Abstract
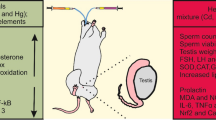
The aim of this study was to evaluate the probable protective effect of quercetin (QUE) against cadmium (Cd)-induced sub-chronic toxicity in rats. Adult male rats were given either Cd (as cadmium chloride; 5 mg/kg) alone or in combination with QUE (50 mg/kg) daily for 4 weeks by oral gavage. At the end of the experimental period, Cd accumulation, and selected hematological, thyroid, and reproductive markers were assessed. Results revealed that Cd treatment significantly increased Cd concentrations in blood, thyroid gland, and testicular tissue of rats. Cd also caused a decline in hemoglobin content, hematocrit value, and total erythrocyte and leucocyte counts. Further, significant suppressions in the blood levels of hormones related to thyroid gland function, and male reproductive hormones (i.e., testosterone, luteinizing hormone and follicle-stimulating hormone), were observed in Cd-treated rats compared to the control. In parallel, low sperm count and sperm motility, increased sperm abnormalities, and marked pathology occurred in testis. Combination with QUE recorded amelioration of the deleterious effects of Cd, involving regulation of hematological toxicity and thyroid hormonal levels and subsequently modulation of testicular function. In conclusion, it appears that dietary QUE can rescue from Cd-induced hematological dysfunctions and testicular damage by reversing the hypothyroid state.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a hazardous heavy metal largely available in the environment, especially in industrial and urbanized regions (Järup and Åkesson 2009). Cd has a very long biological half-life ranging from 15 to 30 years (Satarug et al. 2010). Available epidemiological and experimental investigations revealed that Cd can disrupt thyroid function even at low environmental doses (Nie et al. 2017; Buha et al. 2018). It has been shown that cadmium toxicity has deleterious testicular effects including apoptotic events, decreased steroidogenesis, and male infertility (Marettová et al. 2015). In addition, in vivo experiments showed that high levels of Cd had cytotoxic and genotoxic effects on peripheral blood and bone marrow cells of rats (Çelik et al. 2009; Curcic et al. 2017; Aly et al. 2018). Anemia has been suggested as a risk factor for increased hepatic and renal Cd accumulation after oral Cd intake (Min et al. 2008). Many studies reported the association between Cd intoxication and a biologically relevant inhibition of white blood cell function or formation (Colacino et al. 2014; El-Boshy et al. 2015).
It is well known that Cd exposure results in generation of large amount of reactive oxygen species (ROS) which may contribute to oxidant/antioxidant imbalance, inflammation, membrane protein damage, altered gene expression, and various pathological conditions in humans and animals (Stohs et al. 2001). Therefore, the effects of natural antioxidants such as caffeic acid phenethyl ester (Kobroob et al. 2012) and proanthocyanidins (He et al. 2018) were tested in animal models and demonstrated cytoprotection against Cd-mediated oxidative insult. Quercetin (QUE) is classified as one flavonoid antioxidant of the five subclasses and major dietary flavonoids distributed in both cultivated and wild plants (D'Andrea 2015). The family members of flavonoids include flavones, flavonol, isoflavones, flavanones, flavanonol, flavanol, and anthocyanidin. Flavonols are represented abundantly by QUE (Shahidi and Ambigaipalan 2015). QUE is found in dietary plants like vegetables, onion, broccoli kale and fruits, red grapes, cherries, and blueberries (D'Andrea 2015; Ozgen et al. 2016). QUE is considered as a phytochemical that can modulate different mitochondrial pathways (de Oliveira et al. 2016) and has pivotal role in treatment of diabetes, cancers, and some cardiovascular diseases (Ozgen et al. 2016). Although the antioxidant capacity of QUE has been tested for providing protection to germ cells against the adverse effects of Cd (Bu et al. 2011; Farombi et al. 2012; Kanter et al. 2016; Nna et al. 2017; Ujah et al. 2018), there is a scarcity of information in literature regarding the effect of QUE on Cd-induced thyrotoxicity. Also, the role of QUE in protecting the hematological parameters of Cd-treated animals is still not well elucidated. In view of these considerations, the current study was designed to evaluate the possible ameliorative role of QUE on Cd-induced hematological disorders of adult male rats and its potential as a protector of the thyroid function abnormalities. Furthermore, we also investigated the contribution of QUE in Cd-induced sperm defects and testicular pathology.
Materials and methods
Chemicals
CdCl2 and QUE were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of the highest analytical quality.
Animals and experimental design
A total of 24 male albino rats, weighing 180–200 g (3 months old) were used in the present study. The experimental protocol conforms to the ethical standards of the National Institutes of Health (NIH Publication No. 85-23, revised 1996). Rats were housed under standard laboratory conditions. They were given rat chow and water ad libitum. After 2 weeks of acclimatization, animals were divided into four equal groups (n = 6 in each group) as follow:
-
Group 1 served as control (CTRL) and received dimethyl sulfoxide (DMSO)/saline (0.5 ml per rat), with the final concentration of 1% DMSO (vehicle).
-
Group 2 was treated with CdCl2 (5-mg/kg b.w.).
-
Group 3 was treated with QUE (50-mg/kg b.w.).
-
Group 4 was treated at same time with CdCl2 (5-mg/kg b.w.) in combination with QUE (50-mg/kg b.w.).
Rats were orally (i.e., orogastric gavage) administered by their respective doses every day for 4 weeks on the basis of a previous study by Renugadevi and Prabu (2010).
Blood collection
At the end of the experimental period, animals were sacrificed and trunk blood samples were collected for analysis. The collected blood from each animal was divided into two parts. Ten percent EDTA was added to one part for the evaluation of Cd level and hematological parameters while the second portion was centrifuged at 5000 rpm for 10 min; the separated sera were subjected to different biochemical analysis.
Assay for Cd concentration in serum, thyroid gland, and testis
The concentration of Cd was assessed in serum, thyroid gland, and testis by spectrophotometric method as previously described (Jin et al. 2000).
Hematological analysis
In the whole blood, hemoglobin content (Hb) was determined using a commercial kit according to supplier’s protocol (Biodiagnostics, Cairo, Egypt). Packed cell volume (PCV), white blood cell (WBC) and red blood cell (RBC) counts were determined according to reported methods (Faulkner and King 1970).
Estimation of serum hormonal concentrations
Thyroid hormones were determined by measuring the serum levels of thyroxine (T4), triiodothyronine (T3), free thyroxine (fT4), free triiodothyronine (fT3), and thyroid-stimulating hormone (TSH). T4 was determined following the protocol described in T4 ELISA kit (Cat. No. 60863), Kamiya Biomedical Company, WA, USA. The assay was relied on the competitive enzyme immunoassay technique utilizing a monoclonal anti-T4 antibody and a T4-HRP conjugate. The developed color was measured at 450 nm in a microplate reader (Elisa Microplate Reader (xl8), BioTeck, Bad Friedrichshall, Germany). The data of T4 were measured as ng/ml. The sensitivity of the assay was 1.0 ng/ml, and coefficient of variance (CV) was less that 10%. T3 was estimated using a solid-phase competitive ELISA kit (Cat. No. T3043T-100) obtained from CalbioTech Inc., Spring Valley, Canada. The T3 values were expressed as pg/dl. Levels of fT4 and fT3 were determined by using ELISA kits, Cat. No. MB S700784, 1640, respectively (Alpha Diagnostic Intl. TX, USA). The assays employed the competitive inhibition enzyme immunoassay technique. Values of fT4 and fT3 were expressed as pg/ml. The sensitivity of the assay was less than 1.08 pmol/l for fT4 and 0.3 pg/ml for fT3. The intra- and inter-assay CV was <15% for both fT4 and fT3. TSH was estimated using ELISA kit (Cat. No. CSB-E05115r) obtained from CUSABIO Biotech Co., LTD, Wuhan, China. The records of TSH were given in μIU/ml. The detection range was 0.78–50 μIU/ml, and no significant cross-reactivity or interference was observed with this assay.
Serum levels of testosterone, LH, and FSH were detected according to Sakuma (2009), Shioya and Wakabayashi (1998), and Teerds et al. (1989), respectively. The values of testosterone were expressed as pg/ml whereas LH and FSH values were expressed as ng/ml.
Assessment of sperm concentration, motility, and abnormality
The left testis of each rat was harvested, then minced in pre-warmed saline (37 °C), and the resulted suspension was used in semen analysis. To analyze sperm motility, 1 drop of sperm suspension was placed on a glass slide to analyze 200 motile sperm in 4 different fields. The motility of the sperm was evaluated microscopically within 2–4 min of their isolation from the testis, and data were expressed as percentage motility (Morrissey et al. 1988). The sperms were counted using a hemocytometer following the method of Freud and Carol (1964). The technique Evans and Maxwell (1987) was adopted for sperm abnormality study. Briefly, smears were made by placing a drop from the sperm suspension and one or two drops of the previously warmed (37 °C) eosin–nigrosin stain. The smears were allowed to dry in the air and then examined under the microscope using a high power (100×) oil immersion objective. The normal sperm cells were counted and the percentage calculated.
Light microscopy of the rat testis
Right testis of each experimental animal was fixed in 10% neutral buffered formalin for 24 h, and standard paraffin preparations were stained with conventional hematoxylin and eosin (HE) dye. The testicular tissue was analyzed in random order with standard light microscopy by a pathologist who was unaware as to which group the rat had belonged, and the microscopic scoring was graded on a scale of absence of lesion (−), mild (+), moderate (++), and severe (+++). Also, Johnsen’s tubular biopsy score (JTBS) (Johnsen 1970) was used to evaluate the degree of testicular and spermatogenesis damage in 20 seminiferous tubules (magnification × 400) for each sample. In this respect, a numerical score of 0–10 was assigned to each seminiferous tubule indicating level of germinal epithelial maturation as follow: score (1), no seminiferous epithelium; score (2), Sertoli cells without germ cells; score (3), only spermatogonia; score (4), few spermatocytes; score (5), many spermatocytes; score (6), few early spermatids; score (7), many early spermatids without differentiation; score (8), few late spermatids; score (9), slightly impaired spermatogenesis, many late spermatids; and score (10), full spermatogenesis with regular tubules.
Statistical analysis
All values were expressed as mean ± standard error (SE). Results were analyzed by one-way analysis of variance (ANOVA) followed by LSD post hoc test. In all the cases, statistical significance was accepted when p ≤ 0.05. We used SPSS for Windows (version 17; SPSS Inc., Chicago, IL, USA) for statistical calculations.
Results
Cd accumulation
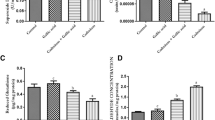
The oral administration of CdCl2 caused a significant increase in Cd level in serum, thyroid gland, and testis compared with the CTRL group. The Cd concentration was also high in Cd+QUE group, but to lesser extent than in Cd-treated rats (Fig. 1).
Hematology
Data in Fig. 2 showed significant decrease in Hb concentration, PCV and RBCs, and WBCs count in the Cd group as compared to the CTRL. But for Cd+QUE group, values of the abovementioned parameters were significantly increased when compared with the Cd group. Specifically, the treatment of Cd-exposed rats with QUE was able to reverse and restore the decrease in PCV and WBCs count to normal levels.
Hormonal measurements
Treating rats with Cd led to a significant decrease in serum T4, T3, fT4, fT3, and TSH when compared to the corresponding CTRL animals (Fig. 3). In fact, thyroid hormone levels of the Cd+QUE group were significantly higher in comparison to the Cd-treated rats but significantly lower when compared to the CTRL (unchallenged) rats.
As can be seen in Fig. 4, Cd administration significantly decreased serum testosterone, LH and FSH when compared to the normal CTRL. Co-treatment by QUE resulted in significant increment in steroid hormone levels versus the Cd-treated rats. Interestingly, simultaneous supplementation of QUE with Cd has shown to restore LH concentration to near the CTRL value.
Sperm quality
Relative to the CTRL, Cd treatment induced a significant decrease in sperm count and sperm motility. While sperm abnormalities increased significantly in response to Cd. However, a significant restoration of sperm parameters was found in animals that received Cd plus QUE when compared to rats treated with Cd alone, although still not reaching the CTRL group levels (Fig. 5).
Testicular histopathology and spermatogenesis
The mean JTBS values and semiquantitative scoring of lesions magnitude for testes in each group are shown in Table 1. The cytoarchitecture of the testicular tissue of CTRL animals exhibited well-preserved seminiferous tubules with active spermatogenesis, as well as prominent interstitial cellularity (Fig. 6a, b). For rats treated with Cd, the affected seminiferous tubules displayed structural atrophy, irregular outlines, spermatogenic dissociation of basal laminae (i.e., desquamation), vacuolization, epithelial gaps, and germ cell destruction (Fig. 6c, d). Moreover, hypocellularity, edema, and congestion were detected in the testicular stroma. In contrast, testes showed better tissue appearance with improved histological findings in the Cd+QUE co-exposed group (Fig. 6e, f). The histological data revealed normal germinal epithelium with considerable numbers of sperms in the seminiferous tubules in rats treated with QUE alone (Suppl. Fig. 1).
Hematoxylin and eosin staining of testicular tissue of different experimental groups. a, b CTRL viewing normal structure of seminiferous tubules with complete spermatogenic series and interstitial tissue. c, d Cd group demonstrating testicular disruption. e, f Cd+QUE group indicating the improvement in the seminiferous tubule structure. Abbreviations/labels inside the image: ST, seminiferous tubules; Sp, spermatozoa; IT, interstitial tissue; arrowheads, atrophied tubules with spermatogenic disturbance; D, desquamation of germ cells; E, interstitial edema; C, congestion; arrows, marked damage of seminiferous tubules. Original magnification × 100 (a, c, e), × 400 (b, d, f)
When testicular spermatogenesis scores (i.e., JTBS) were compared among the groups, the mean JTBS of the Cd group testes was significantly lower than in the CTRL, indicating a substantial maturation arrest or hypospermatogenesis. However, concurrent administration of QUE significantly increased JTBS values over the Cd group.
Discussion
In this study, Cd concentrations significantly increased in blood, thyroid gland, as well as in testis of Cd-treated rats. Cd bioaccumulation had negative impacts on the biological system due to its toxicity at a very low concentration (ATSDR 2012). Previous reports have shown that exposure to Cd causes renal injury (Gonick 2008) thus affecting the rate of elimination of the metal from the body. QUE is considered the most efficient antioxidants of the flavonoids (Ozgen et al. 2016) and recommended for dietary supplements against Cd toxicity (Zhai et al. 2015). According to our results, the combined treatment of Cd+QUE decreased Cd level in serum, thyroid gland, and testis. A probable mechanism for this is through metal chelating ability of QUE (Ravichandran et al. 2014). In addition, recent studies indicated that QUE prevents renal injury in Cd-exposed rats by quenching ROS and/or enhancing cellular antioxidant defenses in the kidneys, thus increasing creatinine, urea, and Cd clearance (Morales et al. 2006; Renugadevi and Prabu 2010). This may partly contribute to decreased Cd accumulation in Cd+QUE-treated rats (Nna et al. 2017).
During the 4-week Cd treatment, we found a significant decrease in the RBCs count, Hb concentration, and PCV. This reflects a sign of anemia related to Cd toxicity. Cd-induced anemia is multifactorial and could be attributed to the following: (1) impairment of erythropoietin formation due to renal failure, (2) inhibition of delta-aminolevulinate dehydratase (δ-ALAD) which is involved in heme biosynthesis, (3) accelerated erythrocyte destruction because of the altered erythrocyte membrane permeability, (4) increased mechanical fragility, and/or (5) failure of the intestinal uptake of Fe because of mucosal lesions (Horiguchi et al. 2011; Dwivedi et al. 2012; Yuan et al. 2014). Furthermore, we observed significant action of Cd, which decreased WBC count. This also was revealed in another study (Simsek et al. 2009). Administration of QUE along with Cd increased the reduced levels of Hb concentration, PCV, and RBCs and WBCs counts, providing protection against Cd-induced hematotoxicity. These findings are supported by the results of Selvakumar et al. (2013) who demonstrated that QUE mitigated the alterations in the RBC, Hb, PCV, and WBC in rats exposed to polychlorined biphenyls. QUE was shown to inhibit RBC hemolysis and oxidative damage to membrane lipoprotein (Kitagawa et al. 2004; Asgary et al. 2005).
The results obtained in this investigation showed that treating rats with Cd induced a decrease in T4, T3, fT4, fT3, and TSH. Similarly, Buha et al. (2013) reported that exposure to low levels of Cd interferes with thyroid function. Cd has been reported to affect synthesis and release of T4 via oxidative phosphorylation disorders in mitochondria of follicular cells (Yoshizuka et al. 1991). Prakash et al. (1997) proposed that thyroid dysfunction under Cd exposure is due to impaired thyroid hormonogenesis under an oxidative stress. Cd also inhibits hepatic peripheral deiodination of T4 to T3 by binding to sulfhydryl groups of 50-monodeiodinase, a seleno-enzyme containing a selenocysteine active site (Matović et al. 2015). It was established that abnormal thyroid hormone levels induce diverse effects on blood cells such as anemia, thrombocytopenia, and leukopenia and even in rare cases cause pancytopenia (in hypothyroidism) (Bremner et al. 2012; Dorgalaleh et al. 2013). Likewise, other blood indices such as MCV, MCH, MCHC, and Hb could change during thyroid dysfunction (Kawa et al. 2010). Furthermore, it is proposed that the prolonged insufficiency of thyroid hormones may be directly related to the impedance of the cellular immune system (Pillay 1998). In this context, studies have suggested that hypothyroidism is associated with a decrease in male sex hormones, abnormal spermatogenesis, and retardation of Sertoli cell differentiation (Krajewska-Kulak and Sengupta 2013; Mohamed and Gawad 2017). T3 hormone increases LH receptors and steroidogenesis of Leydig cells, thus stimulating basal testosterone biosynthesis (Maran et al. 2000). T3 also plays an important role in the regulation of mitochondrial function in several tissues; therefore, hypothyroid condition disturbs intra-mitochondrial glutathione redox status and causes oxidative stress in testis that cannot even be reversed with T3 treatment (Chattopadhyay et al. 2010). Noteworthy, in the present study, QUE showed better improvement of the thyroidal function at the concurrent treatment with Cd. Similarly, earlier study of Nabavi et al. (2011) has verified that QUE may reduce the severity of NaF-induced thyroid dysfunction in male rats.
As expected, the results of the present study clearly demonstrated that oral Cd administration significantly decreased sperm count and motility and increased the percentage of sperm abnormalities compared to CTRL. In addition, it was observed that, in the Cd group, there was a sharp decrease in total serum testosterone, LH, and FSH levels. Cd causes male infertility through alteration in histopathology, sperm characteristics, and oxidant/antioxidant balance (El-Demerdash et al. 2004; El-Neweshy et al. 2013; Djuric et al. 2015). In fact, Cd is likely to substitute Zn2+ in the testicular tissue, which may lead to negative implication on sperm development process (Amara et al. 2008). Cd negatively influences testis structure, by damaging vascular endothelium and blood-testis barrier integrity, and by inducing inflammation and apoptosis within the testis (de Angelis et al. 2017). As a known endocrine disruptor, Cd can modify reproductive hormone levels by affecting the hypothalamic–pituitary–testicular axis (Lafuente 2013). In analyzing the results, it was seen that QUE counteracted the negative effects of Cd on sperm quality and sex hormones production. Other authors have also reported that QUE can attenuate testicular damage in rats following exposure to free-radical promoting agents/drugs such as dioxins (Ciftci et al. 2012), bisphenol A (Jahan et al. 2016), and docetaxel (Altintas et al. 2015). According to Bharti et al. (2014), antioxidant therapy with QUE has the potential to stimulate androgen production, restore redox balance, and prevent germ cell apoptosis subsequently leading to restoration of testicular function in estrogenized rats. Abarikwu et al. (2012) demonstrated that QUE by decreasing atrazine-induced lipid peroxidation and ROS production could promote cell survival in Sertoli/germ cells co-culture. Based on the in vitro study of Hu et al. (2015), QUE is proved to augment Leydig cell viability by downregulating mitochondrial/apoptotic protein-induced by triptolide. Additionally, QUE has high antigenotoxic capacity via diminishing DNA damage of sperm upon in vitro exposure to food mutagens (Anderson et al. 1998).
In conclusion, this study showed that QUE is a powerful/safe phytotherapy to overcome Cd toxicity in rat tissues. On the basis of our results, the regulatory effects of QUE on thyroidal hormones might be the mechanism (at least in part) for its hematological and testicular protective effect against Cd toxicity.
References
Abarikwu SO, Pant AB, Farombi EO (2012) Dietary antioxidant, quercetin, protects Sertoli-germ cell coculture from atrazine-induced oxidative damage. J Biochem Mol Toxicol 26:477–485. https://doi.org/10.1002/jbt.21449
Altintas R, Ciftci O, Aydin M et al (2015) Quercetin prevents docetaxel-induced testicular damage in rats. Andrologia 47:248–256. https://doi.org/10.1111/and.12253
Aly FM, Kotb AM, Haridy MAM, Hammad S (2018) Impacts of fullerene C60and virgin olive oil on cadmium-induced genotoxicity in rats. Sci Total Environ 630:750–756. https://doi.org/10.1016/j.scitotenv.2018.02.205
Amara S, Abdelmelek H, Garrel C et al (2008) Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev 54:129–134. https://doi.org/10.1262/jrd.18110
Anderson D, Dobrzyńska MM, Başaran N et al (1998) Flavonoids modulate comet assay responses to food mutagens in human lymphocytes and sperm. Mutat Res 402:269–277. https://doi.org/10.1016/S0027-5107(97)00306-0
de Angelis C, Galdiero M, Pivonello C et al (2017) The environment and male reproduction: the effect of cadmium exposure on reproductive system and semen quality and its implication in fertility. Reprod Toxicol 73:105–127. https://doi.org/10.1016/j.reprotox.2017.07.021
Asgary S, Naderi G, Askari N (2005) Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp Clin Cardiol 10:88–90
ATSDR (2012) Toxicological Profile for Cadmium
Bharti S, Misro MM, Rai U (2014) Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol Cell Endocrinol 383:10–20. https://doi.org/10.1016/j.mce.2013.11.021
Bremner AP, Feddema P, Joske DJ et al (2012) Significant association between thyroid hormones and erythrocyte indices in euthyroid subjects. Clin Endocrinol. https://doi.org/10.1111/j.1365-2265.2011.04228.x
Bu T, Mi Y, Zeng W, Zhang C (2011) Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat Rec 294:520–526. https://doi.org/10.1002/ar.21317
Buha A, Antonijević B, Bulat Z, Jaćević V, Milovanović V, Matović V (2013) The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol Lett 221:83–90. https://doi.org/10.1016/j.toxlet.2013.06.216
Buha A, Matovic V, Antonijevic B, et al (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 19(5):1501. https://doi.org/10.3390/ijms19051501
Çelik A, Büyükakilli B, Çimen B et al (2009) Assessment of cadmium genotoxicity in peripheral blood and bone marrow tissues of male Wistar rats. Toxicol Mech Methods 19:135–140. https://doi.org/10.1080/15376510802354979
Chattopadhyay S, Choudhury S, Roy A et al (2010) T3fails to restore mitochondrial thiol redox status altered by experimental hypothyroidism in rat testis. Gen Comp Endocrinol 169:39–47. https://doi.org/10.1016/j.ygcen.2010.07.014
Ciftci O, AydinM OI, Vardi N (2012) Quercetin prevents 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia 44:164–173. https://doi.org/10.1111/j.1439-0272.2010.01126.x
Colacino JA, Arthur AE, Ferguson KK, Rozek LS (2014) Dietary antioxidant and anti-inflammatory intake modifies the effect of cadmium exposure on markers of systemic inflammation and oxidative stress. Environ Res 131:6–12. https://doi.org/10.1016/j.envres.2014.02.003
Curcic M, Buha A, Stankovic S et al (2017) Interactions between cadmium and decabrominated diphenyl ether on blood cells count in rats—multiple factorial regression analysis. Toxicology 376:120–125. https://doi.org/10.1016/j.tox.2016.05.011
D'Andrea G (2015) Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 106:256–271. https://doi.org/10.1016/j.fitote.2015.09.018
Djuric A, Begic A, Gobeljic B et al (2015) Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem Toxicol 86:25–33. https://doi.org/10.1016/j.fct.2015.09.004
Dorgalaleh A, Mahmoodi M, Varmaghani B et al (2013) Effect of thyroid dysfunctions on blood cell count and red blood cell indice. Iran J Pediatr Hematol Oncol 3:73–77. https://doi.org/10.1155/2013/385940
Dwivedi VK, Bhatanagar A, Chaudhary M (2012) Prevention of cadmium toxicity by ceftriaxone plus sulbactam with VRP1034 in rats. J Drug Metab Toxicol 3:130. https://doi.org/10.4172/2157-7609.1000130
El-Boshy ME, Risha EF, Abdelhamid FM et al (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol. https://doi.org/10.1016/j.jtemb.2014.05.009
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol 42:1563–1571. https://doi.org/10.1016/j.fct.2004.05.001
El-Neweshy MS, El-Maddawy ZK, El-Sayed YS (2013) Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia 45:369–378. https://doi.org/10.1111/and.12025
Evans G, Maxwell WMC (1987) Handling and examination semen. In: Maxwell WMC (ed) Salamon’s artificial insemination of sheep and goat. Butterworths, Sydney, pp 93–106
Farombi EO, Adedara IA, Akinrinde SA et al (2012) Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia 44(4):273–284. https://doi.org/10.1111/j.1439-0272.2012.01279.x
Faulkner WR, King JW (1970) Manual of clinical laboratory procedures. Published by the chemical rubber company, Cleveland
Freud M, Carol B (1964) Factors affecting haemocytometer count of sperm concentration in human semen. J Report Fertil 8:149–155
Gonick HC (2008) Nephrotoxicity of cadmium and lead. Indian J Med Res 128:335–352
He L, Li P, Yu LH et al (2018) Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ Toxicol Pharmacol 57:1–8. https://doi.org/10.1016/j.etap.2017.11.002
Horiguchi H, Oguma E, Kayama F (2011) Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol Sci 122:198–210. https://doi.org/10.1093/toxsci/kfr100
Hu J, Yu Q, Zhao F et al (2015) Protection of quercetin against triptolide-induced apoptosis by suppressing oxidative stress in rat Leydig cells. Chem Biol Interact 240:38–46. https://doi.org/10.1016/j.cbi.2015.08.004
Jahan S, Ain QU, Ullah H (2016) Therapeutic effects of quercetin against bisphenol a induced testicular damage in male Sprague Dawley rats. Syst Biol Reprod Med 62:114–124. https://doi.org/10.3109/19396368.2015.1115139
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208. https://doi.org/10.1016/j.taap.2009.04.020
Jin G, Kan J, Zhu Y, Lei N (2000) Spectrophotometric determination of cadmium (II) using the chromogenic reagent 4-(o-diazoaminophenylarsonic acid) azobenzene. Indian J Chem Sect A 39:1227–1230
Johnsen SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Horm Res Paediatr 1:2–25. https://doi.org/10.1159/000178170
Kanter M, Aktoz T, Aktas C, Ozen F, Yarali O, Kanter B (2016) Role of quercetin in cadmium-induced oxidative stress, testicular damage, and Apoptosis in Rats. Anal Quant Cytopathol Histpathol 38(1):45–51
Kawa MP, Grymuła K, Paczkowska E et al (2010) Clinical relevance of thyroid dysfunction in human haematopoiesis: biochemical and molecular studies. Eur J Endocrinol. https://doi.org/10.1530/EJE-09-0875
Kitagawa S, Sakamoto H, Tano H (2004) Inhibitory effects of flavonoids on free radical-induced hemolysis and their oxidative effects on hemoglobin. Chem Pharm Bull 52(8):999–1001
Kobroob A, Chattipakorn N, Wongmekiat O (2012) Caffeic acid phenethyl ester ameliorates cadmium-induced kidney mitochondrial injury. Chem Biol Interact 200:21–27. https://doi.org/10.1016/j.cbi.2012.08.026
Krajewska-Kulak E, Sengupta P (2013) Thyroid function in male infertility. Front Endocrinol (Lausanne) 4:174. https://doi.org/10.3389/fendo.2013.00174
Lafuente A (2013) The hypothalamic-pituitary-gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem Toxicol 59:395–404. https://doi.org/10.1016/j.fct.2013.06.024
Maran RRM, Arunakaran J, Aruldhas MM (2000) T3 directly stimulates basal and modulates LH induced testosterone and Oestradiol production by rat Leydig cells in vitro. Endocr J 47:417–428. https://doi.org/10.1507/endocrj.47.417
Marettová E, Maretta M, Legáth J (2015) Toxic effects of cadmium on testis of birds and mammals: a review. Anim Reprod Sci 155:1–10. https://doi.org/10.1016/j.anireprosci.2015.01.007
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. https://doi.org/10.1016/j.fct.2015.02.011
Min KS, Iwata N, Tetsutikawahara N et al (2008) Effect of hemolytic and iron-deficiency anemia on intestinal absorption and tissue accumulation of cadmium. Toxicol Lett 179:48–52. https://doi.org/10.1016/j.toxlet.2008.04.001
Mohamed NA, Gawad HSA (2017) Taurine dietary supplementation attenuates brain, thyroid, testicular disturbances and oxidative stress in streptozotocin-induced diabetes mellitus in male rats. Beni-Suef Univ J Basic Appl Sci 6:247–252. https://doi.org/10.1016/j.bjbas.2017.04.006
Morales AI, Vicente-Sánchez C, Sandoval JMS et al (2006) Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol 44:2092–2100. https://doi.org/10.1016/j.fct.2006.07.012
Morrissey RE, Schwetz BA, Lamb JC et al (1988) Evaluation of rodent sperm, vaginal cytology, and reproductive organ weight data from national toxicology program 13-week studies. Toxicol Sci 11(2):343–358. https://doi.org/10.1093/toxsci/11.1.343
Nabavi SF, Moghaddan AH, Nabavi SM, Eslami S (2011) Protective effects of curcumin and quercetin on thyroid function in sodium fluoride intoxicated rats. Fluoride 44:147–152
Nie X, Chen Y, Chen Y et al (2017) Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ Pollut 230:320–328. https://doi.org/10.1016/j.envpol.2017.06.052
Nna VU, Ujah GA, Mohamed M et al (2017) Cadmium chloride–induced testicular toxicity in male wistar rats; prophylactic effect of quercetin, and assessment of testicular recovery following cadmium chloride withdrawal. Biomed Pharmacother 94:109–123. https://doi.org/10.1016/j.biopha.2017.07.087
de Oliveira MR, Nabavi SM, Braidy N et al (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. https://doi.org/10.1016/j.biotechadv.2015.12.014
Ozgen S, Kilinc OK, Selamoğlu Z (2016) Antioxidant activity of quercetin: a mechanistic review. Turkish J Agric - Food Sci Technol 4:1134–1138. https://doi.org/10.24925/turjaf.v4i12.1134-1138.1069
Pillay K (1998) Congenital hypothyroidism and immunodeficiency: evidence for an endocrine-immune interaction. J Pediatr Endocrinol Metab 11:757–761. https://doi.org/10.1515/JPEM.1998.11.6.757
Prakash P, Kumar PG, Laloraya M et al (1997) Superoxide anion radical production as a cadmium-mediated mechanism of toxicity in avian thyroid: an electron spin resonance study by spin trapping. Comp Biochem Physiol - C Pharmacol Toxicol Endocrinol 118:89–95. https://doi.org/10.1016/S0742-8413(97)00082-0
Ravichandran R, Rajendran M, Devapiriam D (2014) Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem 146:472–478. https://doi.org/10.1016/j.foodchem.2013.09.080
Renugadevi J, Prabu SM (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62:471–481. https://doi.org/10.1016/j.etp.2009.06.006
Sakuma Y (2009) Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol 21:410–414. https://doi.org/10.1111/j.1365-2826.2009.01856.x
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118:182–190. https://doi.org/10.1289/ehp.0901234
Selvakumar K, Bavithra S, Suganya S, et al (2013) Effect of quercetin on haematobiochemical and histological changes in the liver of polychlorined biphenyls-induced adult male Wistar rats. J Biomarkers 2013:960125. https://doi.org/10.1155/2013/960125
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects—a review. J Funct Foods 18:820–897. https://doi.org/10.1016/j.jff.2015.06.018
Shioya N, Wakabayashi K (1998) In vivo bioactivities and kinetic parameters of rat luteinizing hormone components: discrepancy between in vitro and in vivo assays. Endocr J 45:307–314
Simsek N, Karadeniz A, Kalkan Y et al (2009) Spirulina platensis feeding inhibited the anemia- and leucopenia-induced lead and cadmium in rats. J Hazard Mater 164:1304–1309. https://doi.org/10.1016/j.jhazmat.2008.09.041
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20:77–88. https://doi.org/10.1615/JEnvironPatholToxicolOncol.v20.i2.10
Teerds KJ, Closset J, Rommerts FFG et al (1989) Effects of pure FSH and LH preparations on the number and function of Leydig cells in immature hypophysectomized rats. J Endocrinol 120:97–106. https://doi.org/10.1677/joe.0.1200097
Ujah GA, Nna VU, Agah MI, et al (2018) Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male Wistar rats. Andrologia 50(2):e12866. https://doi.org/10.1111/and.12866
Yoshizuka M, Mori N, Hamasaki K et al (1991) Cadmium toxicity in the thyroid gland of pregnant rats. Exp Mol Pathol 55:97–104. https://doi.org/10.1016/0014-4800(91)90021-O
Yuan G, Dai S, Yin Z et al (2014) Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem Toxicol 65:260–268. https://doi.org/10.1016/j.fct.2013.12.041
Zhai Q, Narbad A, Chen W (2015) Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 7:552–571. https://doi.org/10.3390/nu7010552
Funding
This work is financially supported by the Deanship of Scientific Research at King Faisal University (Saudi Arabia) under grant no. 17122008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Suppl. Figure 1
Hematoxylin and eosin staining of testicular tissue of QUE group revealing normal testicular architecture. Abbreviations/labels inside the image: ST, seminiferous tubules; Sp, spermatozoa; IT, interstitial tissue. Original Magnification: 100x (a), 400x (b). (PDF 132 kb)
Rights and permissions
About this article
Cite this article
Badr, G.M., Elsawy, H., Sedky, A. et al. Protective effects of quercetin supplementation against short-term toxicity of cadmium-induced hematological impairment, hypothyroidism, and testicular disturbances in albino rats. Environ Sci Pollut Res 26, 8202–8211 (2019). https://doi.org/10.1007/s11356-019-04276-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04276-1