Abstract
Oxyfluorfen (Goal 24%EC) herbicide is widely used in agriculture for weed control. Biomphalaria alexandrina snails can be used as bioindicator of the chemical pollution in the aquatic environment. The objective of this study was to evaluate the molluscicidal activity of this herbicide on Biomphalaria alexandrina snails and how it affected its biological system. The present study revealed a molluscicidal effect of oxyfluorfen 24%EC on these snails at LC50 5.9 mg/l. After exposure of snails to the sub-lethal concentrations (LC0, LC10, or LC25) of this herbicide, the survival rates, reproductive rate (R0), and fecundity (Mx) of adult B. alexandrina snails were significantly decreased in comparison with the control group. Also, levels of testosterone and estradiol were decreased significantly. It caused alterations in the antioxidant system, where exposure to sub-lethal concentration of this herbicide caused significant increases in levels of lipid peroxide malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD), while it significantly decreased glutathione transferase (GST). Histopathological changes in the digestive gland included severe damage in the digestive cells, where, they lost their tips and some were degenerated, while the secretory cells increased in number. Regarding the hermaphrodite gland, there were losses of the connective tissues, irregular sperms, and the eggs degenerated. These findings concluded that B. alexandrina snails can be used as a bioindicator for pollution with pesticide in the aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are extensively used for agricultural purposes worldwide to reduce pests. These pesticides and their residues found their way to the aquatic environment through water drainage system and lead to toxicological effects on aquatic ecosystems (Moustafa et al. 2016).
Herbicides made up about 40% of the production of pesticides in the world and were intensively used in agriculture (Abdel-Ghaffar et al. 2016). In Egypt, these herbicides that enter the Nile River through runoff from agricultural fields result in the pollution of aquatic habitat, inducing adverse impacts on the living organisms with potential direct and indirect impacts on human health (Hassanein 2002). Globally, glyphosate is the most used herbicide where it is used for inhibition of unwanted weeds in agriculture and also used for aquatic weed control in fish ponds (Tsui and Chu 2008). The 24-h LC50 of Roundup (a glyphosate herbicide) for young Nile Oreochromis niloticus was 17.5 g/l and that for adult tilapia was 46.9 mg/l (Vera-Candioti et al. 2012). Also, the median lethal concentration (LC50) of this herbicide for mosquito fish (Gambusia affinis) was 17.82 mg/l for 72 h (Talib et al. 2018).
Oxyfluorfen (Goal 24%EC) is a diphenyl-ether herbicide and ranks as a light-dependent peroxidizing herbicide (LDPH) used for controlling of monocotyledonous and broadleaf weeds in fields with grapes, onion, or cotton through interference with chlorophyll production, inhibition of photosystem II, and electron transport disrupting photosynthesis (Banhawy et al. 1996). This herbicide was widely used in Egypt and its concentration reached 23.6 mg/l in some branches of the River Nile, and that concentration could adversely affect the nervous system of Gambusia affinis and Oreochromis niloticus fishes and caused reductions in the brain acetylcholinesterase activity after 6, 15, and 30 days of exposure (Hassanein 2002). Also, other studies on freshwater invertebrates as Elliptio complanata clam (Godfrey and Longacre 1990), Eastern oyster, Crassostrea virginica, Palaemonetes pugio, and Daphnia magna investigated the toxic effect of oxyfluorfen herbicide on their nervous, immune, and endocrine systems after exposure either to short- or long-term treatment that led to hyperexcitability and loss of equilibrium (EPA/OPP 2001).
Invertebrates included over 90% of the aquatic species and they were used as biomonitors of environmental pollution (Ibrahim et al. 2018). B. alexandrina snails are a remarkable species that serve as sensitive bioindicators for aquatic ecosystem pollution (Fahmy et al. 2014). It is the snail vector for Schistosoma mansoni in Egypt, distributed throughout the Nile River and in the irrigation canals that are highly polluted. Sharaf-El-Din et al. (2010) proposed that B. alexandrina snails as biomonitors for metal pollution and the snail oviposition have shown to be sensitive to pollution in the surrounding environment. So such snails were used in this study as bioindicators of aquatic pollution because of their abundance, wide geographical distribution, and short lifespan.
Antioxidant enzymes were used as a biomarker for metal and inorganic compound pollution in marine and freshwater organisms. The oxidative stress is the major mechanism through which several pesticides induce their effects producing pro-oxidants in cells (Tellez-Bañuelos et al. 2009). Such stress may be accompanied by developmental alterations, including reproductive effects and teratogenicity in aquatic organisms (Pašková et al. 2011). The extent of alterations depends on the cell’s antioxidant network represented by the antioxidant enzymes and the non-enzymatic antioxidant molecules (Tilton et al. 2008).
Testosterone hormone is required in the gonadal development in B. alexandrina and might be used as a biomarker for insecticide toxicity, so B. alexandrina snails could be used as a bioindicator for endocrine disruption in terms of steroid levels (testosterone and 17β-estradiol), after exposure to sub-lethal concentrations of herbicides (Omran and Salama 2016).
The aim of the present study is to assess the toxic effect of oxyfluorfen 24%EC herbicide on the biological functions of freshwater B. alexandrina snails through studying its deleterious effects on the reproductive, oxidative enzymes, endocrine systems, and histopathology.
Materials and methods
Snails
Mature B. alexandrina (Ehrenberg, 1831) snails (8–10 mm) were maintained in Medical Malacology Laboratory, Theodor Bilharz Research Institute (TBRI), Giza, Egypt. Snails were kept in plastic aquaria (16 × 23 × 9 cm). The aquaria were provided with dechlorinated aerated tap water (10 snails/l), pH 7 ± 0.2 and temperature (25 ± 2 °C) and covered with glass plates and the photoperiod was controlled (12 h/12 h) with two 48 W fluorescent lamps as the light source. Water hardness was optimized with 30 mg/l calcium carbonate and CaCO3 (small pieces of chalk) for the greatest snail fecundity, growth, and shell length (Eveland and Haseeb 2011). Water in the aquaria was changed weekly. Snails were fed with oven-dried lettuce leaves, blue-green algae (Nostoc muscorum), and Tetramin (Fish food). Small pieces of 3 × 5 cellophane sheets that float in aquaria and snails deposit egg masses on them under surface; the sheets are easily transferred to other aquaria.
Herbicide
Goal 24%EC (CAS: 42874-03-3) is a commercial herbicide and has the active constituents called oxyfluorfen 24%EC. Oxyfluorfen is a nitrodiphenyl ether herbicide [2-chloro-1-(3-ethoxy-4- nitrophenoxy)-4-(trifluoromethyl) benzene] and has a similar structure to that of nitrofen and acifluorfen (Keum et al. 2008). It is readily soluble in most organic solvents but emulsifies into water and has a high tendency to bind to soil containing organic matter. It is stable up to 50 °C and it degrades primarily via photolysis (EPA/OPP 2001). Its registration number is 190 and was purchased from Dow Agro Sciences-France.
Investigation of molluscicidal activity of oxyfluorfen 24%EC
A fresh stock solution of 1000 ppm was prepared from oxyfluorfen 24%EC on the basis of V/V using dechlorinated tap water. Series of concentrations (10, 8, 6, 4, 3, 2, 1 mg/l) were prepared to calculate LC50 and LC90 (WHO 1983). One hundred fifty snails were exposed to 24 h of the herbicide followed by another 24 h for recovery and then, mortality rate and lethal doses were recorded for each group. Another snail group of the same size was dipped in dechlorinated water only as control (30 snails). Three replicates were used each of 10 snails for each concentration and the control group. The percentages of observed mortalities were recorded and analyzed (Litchfield Jr and Wilcoxon 1949). LC0 is the concentration of any toxicant, below which no measurable effects take place (Warren 1900), and is estimated as 1/10 LC50 value (WHO 1965).
Experimental design
One hundred B. alexandrina snails (8–10 mm) were used in this experiment, where 10 snails in each aquarium exposed to each sub-lethal concentration of oxyfluorfen 24%EC at LC0 (0.016 mg/l), LC10 (3.14 mg/l), or LC25 (4.48 mg/l) for 24 h (exposure), followed by another 24 h of recovery, and this method is done for 2 weeks and followed by 2 weeks of recovery. Thirty unexposed snails (control) were assayed side by side with the treated groups, and then the following bioassays were done:
Effect on snails’ egg-laying capacity of adult snails
Both the survival and reproductive rates (Lx and R0) of B. alexandrina snails (8–10 mm) that were subjected to each concentration (LC0 (0.016 mg/l), LC10 (3.14 ppm), or LC25 (4.48 mg/l)) of the herbicide and control group were weekly recorded. The egg laying capacity is expressed in the form (Mx) which is the number of eggs/snail/week, and this is determined by dividing the total number of laid eggs in any week by the total number of living snails at the beginning of this week (El-Gindy et al. 1965). To calculate the net reproductive rate (R0) of the snails throughout the experimental period, the following parameters will be considered:
-
Time of exposure in weeks (x).
-
Survivorship (Lx) which is the survived snails at any given week as a fraction of the correct one (1.0 = 100%).
-
Fecundity (Mx) the mean number of eggs/snail/week.
-
The net reproductive rate (R0) at any given period was represented by the term Σ LxMx.
Tissue preparation
From each treated group and the control one, snails were crushed between two slides, and their soft tissues were withdrawn from the shell using a forceps, weighed, and then homogenized in ice cold, twice-distilled water. One-gram tissue of each group/10 ml water was homogenized using a glass Dounce homogenizer. The homogenates were centrifuged at 3000 rpm for 10 min and the supernatants were stored at − 80 °C until used.
Investigation of the antioxidant system responses: the enzymatic responses (superoxide dismutase, catalase), non-enzymatic responses (glutathione), and the oxidative stress marker (malondialdehyde)
These responses were detected in the supernatant of the tissue homogenate for each group. Biodiagnostic kits (Biodiagnostic Dokki, Giza, Egypt) were used for the determination of superoxide dismutase (SOD) and catalase (CAT) (Aebi 1984). In addition, tissue malondialdehyde (lipid peroxide) was done according to Ohkawa et al. (1979), and reduced glutathione (GSH) was done according to the method of (Beutler 1963).
Hemolymph preparation
The hemolymph of some surviving snails from each group at second week of exposure was collected by removing a small portion of the shell and inserting a capillary tube into the heart to collect the hemolymph (Nduku and Harrison 1980). Then, hemolymph was pooled in a glass vial tube (1.5 ml) and stored at − 80 °C until used for the hormonal assay (17β-estradiol and testosterone).
Investigation of testosterone and 17β-estradiol hormones in hemolymph
Hormone concentrations (T and E) were assayed in hemolymph for all groups according to the manufacturer instructions of T EIA kit (Enzo Life Science, MI, USA, ADI-900-065) and E EIA kit (Cayman Chemical Company, MI, USA, item no. 582251).
Histological studies
The digestive and hermaphrodite glands of the surviving B. alexandrina snails after exposure to each sub-lethal concentrations for 24 h followed by another 24 h of recovery for 2 weeks (exposure) were removed from their shells, fixed in Bouin’s fluid, embedded in paraffin wax, and sectioned and stained with hematoxylin and eosin (Mohamed and Saad 1990).
Statistical analysis
Statistical Processor System Support “SPSS” version 20 for Windows software was used for the statistics. Probit analysis was used to define the lethal concentration values (Finney 1971). Statistical analysis was evaluated by one-way analysis of variance (ANOVA) and Duncan’s test to assess the significance of differences among control and treated groups. Values were expressed as mean ± S.E.
Results
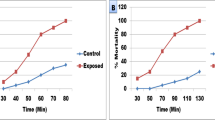
The molluscicidal activity of the tested herbicide Goal 24%EC (oxyfluorfen 24%EC) against adult B. alexandrina snails after 24 h of exposure followed by another 24 h for recovery is represented in (Table 1 and Fig. 1). These results show that Goal 24%EC was toxic to B. alexandrina snails at (LC50 5.96 mg/l; LC90 8.78 mg/l).
The present results (Table 2) indicated that the survival rate of snails exposed to LC0 (0.016 mg/l) for 24 h/ week for 2 weeks followed by another 2 weeks of recovery was slightly affected, being 0.65 at the 4th week of exposure. Also, exposure of snails to LC10 (3.14 mg/l) considerably reduced their survival rate (Lx) to be 0.25 after the 2 weeks of recovery, i.e., at the 4th week of the experiment compared to 0.75 for the control group. Raising the concentration to LC25 (4.48 mg/l) caused a quick and severe death among treated snails throughout the experiment weeks.
The present data (Table 2) indicated that snails exposed for 24 h/week to LC0 (0.016 mg/l) of Goal 24%EC negatively affected their fecundity (Mx). Thus, the snails survived at this concentration laid few eggs/snail/week through the weeks of the experiment in comparison with that of control ones. Raising the concentration to LC10 (3.14 mg/l) highly suppressed the snail’s fecundity at the 2nd and 3rd weeks, compared with control snail. Moreover, at LC25, the survived snails stopped laying egg through the 3rd week until they died at the 4th week.
In vivo exposure of B. alexandrina snails to sub-lethal concentrations of oxyfluorfen herbicide (LC0, LC10, or LC25) exhibited a significant increase (p < 0.05) in malondialdehyde (MDA) and this increase was concentration-dependent. Also, there were significant increases (P < 0.05) in CAT and SOD activities compared with the control group. The highest increase in CAT activity was observed in the snails exposed to LC10 of oxyfluorfen. Referring to SOD activity, there was a significant increase in snails exposed to LC10 and LC25 but a non-significant increase following the exposure to LC0. On the other hand, tissue GSH content was significantly (p < 0.05) decreased after oxyfluorfen exposure in a concentration-dependent manner (Fig. 2).
Antioxidant activity changes (lipid peroxides malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione transferase (GST) in tissue of B. alexandrina after exposure to sub-lethal concentrations of oxyfluorfen herbicide (LC0, LC10, or LC25). Values are mean ± S.E, n = 5 in each group, *p < 0.05: significant, **p < 0.01: highly significant as compared with control
Testosterone (T) and 17β-estradiol (E) were determined in the ovotestes of oxyfluorfen-exposed snails after 2 weeks of exposure. Levels of T and E were decreased significantly (p < 0.05) after exposure to sub-lethal concentrations compared with control (Fig. 3).
Regarding its histopathological changes in adult, treated snails, the normal digestive gland occupies a considerable part of the visceral hump and consists of a number of tubular glands; each tubule is lined with one layer of two cells; the digestive cells and the secretory cells (Fig. 4a). The present results showed that a moderately pathological damage in the digestive gland after exposure to LC0 of the herbicide (Fig. 4b), while at LC10, the connective tissue was broken down so the intertubular space becomes distinctive around the digestive tubules and the lumen inside tubule increased (Fig. 4c). The most destructive effect in the digestive tissue was shown with snails subjected to LC25 (Fig. 4d), where there is a great loss of identical shape of digestive cells (syncytium).
Light micrograph showing sections in digestive gland of snails exposed to sub-lethal concentration of oxyfluorfen 24%EC for 2 weeks (H&E) (× 40). a Normal digestive gland of snails. b Snails subjected to LC0 of tested herbicide. c Snails subjected to LC10. d Snails subjected to LC25 of the herbicide. DDG degenerated digestive cells, IT inter tubular space, DSC degenerated secretory cells, TG tubular gland, L lumen, SC secretory cells, VDC vacuolated digestive cell, RDC ruptured digestive cells
The hermaphrodite gland of the normal B. alexandrina snails is composed of a number of cub-shaped acini connected by connective tissue. The male reproductive cells are differentiated into primary and secondary spermatocytes. The fully developed sperms were observed either in the lumen or attached to Sertoli cell. While the female oogenic cells filled the acinar lumen as primary, secondary oocytes and mature ova (Fig. 5e). The treatment of B. alexandrina snails with oxyfluorfen 24%EC at the sub-lethal concentrations for 2 weeks has marked morphological changes to gland cells in addition to several evacuations of its tubules from gametogenic stages, but still presents a mature ovum and little sperms in snails treated with LC0 (Fig. 5f), while in snails treated with LC10, there are increases in degenerated sperms and ova (Fig. 5g). The great damage to gonadal cells occurs by subjecting snails to LC25 (Fig. 5h), where ova lost their shape and degenerated and sperms are reduced and degenerated. Most of the spermatogenic and oogenic stages disappeared.
Light micrograph showing sections in hermaphrodite glands of snails exposed to sub-lethal concentration of oxyfluorfen 24%EC for 2 weeks (H&E) (× 40): e Normal hermaphrodite gland of snails. f Snails subjected to LC0 of tested herbicide. g Snails subjected to LC10. h Snails subjected to LC25 of tested herbicide. MO mature ovum, SP sperms, SPR spermatocytes, OC oocyte, DO degenerated ovum, DSP degenerated sperms, DSPR degenerated spermatocyte, V vacuole
Discussion
Pesticides are a double-edged sword as they have benefits for agriculture, but they can harm aquatic life and wildlife if used incorrectly. Pesticides and their residues can be easily transferred into aquatic environments through the surface runoff and may alter the development and reproduction in various aquatic invertebrates (Pašková et al. 2011). These pollutants are known to modulate antioxidant defensive systems and to cause oxidative damage in aquatic organisms by the production of reactive oxygen species (ROS) (Fahmy and Sayed 2017).
To be a molluscicidal material, the median lethal concentration (LC50) should not be more than 100 ppm (WHO 1993). The present investigation indicated that oxyfluorfen 24%EC has LC50 5.9 mg/l on B. alexandrina snails. This toxic effect of oxyfluorfen 24%EC herbicide was also investigated after exposure of Crassostrea virginica, Palaemonetes pugio, and Daphnia magna to either short- or long-term treatment, where it caused neurotoxicity and an increase in acetyl cholinesterase concentration (EPA/OPP 2001) and has toxic effects on nervous, immune, and endocrine systems of freshwater invertebrates as Elliptio complanata clam (Godfrey and Longacre 1990).
The present results showed that survival rates of adult B. alexandrina snails were markedly reduced post their exposure to sub-lethal concentrations (LC0, LC10, or LC25) of oxyfluorfen 24%EC herbicide. Comparable perceptions were recorded by Abdel-Ghaffar et al. (2016), who reported that survival rates of B. alexandrina snails were reduced after their exposure to the sub-lethal concentrations (LC0, LC10, or LC25) butralin 48% EC, glyphosate isopropylammonium 48% SL, and pendimethalin 50% EC herbicides. They linked this reduction by histological examinations, which showed severe damage in the digestive and hermaphrodite gland cells of the treated snails. The same results obtained by Barky et al. (2012), who showed that LC10 of two pesticides Atrazine (0.33 ppm) and Roundup (0.84 ppm) caused a considerable reduction in the survival rates and egg production of B. alexandrina snails. They reasoned that reduction due to snails could overcome the destructive effect of tested pesticides through discharging it to the surrounding media or by biodegrading it to non-poisonous by-products.
The reproductive rate (R0) and fecundity (Mx) of adult B. alexandrina snails in the present study were significantly decreased by their exposure to the sub-lethal concentrations (LC0, LC10, or LC25) of oxyfluorfen 24%EC, in comparison with the control group. These low values of R0 and Mx of treated snails could be attributed partially to their high mortality rates and due to severe damage in the digestive and hermaphrodite gland cells of treated snails (Hasheesh and Mohamed 2011).
In the present study, the oxidative stress induced by oxyfluorfen toxicity was indicated by the significantly increased levels of tissue lipid peroxides (MDA), which is the major reactive aldehyde formed by the peroxidation of membrane lipids and one of the most damaging effects of ROS (Ohkawa et al. 1979). Lipid peroxidation was increased in all treated snails especially those exposed to LC25 that explain a concentration-dependent effect and is an indication of the oxidative stress. The present results are in agreement with observations made by Atli and Grosell (2016) who studied the potential effects of acute copper (Cu) toxicity (2–90 g/ml for 48 h) on the antioxidant responses in three tissues—the hepatopancreas, the foot muscle, and the mantle of Lymnaea stagnalis snails—and they stated that all antioxidant enzymes (SOD, CAT, and glutathione peroxidase, GPx) except glutathione reductase (GR) increased after exposure to the highest Cu concentration in mantle and concluded that they could be suitable biomarkers of the metal toxicity in the aquatic environment.
The present study showed that the enzymatic defenses include catalase CAT and SOD was increased in response to the overproduction of reactive oxygen species inside the tissue. These results were consistent with (Peixoto et al. 2006), who found higher CAT activity in tilapia Oreochromis niloticus exposed to 0.3 and 0.6 mg/l oxyfluorfen for 7 and 14 days. In addition, catalase liver activity also showed an increase in the Neotropical Prochilodus lineatus fish exposed to 10 mg/l of Roundup, a glyphosate-based herbicide (Langiano do and Martinez 2008), suggesting the activation of antioxidant defenses after Roundup exposure.
GSH is a non-enzymatic antioxidant acting as a substrate for the antioxidant enzyme, glutathione transferase (GST), and an important free radical quencher. Snail exposure to different concentrations of oxyfluorfen 24%EC herbicide leads to a significant decrease in tissue GSH content which explains the high rates of free radical input. These results were in agreement with work done by Bagul et al. (2016) who showed potential toxicity of oxyfluorfen (23.5% EC) on the earthworm, Eisenia fetida at the concentration of 0.31 mg/kg. The increase in MDA activity accompanied by the decrease in GSH content may clarify the tissue injury occurred after the exposure to all concentrations of oxyfluorfen 24%EC herbicide. Moustafa et al. (2016) confirmed that Roundup, Stomp herbicides either individually or in combination at concentration 1/2 96 h lethal concentration 50 (LC50) elucidated significant elevation in the levels of SOD, CAT, and GPx in Nile catfish (Clarias gariepinus). Meanwhile, the data depicted the reduction in levels of reduced GSH and GST. So, the induction of the SOD/CAT system provides a first-line defense against ROS especially after the increase of lipid peroxide MDA and the diminishing of reduced glutathione GSH.
The present results determined testosterone and 17β-estradiol (T and E) levels in the gonads of oxyfluorfen-exposed snails after 2 weeks of exposure. Levels of T and E were decreased significantly after exposure to sub-lethal concentrations of oxyfluorfen 24%EC (p < 0.05) compared with control indicating the disrupter effect of oxyfluorfen 24%EC herbicide. This is in accordance with the findings of (Omran and Salama 2016) who found that the level of T was decreased significantly after exposure to LC10 sub-lethal concentrations of glyphosate (4.2 ppm) and atrazine (10.1 ppm) herbicides and the level of E was decreased by nearly 50% in both glyphosate and atrazine exposed B. alexandrina snails compared with control. Also, these results come in accordance with Clair et al. (2012) who stated that atrazine and glyphosate acts as an endocrine disrupter, where they were toxic to Sertoli cells in mature rat fresh testicular cells from 1 to 10,000 ppm and induced apoptosis at higher doses in germ cells and in Sertoli/germ cells co-cultures.
The sub-lethal concentrations of oxyfluorfen 24%EC induced histopathological changes in the digestive gland of subjected snails and these damaged increased with increasing the concentrations. The most severe damage in the digestive cells was the presence of a great loss of identical shape of digestive cells. Some digestive cells were ruptured and most of these cells degenerated, also the secretory cells increased in number and lumens inside tubule increased, and the connective tissue between digestive tubules shrank. These results agreed with Abdel-Ghaffar et al. (2016), who stated that butralin 48% EC, glyphosate isopropylammonium 48% SL, and pendimethalin 50% EC herbicides induced histopathological changes in the digestive glands, where the most prominent severe damage was a great loss in shape of digestive cells (syncytium) and some were ruptured or degenerated and secretory cells became denser in color and increased in number. Also, these findings were in accordance with Sharaf et al. (2015), who found that the exposure to sub-lethal concentrations (LC25) of both methiocarb (4.4 ppm) and chlorpyrifos (0.005 ppm) pesticides caused many histological changes in the digestive gland of land snail Helicella vestalis and these alterations included severe tubular disruption, evacuation, pyknotic nuclei, and necrosis of digestive tubules.
Also, the histological examination of the treated hermaphrodite gland showed losses of connective tissues, the presence of a large number of degenerated eggs and sperms after exposure. These results were in accordance with Osman et al. (2008) who found that exposure of B. alexandrina snails to sub-lethal concentrations (10 ppm) of Roundup or Topik herbicides showed complete destruction of gametogenic cells. Also, Langiano do and Martinez (2008) reported that short-term exposure to sub-lethal concentration 10 mg/l of Roundup induced several histological alterations in Neotropical fish, Prochilodus lineatus.
Conclusion
Oxyfluorfen 24%EC, a potent molluscicidal agent, has been shown to cause alterations in the antioxidant system causing oxidative stress, reduce survival rate, and inhibit the reproduction in the freshwater snail B. alexandrina, in addition to endocrine disruptor effect accompanied with histopathological alterations in gonads. Further investigations are urgently needed to assess the current environmental burden of herbicides in riverine ecosystems to determine whether there is an extent need to monitor the use of herbicides in the Nile River Basin aiming to reduce water pollution and studying its harmful effects on non-target organisms.
References
Abdel-Ghaffar F, Ahmed AK , Bakry F, Rabei I, Ibrahim A (2016) The impact of three herbicides on biological and histological aspects of biomphalaria alexandrina, intermediate host of Schistosoma mansoni. Malacologia 59(2):197–210
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Atli G, Grosell M (2016) Characterization and response of antioxidant systems in the tissues of the freshwater pond snail (Lymnaea stagnalis) during acute copper exposure. Aquat Toxicol 176:38–44. https://doi.org/10.1016/j.aquatox.2016.04.007
Bagul PK, More BC, Patole SS (2016) Sub lethal effects of cypermethrin and oxyfluorfen on stress enzyme activities of earthworm species, Eisenia foetida Savigny, 1826. Int J Innov Res Sci Eng Technol. https://doi.org/10.15680/IJIRSET.2016.0512047
Banhawy MA, Soliman FM, Abdel-Rehim SA, Hamada HMA (1996) Metabolic changes in the Nile bolti Oreochromis niloticus exposed to different concentrations of the herbicide (Goal). Proc Egypt Acad Sci 46:99–117
Barky FA, Abdelsalam HA, Mahmoud MB, Hamdi SAH (2012) Influence of Atrazine and Roundup pesticides on biochemical and molecular aspects of Biomphalaria alexandrina snails. Pestic Biochem Physiol 104:9–18. https://doi.org/10.1016/j.pestbp.2012.05.012
Beutler E (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61:882–888
Clair É, Mesnage R, Travert C, Séralini G-É (2012) A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol in Vitro 26:269–279
El-Gindy MS, Radhawy IA et al (1965) Effect of low concentrations of sodium pentachlorophenate on the fecundity and egg viability of Bulinus truncatus from Central Iraq. Bull Endem Dis (Baghdad) 7:44–54
EPA/OPP US (U. SEPA of PP) (2001) Revised Environmental Fate and Effects Division preliminary risk assessment for oxyfluorfen reregistration eligibility decision document. http://fluoridealert.org/wpcontent/pesticides/oxyfluorfen.enveffects.2001.pdf
Eveland LK, Haseeb MA (2011) Laboratory rearing of Biomphalaria glabrata snails and maintenance of larval Schistosomes in vivo and in vitro. In: Biomphalaria snails and larval trematodes. Springer New York, New York, pp 33–55
Fahmy SR, Sayed DA (2017) Toxicological perturbations of zinc oxide nanoparticles in the Coelatura aegyptiaca mussel. Toxicol Ind Health 33(7):564–575
Fahmy SR, Abdel-Ghaffar F, Bakry FA, Sayed DA (2014) Ecotoxicological effect of sublethal exposure to zinc oxide nanoparticles on freshwater snail Biomphalaria alexandrina. Arch Environ Contam Toxicol 67:192–202
Finney DJ (1971) Probit analysis, 3rd edn. Cambrige University Press, Cambrige
Godfrey W, Longacre S (1990) Rohm and Haas Company Phase 3 Summary of MRID 00134452. Goal Technical Herbicide Oxyfluorfen Acute Toxicity to the Freshwater Clam: Rohm and Haas Report 77RC-1103; UCES Project No. 11506–33-02. Prepared by Union Carbide Corp. 14 p
Hasheesh WS, Mohamed RT (2011) Bioassay of two pesticides on Bulinus truncatus snails with emphasis on some biological and histological parameters. Pestic Biochem Physiol 100:1–6
Hassanein HMA (2002) Toxicological effects of the herbicide oxyfluorfen on acetylcholinesterase in two fish species: Oreochromis niloticus and Gambusia affinis. J Environ Sci Health A 37:521–527
Ibrahim MA, Ahmed AK et al (2018) Hematological, physiological and genotoxicological effects of Match 5% EC insecticide on Biomphalaria alexandrina snails. Ecotoxicol Environ Saf:147. https://doi.org/10.1016/j.ecoenv.2017.09.059
Keum YS, Lee YJ, Han KJ (2008) Metabolism of nitrodiphenyl ether herbicides by dioxin-degrading bacterium Sphingomonas wittichii RW1. J Agric Food Chem 56:9146–9151. https://doi.org/10.1021/jf801362k
Langiano do CV, Martinez RC (2008) Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comp Biochem Physiol C Toxicol Pharmacol 147:222–231
Litchfield JT Jr, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
Mohamed SH, Saad AA (1990) Histological studies on the hermaphrodite gland of Lymnaea caillaudi and Biomphalaria alexandrina upon infection with certain larval trematodes. Egypt J Histol 13:47–53
Moustafa GG, Shaaban FE, Hadeed AH, AboElhady WM (2016) Immunotoxicological, biochemical, and histopathological studies on roundup and stomp herbicides in Nile catfish (Clarias gariepinus). Vet World 9:638–647. https://doi.org/10.14202/vetworld.2016.638-647
Nduku WK, Harrison AD (1980) Cationic responses of organs and haemolymph of Biomphalaria pfeifferi (Krauss), Biomphalaria glabrata (Say) and Helisoma trivolvis (Say)(Gastropoda: Planorbirdae) to cationic alterations of the medium. Hydrobiologia 68:119–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omran NE, Salama WM (2016) The endocrine disruptor effect of the herbicides atrazine and glyphosate on Biomphalaria alexandrina snails. Toxicol Ind Health 32:656–665
Osman GY, Mohamed AH, Mohamed AM, Al-Qormuti SA (2008) Effect of Roundup herbicide on biological activity of Biomphalaria alexandrina snails infected with Schistosoma mansoni. Mansoura J Biol 35:147–167
Pašková V, Hilscherová K, Bláha L (2011) Teratogenicity and embryotoxicity in aquatic organisms after pesticide exposure and the role of oxidative stress. In: Reviews of environmental contamination and toxicology, vol 211. Springer, Berlin, pp 25–61
Peixoto F, Alves-Fernandes D, Santos D, Fontainhas-Fernandes A (2006) Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic Biochem Physiol 85:91–96
Sharaf HM, Salama MA, Abd El-Atti MS (2015) Biochemical and histological alterations in the digestive gland of the land snail Helicella vestalis (Locard, 1882) exposed to methiocarb and chlorpyrifos in the laboratory. Int J Sci Res 4:334–343
Sharaf-El-Din AT, Mohamed AM, Mohamed AH et al (2010) Relationship between some heavy metals and Schistosoma mansoni infection rates in Biomphalaria alexandrina snails collected from different Egypt localities. World Appl Sci J 11:38–43
Talib AH, AL-Rudainy JA et al (2018) The acute toxicity of herbicide roundup ultra in mosquito fish Gambusia affinis. J Biodivers Environ Sci 13:9–15
Tellez-Bañuelos MC, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitseva G (2009) Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol 27:105–111
Tilton F, La Du JK, Tanguay RL (2008) Sulfhydryl systems are a critical factor in the zebrafish developmental toxicity of the dithiocarbamate sodium metam (NaM). Aquat Toxicol 90:121–127
Tsui M, Chu LM (2008) Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 71:439–446
Vera-Candioti J, Safety SS-… and environmental, 2013 U (2012) Evaluation of the genotoxic and cytotoxic effects of glyphosate-based herbicides in the ten spotted live-bearer fish Cnesterodon decemmaculatus (Jenyns, 1842). Ecotoxicol Environ Saf 89:166–173
Warren E (1900) Memoirs: on the reaction of Daphnia magna (Straus) to certain changes in its environment. J Cell Sci 2:199–224
WHO (1965) Molluscicide screening and evaluation. Bull WHO 33:567–581
WHO (1983) Report of the scientific working group on Plant Molluscicide & Guidelines for evaluation of plant molluscicides. WHO, Geneva (TDR/SCHSWE (4)/833
WHO (1993) The control of schistosomiasis, Technical Report Series Geneva Switz, pp 1–86
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, A.M., Sayed, D.A. Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) snails. Environ Sci Pollut Res 26, 7960–7968 (2019). https://doi.org/10.1007/s11356-019-04251-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04251-w