Roots of T. domingensis did not extend beyond about 29 cm (Ranieri et al. 2014). A total of 4 to 5 leaves per plant were selected for enzyme activities analysis.
Pilot description and operational conditions
The experimental was conducted in constructed wetlands with horizontal subsurface flow system (HFCWs), in three pilot conditions. Three polyester tanks (0.53 m, 0.52 m, and 0.52 m; L × W × H) were used (Figs. 2 and 3) as HFCWs systems. The three tanks were filled with fine gravel with an average diameter of 8 to 10 mm for approximately 35 cm width. In the inlet and outlet zones, for about 15 to 17 cm width we use coarse gravel sizes (Ø 80 to 100 mm) until 45 cm of high. The 45 cm depth of the filtration bed of the RHFCWs (Fig. S2) allows roots of wetland plants to penetrate the bed and ensure oxygenation of the bed through oxygen release from roots.
A reservoir with 60 L capacity (Fig. 2) was connected to each tank and used for the feeding and the re-circulation of water by peristaltic pump at the flow rate of 10 L/h during 3.0 h each day (from 9 to 11 am). The feeding was for 3 h per day. An outlet siphon system adjusts the water level in the filter so that it is always filled with water where water from the inlet zone passes horizontally through the bed matrix until it reaches the outlet zone. The operated HFCW (tank b) was planted with T. domingensis and irrigated with AM azo dye solution. The second HFCW system (tank a) was irrigated only with tap water and used as a control bed for the determination of the response of these macrophytes species to this oxidant stress caused by amaranth dye by the activation of the antioxidant enzymes. Another control bed in this work was carried with the unplanted bed (tank c), where dye solution has been re-circulated at the same condition of the planted beds. All three beds (tanks a, b, and c) were installed in greenhouse under the same conditions of temperature, humidity and light. (Fig. S2). All experiments were fed with a fixed flow rate of 10 L/h during 3 h.
Samples of 150 mL in total were withdrawn from the outlet of the system for chemical analysis after each cycle of re-circulation (3 h, 27 h,51 h, 75 h, etc.) until the target treatment was reached (maximum of 30 samplings for one experimentation) corresponding to a maximum of sampling volume of 4.5 L.
Residence time test
According to the method outlined by (King et al. 1997), tracer tests were conducted for the planted and unplanted gravel CWs. The objective is to determine the residence time distribution (RTD) through the RHFCW systems and the flow velocity. When the tracer is injected in the inlet, sampling at the selected points takes place over time. Lithium (Li2+) is commonly used as a tracer because it is inert and does not react with the CWs system. Li2+ was accurately analyzed by ionic chromatography using a Metrohm ion chromatography system that was fitted with an anion-exchange column (AS4A-SC, 150 mm × 4 mm) and coupled with a conductivity detector under the control of IC NET software. The injected concentration was 0.3 mg/L based on the work of King et al. 1997; Ranieri et al. 2013, modified. Samples were collected during a period of two to three times the theoretical retention time to ensure that the entire amount of tracer was discharged.
The RTD curves have been calculated using dispersion reactor model given by Levenspiel and Smith (1957):
(eq. 1):
where,
- ∑iCiΔt:
-
Area under the residence time distribution, Pe, peclet number; θ, the residence time distribution
Based on the concentration in the outlet of the tanks, residence time distribution function is generated as given in (eq. 2):
where,
- E (t):
-
RTD function, min−1
- C (t):
-
concentration of tracer at t time, mg/L
- t :
-
time, min
Water samples and analysis
The efficiency of the system was evaluated by measuring organic and inorganic parameters. The following parameters were analyzed for each sample, pH, and chemical oxygen demand (COD) using the standard dichromate method described in standard methods (Rodier et al. 2009). Nitrate (NO3−), nitrite (NO2−) using Metrohm ion chromatography system that was fitted with an anion-exchange column (AS4A-SC, 150 mm × 4 mm), ammonium (NH4+) was determined using the Nessler method by Rodier et al. 2009. Dye coloration was measured using the UV-Visible spectrophotometer method (Perkin Elmer Lambda 25 UV-VIS Spectrometer). The percentage of color removal was calculated by the following equation (eq. 3):
where Abs0 is the absorbance of AM dye before treatment and Abst is the absorbance of Amaranth (AM) dye after t time treatment.
AM degradation mechanism was investigated using an Agilent high-performance liquid chromatography (HPLC) composed of a binary pump coupled to a UV-visible detector. The amaranth dye molecules and by-products were determined according to the method outlined by Elaissaoui et al. 2016. The main characteristic absorbance was obtained at 260 nm for AM dye.
Enzymatic activities essay
Activities of several antioxidant enzymes such as superoxide dismutase (SOD), guaiacol peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) were measured in leaves extract of T. domingensis during treatment. The fresh leaves extract was homogenized in phosphate buffer (0.01 M/L, pH 7.0), containing 0.2% polyvinylpyrrolidone. The homogenate was centrifuged at 2000×g at 4 °C for 20 min. The supernatant was used as the crude extract for the following assay:
Protein content was determined according to the method of Bradford (1976) with bovine serum albumin as a standard. Superoxide dismutase (SOD) activity was determined using the method developed by Winterbourn et al. 1976 and based on the measurement of the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT). Guaiacol peroxidase (GPX) consumes H2O2 using guaiacol as an electron donor. The assay of its activity is followed by the evolution of the enzyme kinetics for 1 min at 470 nm (Khan and Robinson 1994). Catalase (CAT) activity was determined by following the decomposition of H2O2 at 240 nm for 1 min in 1 mL of reaction mixture. Ascorbate peroxidase (APX) activity is determined by the method described by Asada and Bager (1984). One unit of APX activity was defined as l mol of ASC consumed/(min g FW). Glutathione reductase (GR) is quantification from the decrease in absorbance at 340 nm following the oxidation of NADPH. The extinction coefficient used to calculate the activity was 6.22 m M−1 cm−1.
Each enzyme activity was measured three times in units/g of plant/min. The activity was based on the total content of soluble protein (measured in mg protein/g plant), which will determine the specific activity of the enzyme, expressed in units/mg protein.
Statistical analysis
The data were expressed as mean ± SD. In order to compare the results, Student-Newman tests were performed using SPSS data analysis (version.20). In all cases, significance was defined by p < 0.05.
Results and discussions
RHFCW hydraulic system
The residence time distribution (RTD) for the two CWs, planted with T. domingensis and the unplanted one, were measured in order to determine the flow behavior within the gravel-based CWs. Experiences where operated at a flow rate Q = 10 L/h. The planted field had a mean residence time of 3.3 h where the unplanted field had a measured hydraulic residence time (HRT) of 3.1 h.
The theoretical residence time calculated is about 3.39 h (Fig. 3). The slightly decrease in the HRT measured compared to the theoretical in unplanted bed was linked to the dead volumes or dead zones in the tank that reach in this case 2.9 L (EPA 1999) and less for the planted bed (0.9 L). This dead volume could correspond to the stagnant water in the base of the tanks. The slightly increased HRTs values of the planted CWs, compared with the unplanted ones, may be attributed to the root systems of T. domingensis, which probably enhanced the residence time of the tracer in the tank. According to Nemade et al. 2010, the flow can be categorized as flow through porous two-channel dispersion model micro and macro.
Tracers’ curves indicated that experimental and theoretical RTD are approximately equal. In this case, we can suggest that CW was an ideal reactor where the flow at the inlet is completely and instantly mixed.
Efficiency of T. domingensis for azo dye removal
Dye discoloration
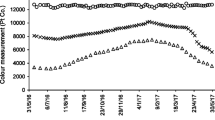
Full spectrum analysis (Fig. 4) showed an example of the UV-Vis spectrum of the effluent samples collected from RHFCW systems for an initial dye concentration of 10 mg/L. Changes on AM dye absorption in the UV and visible range (200–800 nm) at studied initial concentrations were recorded. Decrease in main absorbance (λ max: 520 nm) means that the solution of amaranth dye was being discolored and the AM dye was degraded or transformed in by products. The variation of the absorbance and the decrease of the peak at λ max were monitored to assess the discoloration of the solution.
Based on Fig. 4, there was no modification on the visible part of the spectrum after 3 h of experimentation. After 17 days, high discoloration was achieved (low absorbance at λ max). Discoloration was linked in several works to the cut of the N=N bonds (Hadibarata and Nor 2014; Barros et al. 2014). However, absorbance in UV range (200–400 nm) increased then decreased. Peaks between 280 and 330 nm were related to π → π* transitions in conjugated aromatic rings and was linked to the generation of organic by-products due to the fragmentation of the dye molecule.
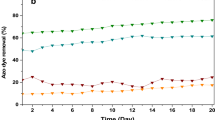
In the unplanted bed (control test, Fig. 5 b) the UV-Vis spectrum did not show high modifications during the treatment. Low level of discoloration was obtained (27% ± 0.19 and 9% ± 0.08 for initial dye concentration of 10 and 25 mg/L respectively). The dye removal efficiency could be mainly linked to the dye adsorption on the gravel with limited effect of microorganisms as the gravel was washed (Jarusipirot 2014).
For the planted system (Fig. 5 a), the highest percentage of discoloration was 92% ± 0.14 reached for the initial dye concentration of 10 mg/L (within 17 days). The discoloration efficiency decreased when the initial dye concentration increased until 83 ± 0.23 for initial dye concentration of 25 mg/L. The obtained efficiencies are high and could be explained by the adsorption of dye on the gravel associated with the absorption /adsorption phenomena on plant roots in the rhisosphere with microorganism development and plant uptake which will enhance the discoloration efficiency (Kamboh et al. 2014), in comparison to the unplanted bed.
The kinetics of the dye discoloration rates can be described by an apparent first order, ln (C0/Ct) = kapp × t (Table 1), where C0 is the initial concentration of dye and Ct is the concentration of dye at time t.
Dye degradation
HPLC analysis was employed to monitor the concentration of AM during the treatment (C0 = 10 mg/L). The HPLC analysis of the AM dye-treated solutions showed clearly that the amaranth had been almost completely disappeared at the end of the treatment (Fig. S3). The chromatograms depicted in Fig. S3 were detected at 260 nm, a wavelength associated with π → π* transitions in conjugated aromatic systems. The intensity of the AM peak at a retention time of 2.43 min diminished during the treatment.
Degradation was accompanied by the appearance of mainly two new peaks at retention times of around 2.022 and 1.798 min. The compounds associated with these peaks could be intermediates/by-products formed by rupture of the dye molecule during the degradation process.
The AM degradation is well fitted with apparent first order kinetic (Fig. 6). The apparent kinetic constant (Kapp) is equal to 0.234 day−1 (R2 = 0.90) for dye initial concentration of 10 mg/L. This value is higher than discoloration apparent constant. The degradation of the dye can lead to by-products with absorption capacity at λ max 520 nm, which will decrease the discoloration efficiency.
The mineralization of the aromatic molecules as dyes lead in general to the opening of the rings at the end of the mineralization process producing oxalic acid and then CO2 and H2O. Indeed, the oxalic acid was identified during the degradation of the AM by oxidation process (Barros et al. 2014). In order to determine whether oxalic acid was formed during biodegradation of the dye, standard solution of oxalic acid (⩾ 95% purity; Sigma-Aldrich) was subjected to HPLC analysis. As shown in Figs. 6 and 7, standard solution showed a well-defined peak at 2.02 min under the HPLC conditions. In order to monitor its appearance and then degradation, the treatment time was increased to 25 days. Figure 7 reports the variation of the oxalic acid concentration showing its increase during treatment when reaching a maximum at day 17, then, it decreases slowly. However, according to Schröder (2013), oxalic acid can be liberated by plant roots in the system solution in small quantities too, which explain the initial value.
Dye mineralization and removal efficiency of COD, BOD5, NO3 −, and NH4 +
In order to monitor the mineralization of the AM dye, the efficiencies of this RHFCW system for the removal of chemical oxygen demand (COD), biological oxygen demand (BOD5), NO3−, and NH4+ by the bed systems were monitored.
COD removal and BOD5 evolution
The COD of the dye solution introduced to the bed system initially increased (Fig. 8 a) due to the composition of the bed (gravel, roots, and bacteria) and the release of carbon materials from plant roots. Then, during the treatment, the COD decreased. This decrease could be linked to the dilution of the COD initially in the bed by the recycling of the dye solution from the reservoir with lower initial COD as underlined by (Ammari et al. 2014). However, an average of 57 ± 1.14% removal was obtained after 22, 24, and 30 days of treatments for 15, 20, and 25 mg/L of initial concentration of AM dye respectively. In this case, and as demonstrated in dye degradation section, the removal of COD was mainly due to plant system than only the explained dilutions when comparing with the unplanted bed, the maximum removal of COD was only 22 ± 0.19% (Fig. 8 b). The COD removal ratio is lower than the dye degradation or discoloration, by-products of degradation can be considered in this case as recalcitrant compounds to the studied treatment system (RHFCW) compared to the dye as demonstrated in other studies (Xu et al. 2018; Severo et al. 2016).
The initial BOD5 values of dye solution were negligible due to the chemical composition of the dye solution and only small variation is observed in the unplanted bed. In this last case, obtained values were in the experimental error range and were not presented. However, for the planted bed, after first circulation during 3 h, the BOD5 increase due to the presence of bacteria in the planted bed related to the rhizosphere (roots and rhizomes) of plant roots and to the released materials (Chen et al. 2016; Aguilar et al. 2019). The BOD5 after 3 h of treatment was noted BOD*0 in this case and Fig. 9 represents the evolution of BOD/BOD*0 during the treatment. After recycling, the obtained ratios of removal were similar in the range 64 ± 0.18% to 68 ± 0.20% for the studied concentrations of dye (Fig. 9). In fact, these improvements in COD and BOD5 removal should be related also to the enrichments of the HFCW system with oxygen. In general, the system filled with gravels allows the mechanical filtration of water and allow roots of T. domingensis to penetrate the whole bed that ensure oxygenation of the whole bed through oxygen release from roots. Roots contain air-filled channels connected to the atmosphere for the purpose of transporting oxygen to the root system (Mitsch and Gosselink 2000). However, in this case, the re-circulation is transporting oxygen to the root system (Mitsch and Gosselink 2000; Vymazal and Kröpfelová 2009). The circulation of dye solution several times within the matrices of the bed improves the contact between the solution and the rhizosphere and brings more oxygen to the HFCW (Rehman et al. 2017).
In another side, in a RHFCW conditions, when the effluents were re-circulated into the feeding tank, dilution of pollution can lead to better overall performance of the system than without recycling (Sun et al. 2005) as this dilution will decrease the negative effect on plants (Arienzo et al. 2009) and improve treatment efficiency.
NO3 −and NH4 +removal
The evolution of nitrate in the outlet of the Typha bed systems during experiments was illustrated in Fig. 10 for planted and unplanted beds. The ammonium and nitrite values were often negligible. A small increase was observed after the first 3 h of circulation followed by a decrease during the treatment. The nitrates elimination in the control reactor (without plant) did not exceed 20 ± 1.21% (Fig. 10 b) and mainly due to dilution effect as already explained. However, NO3− levels through the Typha bed system with re-circulation decreases strongly. The removal efficiencies reached an average of 92 ± 0.34% for the different studied dye concentrations (Fig. 10 a). The high removal efficiency of the RHFCW system show that denitrification was achieved which was linked to the anaerobic/anoxic condition in the HFCW with the presence of organic matter (Albuquerque et al. 2009) and to the Typha nitrogen uptake. However, the re-circulation of the dye solution was expected to provide more oxygen to the bed system and enhance the transformation of NH4+ in nitrates. As the NH4+ concentration is low since the beginning compared to nitrate, the contribution of this process to the total nitrate concentration is limited (Fig. 11). The decrease of ammonia and nitrate concentration in the Typha bed system could be the collective result of nitrification, denitrification, and plant uptake in the wetland system.
The obtained results on the RHFCW system using Typha species for AM degradation were compared to published studies (Table 2). In general, the degradation rate of the dye is strongly linked to the molecule structure and its complexity as well as the corresponding load of COD. However, based in Table 2, the removal capacity seems to follow the same order. The highest discoloration rate was obtained in this study (92%) while the COD removal was between 56 and 68%. By comparison with domestic wastewater (Zhang et al. 2014), the COD removal related to dye degradation is still limited due to generation of by-products as oxalic acid in this study. However, after treatment, the final COD concentration obtained was equal to 50 mg/L for the highest initial dye concentration (C0 = 25 mg/L). This value is under the standards of discharge in the natural system (160 mg/L for textile sector in Tunisia).
Evaluation of enzymatic activities
All results demonstrate the high potential of T. domingensis to degrade the AM azo dye. The role of this species in this degradation pathway of AM dye was studied by evaluating its response to this stress condition. By comparison with the planted bed fed by tap water (uncontaminated bed), it appeared that protein expression in the contaminated plant samples was significantly increased compared to uncontaminated plant samples in agreement with another research (Al-Taee and Witwit 2015). In addition, increase in dye concentrations in the contaminated plant samples causes parallel increase of enzyme antioxidant defenses which are used as biomarkers of oxidative damage (Chandanshive et al. 2017).
The current investigation showed high levels of GPX, SOD, CAT, APX, and GR enzymes activities in all contaminated plant samples as compared to those of uncontaminated ones. These results can be explained by the direct relation between toxic products and increase in oxidative stress as a result of an enhanced level of lipid peroxidation and hydrogen peroxide production in both roots and leaves of stressed plants (Chandanshive et al. 2017; Al-Taee and Witwit 2015).
The variation in enzymatic activities of T. domingensis (GPX, SOD, CAT, APX, and GR) exposed to different concentrations of amaranth dye was presented in Fig. 12, where the value at t = 0 is related to the planted bed fed by tap water. Enzymatic activities showed a rapid increase during treatment followed by stabilization or small decrease compared to control plants that presented same levels of antioxidant enzymes. SOD presented the highest increase and concentration to be the first detoxification enzyme and most powerful antioxidant in the cell. It acts as a component of first-line defense system against ROS (Fig. 12).
The increase and evolution of enzymes activities in leaves of T. domingensis were presented in accordance with the removal efficiency of COD. Maximum activities of antioxidant enzymes were observed at early stage of the treatment for the different concentrations of azo dye as response to the introduced pollution. These results suggest that increased activities of SOD, GPX, CAT, APX, and GR in this species contribute to their enhanced tolerance to this abiotic stress by developing a complex antioxidant defense system. When the color and the COD were decreasing during treatment, the enzymatic activities were stable or decreasing slowly at the end of the treatment (especially for SOD). This decrease was observed after 92 ± 0.14% and 56 ± 1.12% elimination of the color and COD respectively in the case of dye concentration of 25 mg/L. The last trend for SOD was the stabilization despite the continue decrease of COD. The final value of SOD at the end of the treatment is still high compared to the initial one (t = 0) underlining the importance of the residual toxicity in the bed linked to by-products generation and their recalcitrant aspect despite their low concentration compared to the initial dye one. This residual effect corresponds to the 34% of the remained COD in the CW bed corresponding mainly to by-products as all the dye molecules introduced could be totally degraded (see Fig. 8, for initial dye concentration of 10 mg/L). Generated enzymes were expected to contribute to dye degradation by breaking the aromatic rings, especially for azo dye as demonstrated in our previous work related to peroxidase (POD) (Haddaji et al. 2015).
Conclusion
This study was related to the treatment of amaranth azo dye using re-circulated horizontal flow constructed wetland system (RHFCW) planted with T. domingensis. High removal of color (83–92%) was obtained using this system. An extensive passive period followed by re-circulation enhanced organic matter degradation. The HPLC analysis of AM dye treated solutions showed clearly that the amaranth had been almost completely disappeared at the end of the treatment (98% of removal) and demonstrated the appearance of a final by-products including the oxalic acid. The obtained COD degradation of 57% underlined the recalcitrant aspect of generated by-products. High denitrification process was observed leading to low NO3− concentration with limited effect of initial dye concentration. The comparison with unplanted bed demonstrated clearly the contribution of the T. domingensis in the system performances. The dye treatment by T. domingensis acted on the defense system of this species against the introduced stress, which may play an active role in the degradation mechanism of this organic compound (dye) by activating the antioxidative enzymes. Increase of SOD, GPX, CAT, APX, and GR enzymes was observed at early stage of treatment followed by stabilization except for SOD, which registered at the beginning the highest concentration, and increase. The limited decrease of SOD at the end of treatment is in agreement with the limited COD degradation due to the generation of recalcitrant compounds. Indeed, the RHFCW demonstrated a limited degradation of COD when induced by dye pollution.
References
Aguilar L, Gallegos A, Arias C, Ferrera I, Sánchez O, Rubio R, Ben Saad M, Missagia B, Caro P, Sahuquillo S, Pérez C, Morató J (2019) Microbial nitrate removal efficiency in groundwater polluted from agricultural activities with hybrid cork treatment wetlands. Sci Total Environ 653:723–734
Albuquerque A, Oliveira J, Semitela S, Amaral L (2009) Influence of bed media characteristics on ammonia and nitrate removal in shallow horizontal subsurface flow constructed wetlands. Bioresour Technol 100:6269–6277
Al-Taee MMS, Witwit RTA (2015) Evaluation of antioxidant enzyme activities during phytoremediation of textile waste water exposed of structurally different dyes by some aquatic plants. Mesop Environ J 1:35–65
Ammari TG, Al-Zu’bi Y, Al-Balawneh A, Tahhan R, Al-Dabbas A, Rakad A, Raihan Abu-Harb R (2014) An evaluation of the re-circulated vertical flow bioreactor to recycle rural grey water for irrigation under arid Mediterranean bioclimate. Ecol Eng 70:16–24
Arienzo M, Quale WC, Christen E, Jayawardane N (2009) Irrigating with winery wastewater? Developing soil stability thresholds and managing total cations. Aust NZ Grapegrow. Winemak 86–88
Asada K, Bager MR (1984) Photoreduction of 18O2 and H218O2 with concomitant evolution of 16O2 in intact spinach chloroplasts: evidence for scavenging of hydrogen peroxide by peroxidase. Plant Cell Physiol 25:1169–1179
Barros WRP, Steter JR, Lanza MRV, Motheo AJ (2014) Degradation of amaranth dye in alkaline medium by ultrasonic cavitation coupled with electrochemical oxidation using a boron-doped diamond anode. Electrochim Acta 143:180–187. https://doi.org/10.1016/j.electacta.2014.07.141
Bradford (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brix H (1990) Gas exchange through the soil-atmosphere interphase and through dead culms of Phragmites australis in a constructed reed bed receiving domestic sewage. Water Res 24:259–266
Brix H, Schierup H-H (1990) Soil oxygenation in constructed reed beds: the role of macrophyte and soil-atmosphere interface oxygen transport. In: Soil, P.F. and Findlater, B.C. (eds.) Constr Wetl Water Pollut Control. Pergamon Press, London. pp. 53–66
Chandanshive VV, Rane NR, Tamboli AS, Gholave AR, Khandare RV, Govindwar SP (2017) Co-plantation of aquatic macrophytes Typha angustifolia and Paspalum scrobiculatum for effective treatment of textile industry effluent. J Hazard Mater 338:47–56
Chen ZJ, Tian YH, Zhang Y, Song BR, Li HC, Chen ZH (2016) Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol Eng 92:243–250
Ding Y, Song XS, Wang YH, Yan DH (2012) Effects of dissolved oxygen and influent COD/N ratios on nitrogen removal in horizontal subsurface flow constructed wetland. Ecol Eng 46:107–111
El Hamouri B, Nazih J, Lahjouj J (2007) Subsurface-horizontal flow constructed wetland for sewage treatment under Moroccan climate conditions. Desalination 215(1–3):153–158
Elaissaoui I, Akrout H, Grassini S, Fulginiti D, Bousselmi L (2016) Role of SiO x interlayer in the electrochemical degradation of Amaranth dye using SS/PbO 2 anodes. Mater Des 110:633–643
EPA/625/R-99/010 (1999) Manual constructed wetlands treatment of municipal waste waters. National risk management research laboratory
Haddaji D, Bousselmi L, Saadani O, Nouairi I, Ghrabi-Gammar Z (2015) Enzymatic degradation of azo dyes using three macrophyte species: Arundo donax, Typha angustifolia and Phragmites australis. Desalin Water Treat 53:1129–1138
Hadibarata T, Nor NM (2014) Decolorization and degradation mechanism of Amaranth by Polyporus sp. S133. Bioprocess Biosyst Eng 37:1879–1885. https://doi.org/10.1007/s00449-014-1162-0
Jarusipirot C (2014) Removal of reactive dye by adsorption over chemical pretreatment coal based bottom ash. Procedia Chem 9:121–130
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9:215–288
Kagalkar AN, Jagtap UB, Jadhav JP, Govindwar SP, Bapat VA (2010) Studies on phytoremediation potentiality of Typhonium flagelliforme for the degradation of Brilliant Blue R. Planta 232:271–285
Kamboh MA, Bhatti AA, Solangi IB, Sherazi STH, Memon S (2014) Adsorption of direct black-38 azo dye on p-tert-butylcalix[6]arene immobilized material. Arab J Chem 7:125–131. https://doi.org/10.1016/j.arabjc.2013.06.033
Kang H, Freeman C, Lee D et al (1998) Enzyme activities in constructed wetlands: implication for water quality amelioration. Hydrobiologia 368:231–235
Khan AA, Robinson DS (1994) Hydrogen donor specificity of mango isoperoxidises. Food Chem 49:407–410
Khandare RV, Govindwar SP (2015) Phytoremediation of textile dyes and effluents: current scenario and future prospects. Biotechnol Adv 33:1697–1714
Khandare RV, Kabra AN, Kurade MB, Govindwar SP (2011) Phytoremediation potential of Portulaca grandiflora Hook.(Moss-Rose) in degrading a sulfonated diazo reactive dye Navy Blue HE2R (Reactive Blue 172). Bioresour Technol 102:6774–6777
King AC, Mitchell CA, Howes T (1997) Hydraulic tracer studies in a pilot scale subsurface flow constructed wetland. Water Sci Technol 35:189–196
Levenspiel O, Smith WK (1957) Notes on the diffusion type model for the longitudinal mixing of fluids in flow. Chem Eng Sci 6:227–235
Martin J, Hofherr E, Quigley MF (2003) Effects of Typha latifolia transpiration and harvesting on nitrate concentrations in surface water of wetland microcosms. Wetlands 23:835–844
Masi F, Rizzo A, Bresciani R, Martinuzzi N, Wallace SD, Van Oirschot D, Macor F, Rossini T, Fornaroli R, Mezzanotte V (2019) Lessons learnt from a pilot study on residual dye removal by an aerated treatment wetland. Sci Total Environ 648:144–152
Mbuligwe ST (2005) Comparative treatment of dye-rich wastewater in engineered wetland systems (EWSs) vegetated with different plants. Water Res 39:271–280
Meng P, Pei H, Hu W, Shao Y, Li Z (2014) How to increase microbial degradation in constructed wetlands: influencing factors and improvement measures. Bioresour Technol 157:316–326
Mitsch WJ, Gosselink JG (2000) Wetland. Wiley, New York
Nemade PD, Dutta SM, Shankar HS (2010) Residence time distribution and oxygen transfer in a novel constructed soil filter. J Chem Technol Biotechnol 85:77–84
Nilratnisakorn S, Thiravetyan P, Nakbanpote W (2007) Synthetic reactive dye wastewater treatment by narrow-leaved cattails (Typha angustifolia Linn.): effects of dye, salinity and metals. Sci Total Environ 384:67–76
Ong SA, Uchiyama K, Inadama D, Ishida Y, Yamagiwa K (2009) Phytoremediation of industrial effluent containing azo dye by model up-flow constructed wetland. Chin Chem Lett 20:225–228
Parsons S (2004) Advanced oxidation processes for water and wastewater treatment. IWA publishing, London
Patil P, Desai N, Govindwar S, Jadhav JP, Bapat V (2009) Degradation analysis of Reactive Red 198 by hairy roots of Tagetes patula L. (Marigold). Planta 230:725–735
Ranieri E, Gorgoglione A, Solimeno A (2013) A comparison between model and experimental hydraulic performances in a pilot-scale horizontal subsurface flow constructed wetland. Ecol Eng 60:45–49. https://doi.org/10.1016/j.ecoleng.2013.07.037
Ranieri E, Gorgoglione A, Montanaro C, Lacovelli A, Gikas P (2014) Removal capacity of BTEX and metals of constructed wetlands under the influence of hydraulic conductivity. Desalin Water Treat 56(5):1–8
Reddy L, Kumar D, Asolekar SR (2014) Typologies for successful operation and maintenance of horizontal sub-surface flow constructed wetlands. International Journal of Development Research 147(32):2319–6890
Rehman F, Pervez A, Khattak BN, Ahmad R (2017) Constructed wetlands: perspectives of the oxygen released in the rhizosphere of macrophytes. Clean Soil Air Water 45:1
Rodier J, Legube B, Merlet N (2009) L’Analyse de l’Eau. 9è édition. Dunod, Paris 1579 pp
Sarioglu M, Bali U, Bisgin T (2007) The removal of CI Basic Red 46 in a mixed methanogenic anaerobic culture. Dyes Pigments 74:223–229
Schröder LLJKH (2013) Root exudation pattern of Typha latifolia L. plants after copper exposure. Plant Soil 370:187–195
Severo EC, Anchieta CG, Foletto VS, Kuhn RC, Collazzo GC, Mazutti MA, Foletto EL (2016) Degradation of Amaranth azo dye in water by heterogeneous photo-Fenton process using FeWO4 catalyst prepared by microwave irradiation. J Water Sci Technol 73:88–94
Shehzadi M, Afzal M, Khan MU, Islam E, Mobin A, Anwar S, Khan QM (2014) Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res 58:152–159
Sklarz MY, Gross A, Yakirevich A, Soares MIM (2009) A recirculating vertical flow constructed wetland for the treatment of domestic wastewater. Desalination 246:617–624
Stottmeister U, Wießner A, Kuschk P, Kappelmeyer U, Kästner M, Bederski O, Müller RA, Moormann H (2003) Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol Adv 22:93–117
Sudha S, Vasudevan N (2012) Performance studies on constructed wetland for treatment of crocodile pond wastewater. Int J Environ Waste Manag 9:141–153
Sun G, Zhao Y, Allen S (2005) Enhanced removal of organic matter and ammoniacal-nitrogen in a column experiment of tidal flow constructed wetland system. J Biotechnol 115:189–197
Vymazal J (2005) Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol Eng 25:478–490
Vymazal J, Kröpfelová L (2009) Removal of organics in constructed wetlands with horizontal sub-surface flow: a review of the field experience. Sci Total Environ 407:3911–3922
Winterbourn CC, McGrath BM, Carrell R (1976) Reactions involving super- oxide and normaland unstable haemoglobins. Biochem J 155:493–502
Wu S, Kuschk P, Brix H, Vymazal J, Dong R (2014) Development of constructed wetlands in performance intensifications for wastewater treatment: a nitrogen and organic matter targeted review. Water Res 57:40–55
Xu J, Gao Q, Baie X, Wanga Z, Zhu Y (2018) Enhanced visible-light-induced photocatalytic degradation and disinfection activities of oxidized porous g-C3N4 by loading Ag nanoparticles. J Catal Today. https://doi.org/10.1016/j.cattod.2018.07.024
Zhang DQ, Jinadasa KBSN, Gersberg RM, Liu Y, Ng WJ, Tan SK (2014) Application of constructed wetlands for wastewater treatment in developing countries-a review of recent developments (2000-2013). J Environ Manag 141:116–131
Funding
This research work has been developed in the framework of a contract-objective funded by the Tunisian Ministry in charge of Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 512 kb)
Rights and permissions
About this article
Cite this article
Haddaji, D., Ghrabi-Gammar, Z., Hamed, K.B. et al. A re-circulating horizontal flow constructed wetland for the treatment of synthetic azo dye at high concentrations. Environ Sci Pollut Res 26, 13489–13501 (2019). https://doi.org/10.1007/s11356-019-04704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04704-2