Abstract
Removal and recovery of phosphorus (P) from sewage are essential for sustainable development of P resource. Based on the water quality determination of sludge dewatering filtrate from a wastewater treatment plant in Beijing, this study investigated the adsorption and regeneration characteristics of P by magnetic anion exchange resin (MAEX). The experiments showed that the P adsorption capacity of MAEX could reach a maximum of 2.74 mg/mL when initial P concentration was 25 mg/L and dosage of MAEX was 8 mL/L. The P adsorption on MAEX resin was suitable for large temperature range (283–323 K). However, the adsorption capacity was reduced in various degrees due to the interference of different anions (Br−, SO42−, Cl−, NO3−, HCO3−, CO32−) and organic compounds (bovine serum albumin, humic acid). Kinetics studies indicated that the P adsorption process followed the pseudo-second-order model. The MAEX resin had a rapid P adsorption rate and the P adsorption capacity at 30 min could reach 97.7–99.3% of qe. Increase of temperature was favorable to P adsorption on MAEX, and the adsorption isotherm data fitted to Langmuir model more than Freundlich model. Meanwhile, the thermodynamics parameters were calculated; it was shown that the adsorption process was an endothermic reaction. Desorption and regeneration experiments showed that NaHCO3 was a suitable regenerant, and the P adsorption capacity could reach 90.51% of the original capacity after 10 times of adsorption-desorption cycles; this indicated that MAEX resin has an excellent regeneration performance and thus has a very good application prospect of P removal and recovery. Fourier transform infrared spectroscopy (FTIR) analysis confirmed that ion exchange, charge attraction, and hydrogen bonding affected the removal of P by the MAEX resin. The vibrating sample magnetometer (VSM) analysis revealed that MAEX resin was a kind of soft magnetic materials with good magnetism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is essential but non-renewable (Roy 2017). In nature, P only linearly moves from the land to the sea (Hao et al. 2010). Across the globe, P ore depletion is getting worse by relying on limited natural resources (Lwin et al. 2017). However, the unreasonable discharge of P with the domestic sewage and industrial wastewater leads to eutrophication, and it gradually becomes one of the research hotshots all over the world (Xu et al. 2014; Song et al. 2017). Therefore, not only for scanty P resources but also for serious P pollution, P recovery from sewage and sludge has become a particularly promising option (Schütte et al. 2015).
Massive sorts of P-recovery technologies have been researched and applied (Sartorius et al. 2012), including traditional chemical precipitation, crystallization, biological method, and adsorption, etc. (Morse et al. 1998; Shih et al. 2017; Yuan et al. 2012; Loganathan et al. 2014). Of these, the adsorption method is routinely described as a widely wastewater treatment option because of its easy operation, stable treatment effect, reversible process, low-cost absorbents (Bhatnagar and Sillanpää 2011; Dai et al. 2011). A variety of P adsorbents have been developed and evaluated, such as fly ash (Ugurlu and Salman 1998), slag (Ozacar 2003), MgMn-layered double hydroxides (Chitrakar et al. 2005), red mud (Huang et al. 2008), zirconium (Chitrakar et al. 2006), Fe-EDA-SAMMS (self-assembled monolayers on mesoporous supports, a registered trademark of Steward Advanced Materials) (Chouyyok et al. 2010), and palygorskite (Gan et al. 2009). Recently, using magnetic anion exchange resin (MAEX) to remove pollution from water has attracted much attention.
MAEX is a strong base anionic resin with some ammonia functional groups and a magnetized component within its structure that enables this resin to act as a weak individual magnet (Mergen et al. 2008). In contrast with the traditional anion exchange resins, MAEX has two distinctive features. First, the particle size of MAEX, ranging from 150 to 180 μm, is small and the specific surface area is relatively large. This has contributed to rapidly adsorb the negative charge pollutants in water by MAEX (Boyer and Singer 2005). Second, the resin is applied to water and wastewater in a slurry form and will be mixed well with adsorbate by mechanical stirring, thus decreasing the resistance to liquid phase mass transfer (Liu et al. 2011). Initially, MAEX was designed specifically to remove the dissolved organic carbon (DOC) from wastewater, while it recently has become concerned to remove inorganic anions. MAEX had a sulfate removal ratio of 9–24% and a nitrate removal ratio of 17–42% (Kitis et al. 2007). Besides, MAEX had a good adsorption effect on P, while the removal rate was about 70% (Ding et al. 2012). However, the previous study was incomplete since neither the reutilization effect of resin nor the adsorption mechanism was evaluated and explored.
Accordingly, this study investigated the P adsorption and regeneration characteristics of MEAX based on the water quality determination of sludge dewatering filtrate from a wastewater treatment plant (WWTP) in Beijing. For this purpose, the effects of initial P concentration, adsorbent dosage, initial pH, temperature of solution, ion strength, coexistent anions, and organic compounds on P adsorption were investigated. Adsorption kinetics, isotherm, and thermodynamics were also performed. Moreover, the regeneration characteristics of MEAX were analyzed by exploring the kinds of regenerants and regeneration times. In addition, the characterizations of MAEX resin before and after adsorption were investigated by Fourier transform infrared spectroscopy (FTIR) and vibrating sample magnetometer (VSM). The outcomes will be important for providing the fundamental basis for practical application in P recovery by magnetic resin.
Materials and methods
Sampling and analysis of sludge dewatering filtrate
Sludge dewatering filtrate samples were obtained from a WWTP with the anaerobic-anoxic-oxic (A/A/O) process in Beijing. Then the samples were stored at 4 °C and transported to the laboratory.
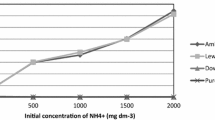
Table 1 and Fig. 1 presented the water quality of the sludge dewatering filtrate samples. The amount of P in the filtrate was considerable and the proportion of soluble total P in total P was high. As shown in Fig. 1, tryptophanlike was the main organic pollutant, consistent with the research result of Liu et al. (2016). In the meantime, according to the amount of P in the water samples, the P concentration of the simulated wastewater in the follow-up experiments was determined to be 25 mg/L.
P adsorption experiments by MAEX resin
All MAEX resins, the adsorbent of this study, were purchased from Jiangsu Guochuang New Material Research Center with a wet superficial density of 1.058 g/mL. MAEX was stored in plastic bottle. Before using, it would be rinsed with deionized water for several times in order to remove impurities, then after settled for about 30 min, a certain amount of MAEX resin was used according to the adsorption experiment requirement.
Table 2 showed the size distribution of MAEX resin measured by Malvern Laser Particle Size Analyzer. The particle size of MAEX resin mainly ranged from 244 to 445 μm. The morphological features and the surface characteristics of MAEX resin were obtained by scanning electron microscopy (SEM), as shown in Fig. 2. From Fig. 2, we can conclude that the surface of MAEX resin appears to be ragged, due to Fe2O3 loaded on the surface of the resin for magnetic purpose.
Potassium dihydrogen phosphate (KH2PO4) was used as the adsorbate. KH2PO4 solutions and MAEX resin were added into a set of 50 mL conical flasks, and the flasks were sealed and agitated in an incubator for some time. Then the mixture was filtered by 0.45 μm membranes, and the P in filtrates was analyzed by the molybdenum blue colorimetry.
The P removal rate and the P adsorption capacity of MAEX resin were calculated as Eqs. (1) and (2), respectively:
where C0 and Ct stand for P concentration before and after adsorption (mg/L), W stands for dosage of MAEX resin (mL), and V stands for volume of the mixed solution (L).
The effect experiments of different factors on P adsorption by MAEX resin were employed by varying resin dosage, initial pH, temperature, ionic strength, coexistent anions, and organic compounds. The effect experiments of resin dosage were conducted with an electric mixer. However, as to accurately control the experimental parameters, the later experiments were conducted in a thermostatic oscillator. The initial pH values were adjusted by adding 0.1 mol/L NaOH or HCl if necessary. Table 3 showed the experimental conditions from the effect experiments of different factors on P adsorption by MAEX resin.
Adsorption kinetic and thermodynamic experiments
Batch adsorption experiments were carried out to explore adsorption kinetics and diffusion mechanism. P solutions with different initial concentration (10 mg/L, 25 mg/L, and 50 mg/L) and 10 mL MAEX resin were added into a set of 500 mL beakers. The mixture was stirred using the motor agitators for 2 h when the adsorption reached equilibrium. In the meantime, the mixtures sampled from the beakers at regular intervals were filtered by 0.45 μm membranes, and the P concentration infiltrates were determined. Each experiment was performed three times under identical conditions.
As for the adsorption thermodynamic research, the experiments were conducted at pH 8, MAEX resin dosage of 8 mL/L, and shaking for 30 min, while the adsorption temperatures were 293, 303, and 313 K and the initial P concentrations were 5, 10, 15, 20, 25, 50, 100, 150, 200, and 250 mg/L. The amount of P adsorption at equilibrium, qe (mg/mL), was computed as follows:
where C0 and Ce are the initial and the equilibrium P concentrations (mg/L), respectively, V is the volume of the solution (L), and W is the dosage of MAEX resin (mL). Thermodynamic parameters could be calculated by experiment data of the temperature effect on P adsorption by MAEX resin. Each experiment was also performed three times under identical conditions.
Regeneration study on MAEX resin
Regeneration experiments were first carried out with six different regenerants (H2O, NaCl, Na2CO3, NaOH, HCl, and NaHCO3) in the same concentration of 1.0 mol/L, and the adsorption capacities of MAEX resins after regeneration were compared to choose suitable regenerants. Then, the effect experiments of regenerant concentrations (NaHCO3 and NaCl) were discussed. In order to investigate the stability and the readsorption capacity of MAEX resin, ten times of adsorption-desorption cycle experiments were carried out by using regenerants of NaHCO3 and NaCl.
Regenerative effect of reagents for MAEX resin, E, was computed as follows:
where q0 is the initial adsorption capacity of MAEX resin (mg/mL), and qR is the adsorption capacity of MAEX resin after regenerated (mg/mL).
FTIR and VSM analysis
To further elucidate the process of P adsorption and the stability of MAEX resin, MAEX resin, before and after once adsorption, was characterized by Fourier transform infrared spectrometer (FTIR) and vibrating sample magnetometer (VSM). The FTIR spectrum was recorded at room temperature ranging from 400 to 4000 cm−1 by FTIR spectrometer (Vertex 70, Germany). The magnetic properties of MAEX resin were assessed with a multifunctional vibrating sample magnetometer (Versa Lab, USA).
Results and discussion
Effects of different parameters on P adsorption by MAEX resin
Monofactor experiments were carried out by the control variable method to investigate the effect of different factors on the P adsorption by MAEX resin.
From Fig. 3a, as the resin dosage was reached to 8 mL/L, it may be noted that there was no significant increase in the removal rate of P when the initial P concentration is 25 mg/L (closed to the P concentration in the actual sludge dewatering filtrate). The resin dosage of 8 mL/L was selected for the following experiments.
According to Fig. 3b, the suitable pH is 8 and the removal rate of P could reach to 87.8%. Besides, both the removal rate and the adsorption capacity all increased first and then decreased as pH was increasing. The removal rate of P and the adsorption capacity of MAEX resin reached to the maximum at pH = 8. The existing state of phosphate in aqueous medium is closely related with pH of the solution. Under the condition of low pH value, phosphate mainly exists in the forms of H3PO4 and H2PO4− (Ozmen et al. 2011), and these two forms are more difficult to exchange the chloride ions from the active adsorption sites, which could lead to the low adsorption capacity of MAEX resin. In addition, since the pH of solution was mainly adjusted by hydrochloric acid or sodium hydroxide, the introduction of chloride ions into the solution can inhibit the exchange between phosphate and chloride. When the pH of solution increases to 8, phosphate mainly exists in the forms of HPO42− and PO43−. The increase of ionic charge can lead to the increase of ion exchange capacity between phosphate and chlorine at the active adsorption sites, resulting in the increase of the adsorption capacity. However, as the pH of solution increased to 12, the competitive adsorption between phosphate and hydroxyl ions in solution results in the reduction of P adsorption capacity on MAEX resin. As a result, the optimum pH for P adsorption by MAEX resin was 8.
From Fig. 3c, the increase of temperature was favorable to the P adsorption by MAEX resin, which indicated that the P adsorption process by MAEX resin might be an endothermic process. Besides, the P adsorption capacities of MAEX resin changed a little during the wide temperature range (283–323 K), which showed that the resin can deal well with the temperature change of the wastewater. In Fig. 3d, along with the concentration of sodium chloride increase from 0 to 1 mmol/L, the removal rate of P by MAEX resin decreased from 79.6 to 65%. It confirmed that the existence of Cl− would reverse the original ion exchange equilibrium. As shown in Fig. 3e–g, the P adsorption capacities by MAEX resin were reduced in various degrees due to the interference of anions or organic compounds, which were mainly because of the competitive adsorption between anions or organic compounds and P. According to Table 4, we can conclude that pH was almost not changed after adding Cl−, SO42−, NO3−, and Br−; however, pH was a little higher after adding CO32 and HCO3−. The order of the inhibition of anion was as follows: Br−> SO42−> Cl−> NO3−> HCO3−> CO32−. Compared Fig. 3 f and g, it was found that the inhibition of the humic acid was stronger than that of the bovine serum albumin. The reason may be that the humic acid has a smaller molecule than the bovine serum albumin (Liu et al. 2016), resulting in more easily adsorbed by MAEX resin.
Adsorption kinetics of P on MAEX resin
To investigate the mechanism and the dynamic of P adsorption process, several kinetic models (pseudo-first-order, pseudo-second-order, and Elovich equation) were tested to fit the experimental data obtained from the batch adsorption experiments (Hameed et al. 2007).
Adsorption kinetic models
Pseudo-first-order kinetic model has been widely used to describe the process of adsorption (Hameed et al. 2008). The equation is generally presented as follows:
where k1 stands for the rate constant of pseudo-first-order adsorption, qe and qt represent the adsorption capacity (mg/mL) at equilibrium and at any time, respectively. Integrating Eq. (5) with respect to boundary conditions q = 0 at t = 0 and q = qt at t = t, Eq. (5) becomes:
According to Eq. (6), the plot of ln (qe − qt) versus t could fit a straight line. From the line, k1 and qe can be determined from the slope and intercept, respectively.
Pseudo-second order assumes that the rate-limiting step is chemisorption (Kan et al. 2017). The equation is generally presented as follows:
where k2 stands for the rate constant of pseudo-second-order adsorption, qe and qt represent as same as above. Integrating Eq. (7) and applying the initial conditions, Eq. (7) becomes:
According to Eq. (8), the plot of t/qt versus t could give a straight line. From the line, k2 and qe can be calculated from the slope and intercept, respectively.
Elovich equation, one of the most useful models, describes the kinetics of activated chemisorption process (Ali et al. 2016). The equation is generally presented as follows:
From Eq. (9), α and β are the constants obtained from the slope and intercept of the linear plot of qt versus ln (t).
Analysis of the experimental data
The adsorption kinetic parameters on MAEX resin at different initial P concentrations were given in Table 5, and the fitted curves for different systems were shown in Fig. 4. According to Table 5, the adsorption process most closely followed pseudo-second-order kinetics, by which the highest correlation coefficient (R2 > 0.99) was obtained as well as the very accurate estimation of qe. From Fig. 4, we also could find that pseudo-second-order kinetic curves appeared to fit well with the experimental data. This suggested that the adsorption of P on MAEX resin may be controlled by the chemical sorption, which involved covalent forces through exchanging or sharing the electrons between adsorbate and adsorbent (Wang et al. 2018). According to Fig. 5, the main process of adsorption was completed in 30 min and the P adsorption capacity at 30 min could reach 97.7–99.3% of qe. Table 6 showed us the comparison of adsorption equilibrium time between some common adsorbents for P adsorption. We could deduce that the P adsorption by MAEX resin reached to equilibrium within a very short time, resulting in shortening treatment time and improving efficiency of wastewater treatment.
Intraparticle diffusion model and analysis
The adsorption process is controlled by several steps including (i) diffusion of the solute from solution to the film on particle surfaces, (ii) diffusion from the film to the particle surface after overcoming liquid film resistance (external diffusion), (iii) diffusion from the surface to the active adsorption sites on the inner surface of particles (surface diffusion or pore diffusion), and (iv) uptake which can involve several mechanisms such as ion exchange, physicochemical sorption, complexation, or precipitation (Wu et al. 2001; Gercel et al. 2007).
Adsorption kinetics is usually controlled by different mechanisms, of which the most limiting ones are the diffusion mechanisms (Toor and Jin 2012). As the diffusion mechanism cannot be identified by the above kinetic models, the intraparticle diffusion model based on the theory proposed by Weber and Morris was widely studied (Weber and Morris 1963). This model assumes that diffusion controls the sorption process if the whole reaction rate is dependent upon the diffuse rate between adsorbate and adsorbent. The model was characterized by Eq. (10):
where kid is the intraparticle rate constant (mg/(g·s t1/2)) and C is the intercept. According to Eq. (10), the plot of qt versus t1/2 should be linear. If this line passed through the origin, the intraparticle diffusion is the rate-limiting step to the adsorption process. If not, there are other factors controlling the process, such as liquid film (Bhattacharyya and Gupta 2006).
Table 7 presented the constants and the correlation coefficients of intraparticle diffusion model for P adsorption on MAEX resin, while Fig. 6 showed the adsorption profiles by plotting qt versus t1/2 for three initial P concentrations.
As illustrated in Fig. 6, during the whole reaction time, the adsorption process was divided into three different stages:
(i) The first stage was surface adsorption stage at the initial adsorption, by which the linear slope was the largest and the adsorption rate was the fastest. During this stage, the process of P transferring from the solution to the adsorbent surface was the mainly material transfer process which had a small resistance.
(ii) The next stage was the internal diffusion process. At this time, the adsorption on the adsorbent surface was basically completed with gradually smaller adsorption rate, and the adsorption process started to transfer into the holes of adsorbent.
(iii) The final stage was the adsorption saturation process, where the adsorption capacity of adsorbent was no longer changed with time, indicating that the adsorption process had reached saturation.
Although there was a high correlation coefficient between qt and t1/2 as shown in Table 7, the fitting curves were not through the coordinate origin at different stages of the adsorption process. It indicated that the adsorption reaction was mainly not only controlled by intraparticle diffusion, while the adsorption rate was also affected by particle diffusion, such as surface adsorption or liquid film diffusion. These three diffusion stages have also been observed form another P-adsorption process in which the soils from diverse ecological zones were used as the adsorbent (Zhang et al. 2015).
Adsorption thermodynamic characteristics of P on MAEX resin
Adsorption isotherm models and analysis
Adsorption isotherm is the equilibrium curve describing the relationship between the adsorbate in solution and adsorbate adsorbed on the surface of adsorbent at a given temperature (Treybal 1981). Establishing the most appropriate correlation for the equilibrium curves is important to optimize the design of an adsorption system (Hameed et al. 2008).
In this study, two adsorption isotherms, namely, Langmuir and Freundlich isotherms, were applied to the equilibrium data of P adsorption on MAEX resin.
The widely used Langmuir isotherm has found successful application in many real sorption processes (Agyei et al. 2000; Bajpai et al. 2000). Langmuir isotherm model is valid for monolayer sorption on a surface with a finite number of identical sites and is generally expressed as follow (Sheshdeh et al. 2014):
In the above equations, ce and qe are respectively the concentration of adsorbate (mg/L) and the adsorption capacity of adsorbent (mg/mL) at equilibrium, qm stands for Langmuir sorption capacity (mg/mL), and KL is the parameter of Langmuir isotherm equation which can reflect adsorption capacity of adsorbent.
Freundlich isotherm is often used for heterogeneous surface energy systems (Agyei et al. 2000; Baup et al. 2000). The equation is expressed as follow:
where KF is the parameter of Freundlich isotherm equation which is related to binding energy and adsorption capacity. The shape of the isotherm depends on the value of 1/n as follows (Toor and Jin, 2012): the isotherm is unfavorable (1/n > 1), ineffective (1/n = 1), favorable (0 < 1/n < 1), or reversible (1/n = 0).
The parameters of Langmuir and Freundlich isotherm models at three temperatures were listed in Table 8, while Figs. 7 and 8 described the fitting curves of two isotherm models. As shown in Table 8 and Fig. 7, Langmuir model with the higher regression coefficients than those of Freundlich model was more suitable for the adsorption behaviors of P on MAEX resin. This indicated that adsorption of P is a monolayer adsorption on the surface of MAEX resin. Furthermore, as presented in Fig. 8, there were no significant increases in the equilibrium adsorption capacity of P when the initial P concentration reached to 50 mg/L, further proving the formation of monolayer coverage of P at the surface of MAEX resin. Similar phenomenon was observed in the adsorption of P on Fe3O4 magnetic nanoparticles (Li et al., 2013), ternary (Fe-Mg-Al)-layered hydroxides (Liu et al. 2015), and modified rectorite (Xu et al. 2016). However, some studies found that the Freundlich model gave a better fit on the adsorption of P using different adsorbents (Shen and Huang 2011; Kuwahara et al. 2016). This might be affected by the characteristics of the adsorbents chosen.
Adsorption thermodynamic parameters
The thermodynamic parameters, such as standard free energy (ΔG), enthalpy (ΔH), and entropy (ΔS), could be helpful to further explain the mechanism of adsorption process (Sarkar et al. 2017). These parameters can be determined from the following equations:
In Eqs. (14) and (15), R is the gas constant (8.314 J/mol K), T is the temperature (K), and KD is the standard thermodynamic equilibrium constant (L/mol) which is calculated by Eq. (16):
In Eq. (16), C0 is the initial P concentration (mg/L), Ct is the P concentration (mg/L) in the solution at equilibrium, V is volume of the solution (mL), and W is dosage of MAEX resin (mL). The detailed computation processes of thermodynamic parameters are as follows: (i) calculating the values of KD at different temperatures, (ii) linear fitting of lnKD versus 1/T (Fig. 9), (iii) calculating ΔS and ΔH from the slope and the intercept, respectively, and (iv) determining ΔG at different temperatures.
From Table 9, the positive value of ΔH (5.3226 KJ/mol) denoted that the adsorption reaction was endothermic, which was also proved by Fig. 3c. The positive value of ΔS (68.6021 J/(mol·K)) suggested that the degree of confusion on the solid-liquid interface was increased and the adsorption reaction can be carried out spontaneously. The value of ΔS was positive, which indicates that the dissociative mechanism was involved in the sorption process. (Abbasizadeh et al., 2014; Talebi et al., 2017). The values of ΔG at different temperatures were all negative (range from − 14.1309 to − 16.9689 KJ/mol), further indicating that the adsorption process of P on MAEX resin was spontaneous thermodynamically. Moreover, as the increase of temperature, the value of ΔG decreases gradually, which showed that increasing of temperature is beneficial to the adsorption process of P on MAEX resin. Besides, according to the absolute value of ΔG at five temperatures ranged from 14 to 17 KJ/mol, we could infer that the adsorption is not only chemical adsorption, but also physical adsorption.
Regeneration and reuse of MAEX resin
Figure 10 showed the regeneration effects of MAEX by different reagents. As seen, the order of the regenerative ability was as follows: NaHCO3>NaCl>NaOH>HCl>H2O>Na2CO3, while the adsorption capacities of MAEX resin after regeneration were respectively 98.12%, 82.04%, 48.74%, 46.34%, 17.43%, and 15.97% of the original capacities. The result showed that NaHCO3 and NaCl have good performance on MAEX resin regeneration, indicating that chloride (Cl−) and bicarbonate (HCO3−) could exchange phosphate at the surface reaction sites of resin more effectively. Cl− at the surface of the resin is the functional point which can exchange the contaminant ions from solution. In addition, OH− hydrolyzed by HCO3− might be reacted with H2PO4− or HPO42− and formed to PO43−, leading to the charge increasing of phosphate ions, which promoted P adsorption on MAEX resin.
Figure 11 depicted the regeneration process of MAEX resin by NaCl and NaHCO3 with different concentrations. From Fig. 11a, P desorbed from MAEX resin was basically stable after 30 min of reacting and increased as the increasing of NaCl concentration. When the concentration of NaCl increased from 0.1 to 1.0 mol/L, the concentration of P in the solution increased from 18.76 to 20.03 mg/L. However, when the concentration of NaCl continued increasing until 4.0 mol/L, the concentration of P just increased to 20.64 mg/L, with no visible difference. Therefore, 1.0 mol/L of NaCl was suitable for the regeneration process of resin. As for Fig. 11b, when the concentration of NaHCO3 increased from 0.1 to 1.0 mol/L, the concentration of P in the solution desorbed from the resin increased from 32.13 to 36.97 mg/L. Since 1.0 mol/L is close to the solubility of NaHCO3 at the room temperature, 1.0 mol/L of NaHCO3 was chased for the subsequent adsorption-desorption cycle experiments.
Figure 12 showed us the adsorption capacities of MAEX resin during ten times of adsorption-desorption cycle experiments by NaHCO3 and NaCl. After ten times of regeneration by 1.0 mol/L NaHCO3 and NaCl, the adsorption capacities of MAEX resin reduced by 9.48% and 24.2%, respectively. The removal rates of P after ten times of regeneration by NaHCO3 and NaCl were 90.51% and 75.8% of the original rate, respectively. The result showed that the regenerating performance of NaHCO3 was better than that of NaCl. Furthermore, no significant loss of adsorption capacity of MAEX resin was observed after 10 cycles of adsorption-desorption, indicating that MAEX resin could be reused for many times and MAEX resin has good physical stability. Table 10 was the comparison of P adsorption by the MAEX resin and with other adsorbents after multiple regeneration. According to Table 10, we could conclude that MAEX resin had little adsorption capacity loss after 10 cycles of adsorption-desorption and had a high regeneration efficiency, which can save the cost of P removal from wastewater.
Characterization of MAEX resin before and after adsorption
FTIR could provide detailed information about the change of ions and functional groups in resin. As shown in Fig. 13, there were obvious absorption bands appeared at 1074 cm−1, 968 cm−1, and 634 cm−1, while the absorption bands around 1070 cm−1, 970 cm−1, and 630 cm−1 were respectively attributed to PO43− and HPO42− (Weng 2010), indicating that MAEX resin had an effective adsorption of P in the solution. The absorption band around 463 cm−1 (the symmetrical telescopic vibration band of C–Cl) (Weng 2010) decreased significantly after adsorption, which represented that the content of chloride in MAEX resin decreased, presumably since the chloride was exchanged by P and transferred into solution. In addition, the absorption bands around 3400 cm−1 to 3230 cm−1 and 3075 cm−1 to 3030 cm−1, attributed to the stretching vibration of alcoholic hydroxyl (Liu et al. 2018) and symmetric stretching vibration of ammonia (Ma et al. 2005), respectively, shifted or changed the intensity after adsorption, which implied that, besides ion exchange, there are other adsorptions (e.g., charge attraction and hydrogen bonding) which affected the removal of P by MAEX resin.
The magnetic hysteresis loop describing the magnetization versus magnetic field was presented in Fig. 14. The coercive force (Hc) value of original MAEX resin was 58.22 Oe (0.73 A/m). According to the value of coercive force, the ferromagnetic materials are divided into soft magnetic materials with low coercive force (Hc < 102 A/m) and hard magnetic materials with high coercive force (Hc > 102 A/m) (Li 2014), explaining that MAEX resin is a kind of soft magnetic materials. Moreover, the saturation magnetization (Ms) values of MAEX resins before and after adsorption were 3.05 emu/g and 2.62 emu/g, respectively. Although the value of Ms decreased slightly after adsorption, the resin still had good magnetic properties and did not affect the application of MAEX resin.
Adsorption mechanism
Based on the results of kinetics, thermodynamics, and infrared analysis, it could be speculated that during the initial stage of adsorption, ion exchange had acted a leading role since there were enough active ion-exchange sites on the surface of the resin to exchange P from the solution. However, along with reacting, the limited active ion-exchange sites could not meet the need for adsorption of P. Once the adsorption reach to the saturation state of monomolecular adsorption, MAEX resin adsorbed P mainly through Van der Waals force and hydrogen bond. Nevertheless, compared with ion exchange, the reaction rate of physical adsorption was slower. The adsorption reaction would proceed slowly until reaching the saturation adsorption (shown in Fig. 15).
Conclusions
This work demonstrated that MAEX resin has a high phosphorus removal and recovery potential. Compared with traditional anion exchange resins, the MAEX resin had a stable magnetic property, which made it easy to aggregate and settle from water after adsorption. An important finding was that temperature had little effect on the adsorption capacity of P on MAEX resin, which illustrated this resin had a good P removal efficient in a wide range of temperature and could be used against the temperature fluctuation of wastewater in some circumstance. The MAEX resin had a rapid adsorption rate of P comparing with the other adsorbents. In addition, this resin could be successfully regenerated and had little adsorption capacity loss after 10 cycles of adsorption-desorption, which could reduce the overall cost of P removal.
All these results revealed that MAEX resin was a promising adsorbent in removing P from wastewater. Further work aiming at the evaluation of P removal effect in actual sludge dewatering filtrate and deep exploring of P adsorption mechanism by MAEX resin is currently in progress.
References
Abbasizadeh S, Keshtkar AR, Mousavian MA (2014) Sorption of heavy metal ions from aqueous solution by a novel cast PVA/TiO2 nanohybrid adsorbent functionalized with amine groups. J Ind Eng Chem 20:1656–1664
Agyei NM, Strydom CA, Potgieter JH (2000) An investigation of phosphate ion adsorption from aqueous solution by fly ash and slag. Cem Concr Res 30(5):823–826
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332
Bajpai AK, Rajpoot M, Mishra DD (2000) Static and kinetic studies on the adsorption behavior of sulfadiazene. Adsorption 6(4):349–357
Baup S, Jaffre C, Wolbert D, Laplanche A (2000) Adsorption of pesticides onto granular activated carbon: determination of surface diffusivities using simple batch experiments. Adsorption 6(3):219–228
Bhatnagar A, Sillanpää M (2011) A review of emerging adsorbents for nitrate removal from water. Chem Eng J 168(2):493–504
Bhattacharyya KG, Gupta SS (2006) Adsorption of Fe (III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption 12(3):185–204
Boyer TH, Singer PC (2005) Bench-scale testing of a magnetic ion exchange resin for removal of disinfection by-product precursors. Water Res 39(7):1265–1276
Chen N, Feng CP, Zhang ZY, Liu RP, Gao Y, Li M, Sugiura N (2012) Preparation and characterization of lanthanum (III) loaded granular ceramic for phosphorus adsorption from aqueous solution. J Taiwan Inst Chem E 43(5):783–789
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Ooi K, Hirotsu T (2005) Adsorption of phosphate from seawater on calcined MgMn-layered double hydroxides. J Colloid Interface Sci 290(1):45–51
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Ooi K, Hirotsu T (2006) Selective adsorption of phosphate from seawater and wastewater by amorphous zirconium hydroxide. J Colloid Interface Sci 297(2):426–433
Chouyyok W, Wiacek RJ, Pattamakomsan K, Sangvanich T, Grudzien RM, Fryxell GE, Yantasee W (2010) Phosphate removal by anion binding on functionalized nanoporous sorbents. Environ Sci Technol 44(8):3073–3078
Sartorius C, Von HJ, Tettenborn F (2012) Phosphorus recovery from wastewater—expert survey on present use and future potential. Water Environ Res 84(4):313–322
Dai J, Yang H, Yan H, Shangguan Y, Zheng Q, Cheng R (2011) Chem Eng JPhosphate adsorption from aqueous solutions by disused adsorbents: chitosan hydrogel beads after the removal of copper (II). Chem Eng J 166(3):970–977
Ding L, Wu C, Deng H, Zhang XX (2012) Adsorptive characteristics of phosphate from aqueous solutions by MIEX resin. J Colloid Interface Sci 376(1):224–232
Roy ED (2017) Phosphorus recovery and recycling with Ecol Eng: A review. Ecol Eng 98:213–227
Gan F, Zhou J, Wang H, Du C, Chen X (2009) Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Res 43(11):2907–2915
Gercel O, Ozcan A, Ozcan AS, Gercel HF (2007) Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl Surf Sci 253(11):4843–4852
Hameed BH, Ahmad AA, Aziz N (2007) Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem Eng J 133(1):195–203
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 144(2):235–244
Hao XD, Yi LK, Wang CC, Qiu FG (2010) Situation and prospects pf phosphorus recovery techniques. J Environ Sci-China 30(5):897–907
Huang W, Wang S, Zhu Z, Li L, Yao X, Rudolph V, Haghseresht F (2008) Phosphate removal from wastewater using red mud. J Hazard Mater 158(1):35–42
Jiang L (2016) Performance and mechanism of aqueous phosphorus removal by magnetic zeolite. Dissertation, Anhui University of Technology
Kan CC, Ibe AH, Rivera KKP, Arazo RO, Luna MDGD (2017) Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sus Env Res 27(4):163–171
Kitis M, Harman BI, Yigit NO, Beyhan M, Nguyen H, Adams B (2007) The removal of natural organic matter from selected Turkish source waters using magnetic ion exchange resin (MIEX®). React Funct Polym 67(12):1495–1504
Kuwahara Y, Tamagawa S, Fujitani T, Yamashita H (2016) Removal of phosphate from aqueous solution using layered double hydroxide prepared from waste iron-making slag. Bull Chem Soc Jpn 89(4):472–480
Li JM (2014) The investigations on the electrochemical fabrication and related properties of nanocrystalline magnetic thin films. Dissertation, Shanghai Jiao Tong University
Li Y, Zhao XL, Huang YM (2013) The adsorptive removal of phosphorus by Fe3O4 magnetic nanoparticles as an adsorbent. J Southwest Univ N 35(5):127–130
Liu G, Li J, Yang X, Han GR (2015) Study on the adsorption capacity of ternary (Fe-Mg-Al) layered hydroxides for phosphorus. Ind Water Treat 35(1):62–64
Liu W, Yan XD, Wu M, Sun MX, Liu HY, Dai J (2016) Fluorescence spectroscopy analysis of domestic wastewater based on molecular weight distribution. Hubei Agric Sci 55(4):872–877
Liu X, Chen ZQ, Han B, Su CL, Han Q, Chen WZ (2018) Biosorption of copper ions from aqueous solution using rape straw powders: optimization, equilibrium and kinetic studies. Ecotoxicol Environ Saf 150:251–259
Liu ZQ, Yan XM, Drikas M, Zhou DN, Wang DS, Yang M, Qu JH (2011) Removal of bentazone from micro-polluted water using MIEX resin: kinetics, equilibrium, and mechanism. J Environ Sci 23(3):381–387
Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS (2014) Removal and recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol 44(8):847–907
Lwin CM, Murakami M, Hashimoto S (2017) The implications of allocation scenarios for global phosphorus flow from agriculture and wastewater. Resour Cnsv Recycl 122:94–105
Ma FF, Zhao BW, Zhong JK, Diao JR, Zhang Y (2015) Characteristics phosphate adsorption onto biochars derived from dairy manure and its influencing factors. China Environ Sci 35(4):1156–1163
Ma ZY, Guan YP, Liu HZ (2005) Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J Polym Sci A Polym Chem 43(15):3433–3439
Mergen MR, Jefferson B, Parsons SA, Jarvis P (2008) Magnetic ion-exchange resin treatment: impact of water type and resin use. Water Res 42(8–9):1977–1988
Morse GK, Brett SW, Guy JA, Lester JN (1998) Review: phosphorus removal and recovery technologies. Sci Total Environ 212(1):69–81
Ozacar M (2003) Adsorption of phosphate from aqueous solution onto alunite. Chemosphere 51(4):321–327
Ozmen F, Kavakli PA, Guven O (2011) Removal of phosphate by using copper-loaded poly (N-vinylimidazole) hydrogels as polymeric ligand exchanger. J Appl Polym Sci 119(1):613–619
Sarkar K, Sen K, Lahiri S (2017) Radiometric analysis of isotherms and thermodynamic parameters for cadmium (II) adsorption from aqueous medium by calcium alginate beads. J Radioanal Nucl Chem 312(4):1–12
Schütte T, Niewersch C, Wintgens T, Yüce S (2015) Phosphorus recovery from sewage sludge by nanofiltration in diafiltration mode. J Membr Sci 480:74–82
Shen W, Huang H (2011) Study on phosphorus in municipal wastewater adsorbed by sulfuric acid-activated coal-measures kaoline. Coal Convers 34(1):78–61
Sheshdeh RK, Abbasizadeh S, Nikou MRK, Badii K, Sharafi MS (2014) Liquid phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J Environ Health Sci Eng 12:148
Shih YJ, Abarca RRM, MDG d L, Huang YH, Lu MC (2017) Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: effects of pH, phosphate concentration and coexisting ions. Chemosphere 173:466–473
Song MY, Li M, Liu JJ (2017) Uptake characteristics and kinetics of inorganic and organic phosphorus by Ceratophyllum demersum. Water Air Soil Pollut 228(11):407
Talebi M, Abbasizadeh S, Keshtkar AR (2017) Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf Environ Prot 109:340–356
Toor M, Jin B (2012) Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem Eng J 187(2):79–88
Treybal ER (1981) Mass transfer operations. McGraw-Hill International editions, Singapore
Ugurlu A, Salman B (1998) Phosphorus removal by fly ash. Environ Int J Environ Sci Risk Health 24(8):911–918
Wang SD, Kong LJ, Long JY, Su MH, Diao ZH, Chang XY, Chen DY, Song G, Shih K (2018) Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 195:666–672
Wang YX (2012) Study on phosphorus recovery from excess sludge by ferric salt modified oyster shell. Dissertation, Dalian University of Technology
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. Asce Sanit Eng Div J 1(2):1–2
Weng SP (2010) Fourier transform infrared spectrometric analysis. Chemical Industry Press, Beijing
Wu FC, Tseng RI, Jung RS (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35(3):613–618
Xu GY, Yin HQ, Hu JL, Sun QW (2016) Studies on the preparation of modified rectorite and its adsorbing performance for phosphorus in water. Chem Res Appl 28(07):1025–1029
Xu XM (2013) Synthesis of magnetic CMC-CoFe2O4 compounds and research on the phosphate removal and recovery from wastewater. Dissertation, Harbin Engineering University
Xu Y, Peng H, Yang Y, Zhang Y, Wang S (2014) A cumulative eutrophication risk evaluation method based on a bioaccumulation model. Ecol Model 289(289):77–85
Yuan ZG, Pratt S, Batstone DJ (2012) Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol 23(6):878–883
Zhang L, Loáiciga HA, Xu M, Du C, Du Y (2015) Kinetics and mechanisms of phosphorus adsorption in soils from diverse ecological zones in the source area of a drinking-water reservoir. Int J Environ Res Public Health 12(11):14312–14326
Zhao BQ (2007) Study on adsorption and recovery of phosphate by magnetic hydrotalcite-like compounds in aqueous solution. Dissertation, Harbin Institute of Technology
Funding
This work was supported by the National Natural Science Foundation of China (No. 51279004) and the Fundamental Research Funds for the Central Universities (No. 2015ZCQ-HJ-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, M., Li, M. Adsorption and regeneration characteristics of phosphorus from sludge dewatering filtrate by magnetic anion exchange resin. Environ Sci Pollut Res 26, 34233–34247 (2019). https://doi.org/10.1007/s11356-018-4049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-4049-9