Abstract
The source-receptor relationship of volatile organic compounds (VOCs) is an important environmental concern, particularly in large industrial cities; however, only a few studies have identified VOC sources using high spatial resolution data. In this study, 28 VOCs were monitored in Ulsan, the biggest multi-industrial city in Korea. Passive air samplers were seasonally deployed at eight urban and six industrial sites. The target compounds were detected at all sites. No significant seasonal variations of VOCs were observed probably due to the continuous emissions from major industrial facilities. Benzene, toluene, ethylbenzene, xylenes, and styrene accounted for 66–86% of the concentration of Σ28 VOCs. The spatial distribution of the individual VOCs clearly indicated that petrochemical, automobile, non-ferrous, and shipbuilding industries were major VOC sources. Seasonal wind patterns were found to play a role in the spatial distribution of VOCs. Diagnostic ratios also confirmed that the industrial complexes were the dominant VOC sources. The results of principal component analysis and correlation analyses identified the influence of specific compounds from each industrial complex on individual sites. To the best of our knowledge, this is the first comprehensive report on the seasonal distribution of VOCs with high spatial resolution in a metropolitan industrial city in Korea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs), a major type of hazardous air pollutants (HAPs), significantly affect air quality, and they can have an adverse effect on human health (Jo et al. 2012; Valach et al. 2014). VOCs are emitted from both natural sources such as shrubs and grasslands and anthropogenic sources such as industrial activities and vehicle emissions (Bhattacharya et al. 2015; Tassi et al. 2013). VOCs react with nitrogen oxides (NOX) in the presence of sunlight, then ozone (O3) and secondary organic aerosols (SOA) are produced (Sarkar et al. 2014; Warneke et al. 2014). They can adversely affect crop yield, air quality, and human health (Pugliese et al. 2014; Villanueva et al. 2014), causing complications such as asthma, allergies, headaches, and chronic obstructive pulmonary diseases (Chen et al. 2015, 2016; Saalberg and Wolff 2016). On account of these possible severe health effects, some carcinogenic compounds (e.g., 1,3-butadiene, benzene, and vinyl chloride) (IARC 2016) have been listed in the US-EPA HAPs list (US-EPA 1999).
Passive air samplers, which collect VOCs through molecular diffusion onto activated charcoal in an adsorption cartridge, are generally used to monitor both indoor and outdoor VOCs (Demirel et al. 2014; Geiss et al. 2011; Tovalin-Ahumada and Whitehead 2007). Performance evaluations (Pennequin-Cardinal et al. 2005) and comparisons of samplers (Lan and Binh 2012) have also been conducted. Passive air samplers provide relatively high spatial resolution data of VOCs because they are cost-effective and easy to operate without electricity (Bruno et al. 2008; Gallego et al. 2011). Seasonal monitoring studies using passive air samplers were conducted on 58 VOCs at 40 industrial sites in Aliaga, Turkey (Dumanoglu et al. 2014), on benzene, toluene, ethylbenzene, and xylenes (BTEX) at 50 industrial sites in Windsor, Canada (Miller et al. 2012), and on BTEX at four urban sites in Delhi, India (Hoque et al. 2008); however, the results of these studies did not fully reflect the seasonal VOC variations because the samplers were only used for short periods of only 1 or 2 weeks in each of the four seasons or were deployed for only one or two seasons (Bruno et al. 2008; Kerchich and Kerbachi 2013; Miller et al. 2009; Roukos et al. 2009; Terrés et al. 2010).
A variety of methods have been applied to source identification of VOCs. For example, diagnostic ratios are frequently used to characterize traffic emission, photochemical age, and long-range transport (Miller et al. 2012; Tiwari et al. 2010). A simple statistical approach, such as correlation analysis, can provide information on potential sources of VOCs (Hoque et al. 2008; Miller et al. 2009). In addition, multivariate statistics can be used; principle component analysis (PCA) extracts several factors related to the main sources of target compounds (An et al. 2014; Hsieh et al. 2006). Furthermore, positive matrix factorization (PMF) can be applied to identify VOC sources and quantify their contributions (Civan et al. 2015; Dumanoglu et al. 2014; Zhang et al. 2014).
According to the Pollutant Release and Transfer Registers (PRTR) system in Korea (MOE 2014e), the metropolitan city of Ulsan had the highest emission density (7.7 t/km2) in Korea, emitting 8139 t of toxic chemicals annually. Ulsan is basically an industrial city that has many large-scale industrial complexes (e.g., petrochemical, automobile, non-ferrous, and shipbuilding industries), all of which have been found to be major VOC emission sources. Recent studies on ambient air pollution in Ulsan have focused on polycyclic aromatic hydrocarbons (PAHs) (Choi et al. 2012) and criteria air pollutants (CAPs) (Clarke et al. 2014), such as ozone (Lee et al. 2007; Susaya et al. 2013) and particulate matter (PM) (Lee et al. 2004); however, very few VOC studies have been conducted using active air samplers at several sites (Na et al. 2001; Shin and Jo 2012). Although there are two national HAPs monitoring network stations in Ulsan, it has been difficult to determine the spatial distribution of VOCs. Therefore, the source-receptor relationship of VOCs in Ulsan based on their spatial distributions has not been fully investigated yet.
In this study, passive air sampling of VOCs was seasonally conducted at 14 sites in Ulsan, Korea. The objectives of this study were (1) to investigate the levels and patterns of VOCs, (2) to determine spatial and temporal distributions of VOCs, and (3) to identify the main sources of VOCs. To the best of our knowledge, this is one of the most comprehensive VOC monitoring studies conducted in industrial cities in Korea and the first to conduct VOC monitoring using passive air samplers in Ulsan.
Materials and methods
Passive air sampling
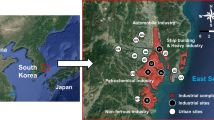
The study area (1061 km2) is located in the southeastern part of South Korea along the East Sea (Fig. 1). Passive air samplers were deployed at 14 sites, which were classified into eight urban (U1–U8) and six industrial (I1–I6) sites (Fig. S1 in the Supplementary Information). Among the 14 sites, 11 sites were part of the urban air monitoring network operated by the Ulsan Institute of Health and Environment (UIHE). Three further sites were selected from two industrial areas (I5 and I6) and one urban area (U2) to increase the spatial resolution of the VOC data. The three sites were classified by their distance from industrial complexes and population.
The passive air samplers (Radiello™ diffusive sampling system) were purchased from SIGMA-ALDRICH (https://www.sigmaaldrich.com). It has a diffusive body, a supporting plate, and an adsorption cartridge made up of a stainless steel net cylinder with a 100 mesh-grid opening of 5.8 mm diameter. The net cylinder was packed with 530 mg of activated charcoal with a particle size of 35–50 mesh (Fig. S2). The samplers were deployed over four seasons, with two monitoring periods: summer (July 2–31 and July 31–August 29, 2014), fall (October 8–November 7 and November 7–December 5, 2014), winter (January 8–February 3 and February 3–March 3, 2015), and spring (April 10–May 8 and May 8–June 7, 2015). Back diffusion is likely to occur for long sampling time, but the sampling time in this study was about 30 days, which is within the range of sampling time (15 min to 30 days) using the Radiello™ diffusive sampling system. In addition, back diffusion was not considered based on the reference sampling volume of 3050 L at 10 μg/m3 of benzene before back diffusion occurred. Duplicate samples were collected from all sampling sites in spring 2015 and from four industrial sites in the other seasons. As samples at one industrial site (I5) were lost during the first spring 2015 monitoring period, a total of 162 samples were collected for assessment. After the sampling was completed, all cartridges were stored in glass containers at − 4 °C until analysis.
Analysis and QA/QC
There were 28 compounds targeted in this study: benzene, ethylbenzene, m,p-xylenes, o-xylene, styrene, toluene, 1,1,1-trichloroethane, 1,1,2-trichloroethane, 1,1-dichloroethylene, 1,2,4-trichlorobenzene, 1,2-dichlorobenzene, 1,2-dichloroethane, 1,2-dichloropropane, 1,4-dichlorobenzene, bromodichloromethane, bromoform, carbon tetrachloride, chlorobenzene, chloroform, cis-1,2-dichloroethylene, dibromochloromethane, dichloromethane, tetrachloroethylene, trans-1,2-dichloroethylene, trichloroethylene, vinyl chloride, 1,3-butadiene, and methyl tert-butyl ether (MTBE). These compounds are classified into aromatic, halogenated, and alkane and oxygenated groups. Detailed information on the target compounds is shown in Table S1 in the Supplementary Information.
After sampling, the target compounds were extracted from the cartridges using 2 mL of carbon disulfide (CS2). As surrogate standards, deuterated compounds (methylene chloride-d2, 1,2-dichloroethane-d4, benzene-d6, toluene-d8, chlorobenzene-d5, ethylbenzene-d10, 1,2-dichlorobenzene-d4, and 1,4-dichlorobenzene-d4) were inserted into the cartridges before extraction, after which the samples were extracted for 30 min using an ultrasonic bath, and a 1 mL extract was transferred to a vial. Before the analyses, fluorobenzene was added to the vial as an internal standard.
After the pretreatment procedure, the samples were analyzed using a gas chromatograph/mass spectrometer (GC/MS, Agilent 7890N/5975C). A DB-624 column (60 m × 0.25 mm i.d. and 1.4 μm film thickness) was used to separate the target compounds, and a helium carrier gas with a flow rate of 1.0 mL/min was employed. Of the final sample, 1 μL was injected into the GC in split mode (25:1 and 30 mL/min), and selective ion monitoring (SIM) mode was applied. The oven temperatures were as follows, 40 °C for the initial 3 min, which was then increased at 8 °C/min up to 90 °C and maintained for 4 min, and then increased at 6 °C/min up to 200 °C and maintained for 5 min. The ions were observed in electron ionization (EI) mode.
Field blanks were taken (n = 24 for the whole monitoring periods), and data were blank corrected. The average sample recovery ranged from 40 to 70%. The method detection limits (MDLs) were calculated using the following equation.
where ts is the Student’s t value at 99% confidence, and SD is the standard deviation for each target chemical’s concentration in spiked samples (n = 7, spiked amount 0.1 μg). The MDL samples were analyzed using the same analytical procedure as for the real samples. The MDLs were in a range of 0.017–0.200 μg/m3. The mean coefficient of variation (CV) for total VOCs between the duplicated samples was 8.2 ± 4.0%.
Calculation of VOC concentrations
Time-averaged concentrations (C) were calculated using Eq. 2 (Maugeri-IRCCS 2006).
where M is the mass of chemical adsorbed by the cartridges (μg), t is the sampling period (min), and Qk is the chemical-specific sampling rate calculated using Eq. 3.
where Q298 is the sampling rate in the normal condition (298 K) provided by the manufacturer (Maugeri-IRCCS 2006), and K is the absolute temperature during the sampling period.
Statistical analysis
A rank sum test using SigmaPlot 12.0 (Systat Inc., USA) was conducted to examine the statistical differences between the groups. Correlation and principal component analyses (PCA) using a VARIMAX rotation were performed using IBM SPSS statistics 20.0 (IBM, USA) for source identification. The input data from each season were slightly different because several VOCs that had less than a 50% detection were excluded. For the PCA, the top 10 VOCs with the highest concentrations for the individual seasons were selected as input data, which were normalized using the sum of the ten selected compounds for each sample.
Results and discussion
Seasonal variations of total VOCs
The concentrations of total VOCs were in a range of 7.8–62.0 μg/m3 (mean 23.6 μg/m3, median 18.2 μg/m3) in summer, 12.7–54.8 μg/m3 (mean 28.1 μg/m3, median 25.9 μg/m3) in fall, 11.0–40.0 μg/m3 (mean 19.6 μg/m3, median 16.8 μg/m3) in winter, and 11.2–67.0 μg/m3 (mean 32.3 μg/m3, median 29.5 μg/m3) in spring (Fig. 2). The average concentration of total VOCs was the highest in spring, followed by fall, summer, and winter. Statistical differences were found between spring and summer, spring and winter, and fall and winter (rank sum test, p < 0.05). The same seasonal patterns of total VOCs were observed for the industrial and residential sites.
The concentrations of VOCs can increase in the warmer seasons because of the enhanced volatilization from the surface environment, and elevated levels of VOCs in the cold seasons can occur in stable atmospheric conditions (i.e., limited dilution). For these reasons, previous studies have reported different seasonal patterns of VOC levels: spring>summer>winter>fall in rural areas in Luding, China (Zhang et al. 2014), fall>spring>summer>winter in industrial areas in Aliaga, Turkey (Dumanoglu et al. 2014), summer>spring>fall>winter in industrial areas in Nanjing, China (An et al. 2014), and winter>spring>fall>summer in urban areas in Rome, Italy (Fanizza et al. 2014). Consequently, it is difficult to generalize a seasonal trend of VOCs from former studies.
Even though the concentrations of total VOCs in Ulsan were seasonally different, the concentrations in spring were only 1.6 times higher than those measured in winter. This result indicates that the seasonal variations of VOCs in Ulsan were not severe, possibly because of the relatively constant emissions from industrial facilities throughout the year and meteorological conditions (Table S2). For example, evaporation of VOCs can be enhanced in the summer by increased temperature, but more precipitation also results in a decrease in VOC concentrations. The influence of seasonal wind patterns will be discussed in the section “Spatial distribution of VOCs”. Also, the HAPs monitoring network of Korea indicated weak seasonal variations of VOCs in Ulsan (MOE, 2014a, b, c, d, 2015a, b, c, d).
Levels of individual VOCs
The annual mean concentrations for each compound are shown in Fig. 3, and the seasonal mean concentrations for each compound are shown in Fig. S3. The aromatic group was found to have the highest mean concentration (20.1 μg/m3), followed by the halogenated (5.9 μg/m3) and the alkane and oxygenated groups (0.7 μg/m3). The aromatic group accounted for 75% of the annual concentration of total VOCs. Toluene was found to have the highest mean concentration (8.4 μg/m3), followed by ethylbenzene (3.2 μg/m3), m,p-xylenes (3.0 μg/m3), o-xylene (2.7 μg/m3), and benzene (2.2 μg/m3) (Fig. 3). Three compounds (1,1-dichloroethylene, bromodichloromethane, and chlorobenzene) were not detected in the 28 target compounds. The seasonal patterns for the target compounds were generally similar (Fig. S3).
The concentrations of BTEX in this study were compared with data from previous studies (Table 1). Toluene is also a dominant compound in the previous studies. The results in this study (19.6 μg/m3 of Σ BTEX) were similar to or higher than the results from other countries except for Italy (41.9 μg/m3) (Bruno et al. 2008), which was directly influenced by vehicular emissions. In contrast, a clear difference was found between Ulsan and Seoul in Korea, with the Σ BTEX concentrations in this study being two times higher in Ulsan than in Seoul (9.9 μg/m3) (Kim et al. 2012); however, the levels of toluene in both cities were similar because of common toluene sources (e.g., vehicle fuels, paints, paint thinners, rubbers, and adhesives) in the urban and industrial areas. In Ulsan, the concentrations (40.5 μg/m3) in 1997 (Na et al. 2001) were reported to be much higher than those in Seoul and the results from this study, which could suggest that BTEX emissions in Ulsan had decreased substantially by enforced governmental regulations through the Clean Air Conservation Act. Since 1996, the emissions of VOCs from industrial facilities have been regulated by this act; however, recent data from the HAPs monitoring network (MOE, 2014a, b, c, d, 2015a, b, c, d) showed no apparent VOC decreases at the two sites (urban: Sinjeong (U4), industrial: Yeocheon (I2)) in Ulsan after the mid-2000s, which may have been because of the larger population, the greater number of registered vehicles, and petrochemical products.
The HAPs monitoring network data (13 compounds) in Ulsan over the same period (July 2014–June 2015) were compared with our data (Fig. S4), from which it was found that the VOC concentrations (especially BTEX) in this study were higher than those measured at the urban site. An opposite result, particularly for m,p-xylenes, was observed at the industrial site in fall 2014. These differences could be explained from the time-averaged concentrations derived for this study, whereas 13 VOCs were measured for 1 day (24 h) per month using active air samplers in the HAPs monitoring network. Nonetheless, the overall levels and patterns for the 13 VOCs in the two studies were comparable.
Spatial distribution of VOCs
The annual and seasonal mean concentrations of total VOCs and BTEX at each sampling site are shown in Fig. S5. As expected, the industrial sites showed higher levels of VOCs than the urban sites; the annual concentrations of total VOCs and BTEX were 34.5 μg/m3 and 24.9 μg/m3 at the industrial sites and 20.7 μg/m3 and 15.7 μg/m3 at the urban sites, respectively. Similar spatial distributions were observed during all seasons except for Sites I2 and U2. Although Site I2 is close to a petrochemical industrial complex, the VOC concentrations were lower than those at other industrial sites and similar to the concentrations at the urban sites possibly due to the predominant wind directions (northwesterly). On the contrary, high VOC concentrations similar to those at the industrial sites were found at an urban site (U2), possibly because of a nearby automobile industrial complex with painting, adhesion, cleaning, and coating processes.
The spatial distributions of VOCs were also examined using a geographic information system (ArcGIS 10.4.1, ESRI Inc., USA). As an interpolation method, inverse distance weighting (IDW) was employed. Contour plots of seasonal distributions of total VOCs and wind roses at the Ulsan meteorological observatory (MO) are presented in Fig. 4, and the annual distributions of total VOCs are shown in Fig. S6. The annual levels of total VOCs at all industrial sites and one urban site (U2) were higher than at the other urban sites, indicating that the four major industrial complexes (petrochemical, automobile, non-ferrous, and shipbuilding industries) are the primary sources of VOCs. This result was also supported by similar spatial distributions of the annually measured BTEX and the PRTR emission data, as shown in Fig. S7. More specifically, the levels of benzene were relatively high at Site I6 in the petrochemical industrial complex and at Site I4 in the non-ferrous industrial complex, and these areas also had larger benzene emissions. Meanwhile, the emission data from the petrochemical industrial complex did not strongly match the measured data because benzene emissions may have mixed sources including vehicle exhaust. For toluene, both the measured data and the emission data showed higher levels at the automobile industrial complex. The ethylbenzene and xylene concentrations were higher in the automobile, shipbuilding, and petrochemical industrial complexes. These measured data were also supported by the emission data.
Sites U2 and I6 were found to be hot spots of VOC pollution in summer (Fig. 4a), probably because the toluene-emitting automobile industrial facilities are densely distributed around Site U2 (also I1) (Fig. S7). The BTEX measured data and emission data were found to have similar spatial distributions; however, Site I6 has a benzene concentration (15.06 μg/m3) three times higher than the annual European Union limit of 5 μg/m3 because of the strong influence of the petrochemical processes as well as vehicle exhaust, which was also suspected to be a major benzene source (Miller et al. 2012). This result is discussed further in the next section of source identification. The concentrations of total VOCs in spring (Fig. 4d) were higher than in summer at all sampling sites except I6, with the similar spatial distributions in both seasons because of the prevailing easterly and westerly winds. In fall and winter, the northwesterly winds were speculated to bring large amounts of VOCs emitted from the industrial complexes to the East Sea. As northeasterly winds were also observed in fall (Fig. 4b), this wind pattern appeared to be the reason for the elevated ethylbenzene and xylene concentrations at Site I5, with the major ethylbenzene and xylene sources located in a northeast direction from Site I5 (Fig. S7). These results indicate that wind patterns played an important role in the spatial distributions of VOCs. More discussion on the distributions of the other minor compounds can be found in Text S1 in the Supplementary Information.
Source identification of VOCs
The monitoring data can be used to identify the major air pollutant sources using various diagnostic ratios and statistical analyses (e.g., PCA and correlation analysis). Toluene/benzene (T/B) and m,p-xylenes/ethylbenzene (X/E) ratios have been widely used to obtain preliminary source information. The T/B ratio can be used to evaluate the influence of traffic and non-traffic sources (Shi et al. 2015). Both benzene and toluene are emitted from vehicles, but benzene is a well-known marker of the vehicular exhaust (Miller et al. 2012), and toluene is mainly evaporated from paint solvents (Liu et al. 2008). Several previous studies have reported that ratios lower than 1 (Tiwari et al. 2010), 2 (Yurdakul et al. 2013), 3 (Jaars et al. 2014), 4.3 (Miller et al. 2012) or in a range of 1.5–3.0 (Miller et al. 2011) indicate a high traffic source contribution, and higher ratios indicate non-traffic sources (e.g., solvent evaporation); a much higher T/B ratio could also indicate the effect of industrial facilities (Ho et al. 2004). Based on these previous studies, in this study, a T/B ratio of three was used to distinguish the traffic (< 3.0) and non-traffic effects (> 3.0).
The T/B ratios for all data are shown in Fig. 5a. The ratios were in ranges of 0.8–23.4 (mean 6.9) in summer, 1.7–12.9 (mean 7.0) in fall, 1.2–6.9 (mean 3.9) in winter, and 1.0–18.3 (mean 5.9) in spring, with the non-traffic effects being more dominant in all seasons. Much higher T/B ratios were observed at two urban sites (U2 and U3) close to automobile and shipbuilding industrial complexes, indicating that these industries were a primary toluene source. Meanwhile, much lower T/B ratios were found at Sites I3, I4, and I6. As Sites I3 and I4 were respectively next to roads in the petrochemical and non-ferrous industrial complexes, there would be higher benzene concentrations from both the traffic and the industrial effects. Site I6, which was also near the petrochemical industrial complex, was possibly more influenced by traffic emissions in spring and summer due to the whale museum, one of the most famous tourist attractions in Ulsan; Korean tourism statistics (http://www.tour.go.kr/) reported that 5.4 times more tourists visit the museum for whale watching cruises in summer and spring (18,666 people) than in fall and winter (3459 people). In Fig. 5b, data from Sites U2, U3, I3, I4, and I6 were excluded to better understand the differences between the industrial and urban sites, from which it can be seen that the ratios at several of the industrial sites were significantly higher than those at the urban sites. The T/B ratios in winter were generally lower than those in the other seasons. This observation can be explained by the seasonal variation of fuel formulations, which is more volatile in winter (Miller et al. 2012), and more evaporation of gasoline with a high content of toluene in summer (Ho et al. 2004). Furthermore, the major northwesterly winds (Fig. 4c) in the winter could reduce the influence of toluene emissions from the industrial complexes.
The X/E ratio indicates the aging of VOCs in the atmosphere; that is, it can diagnose the effects of local pollution, transport, or photochemical reactions (Nelson and Quigley 1983). The atmospheric lifetimes of benzene and toluene are 12.5 and 2.0 days, respectively (Liu et al. 2008), which are rather stable, while those of m,p-xylenes and ethylbenzene are 3 and 8 h, respectively (Shi et al. 2015). As m,p-xylenes react more rapidly than other compounds, a lower X/E ratio indicates the aging of VOCs in the atmosphere. The X/E ratios in this study were in ranges of 0.8–1.7 (mean 1.0) in summer, 0.8–1.2 (mean 0.9) in fall, 0.7–1.6 (mean 1.0) in winter, and 0.7–1.2 (mean 0.9) in spring; therefore, there were no significant differences in X/E ratios between the seasons and the sites. This result suggests that they are emitted continually from the industrial facilities regardless of the season. In previous studies, X/E ratios were 2.6–3.7 (mean 3.0) at urban sites in Canada (Miller et al. 2012), 1.1–3.0 (mean 2.0) at industrial sites in China (Shi et al. 2015), and 0.4–0.7 (mean 0.5) at urban and industrial sites in Taiwan (Liu et al. 2008). The ratios in this study were a little lower or similar to those reported in other studies.
The results of the PCA for spring are shown in Fig. 6, and the results for the other seasons are shown in Fig. S8. The PCA results for the different seasons were similar. In the spring data, three components (X-axis: PC1, Y-axis: PC2, and Z-axis: PC3) with Eigenvalues over 1 were selected, which respectively accounted for 37%, 21%, and 15% of the total variances. Three groups were classified in the score plot (Fig. 6a): Group 1 (U2, U3, I1, and I5), Group 2 (urban sites), and Group 3 (I2, I4, and I6). The loading plot (Fig. 6b) shows that toluene, o-xylene, ethylbenzene, and m,p-xylenes were more influential in Group 1. Actually, Sites I1 and U2 are located in the automobile industrial complex, which mainly emits toluene (Fig. S7b). Meanwhile, Sites I5 and U3 were contaminated mainly with xylenes and ethylbenzene from the shipbuilding industrial complex, and Group 3 had higher contributions of cis-1,2-dichloroethylene, benzene, and chloroform. As discussed, relatively high benzene concentrations were detected at Sites I4 and I6. The urban sites in Group 2 were possibly influenced by the various sources of Groups 1 and 3 and Site I3 (petrochemical facilities). The urban sites were also characterized by vinyl chloride, carbon tetrachloride, and MTBE (Figs. 6 and S8). As Site I2 is at the edge of the petrochemical industrial complex and close to the urban sites, this site had similar characteristics to the urban sites. Site I3, characterized by 1,2-dichloropropane, was found to vary significantly from the three groups in all seasons. 1,2-dichloropropane is widely used in industrial coatings (Li et al. 2016), lacquers, and varnishes (Vega et al. 2011), implying that the compound can be an indicator for the petrochemical industry.
The results for the Pearson’s correlation analysis in all seasons are shown in Tables S3–6, in which three groups are shaded (strong: r > 0.8, good: r = 0.6–0.8, and negative: r < − 0.5). Aromatic compounds showed relatively strong (r > 0.8) and good (r = 0.6–0.8) correlations with each other except for benzene and styrene. Ethylbenzene and m,p-xylenes were strongly correlated (r = 0.914, 0.968, 0.939, and 0.962 in summer, fall, winter, and spring, respectively) as these two compounds were primarily emitted from the same source, the shipbuilding industrial complex.
In addition, a strong correlation (r > 0.8) was found between styrene and 1,2-dichloropropane in spring, summer, and fall, with these two compounds showing much higher concentrations at Site I3 except in winter, which implies that the compounds are indicators of emissions from the petrochemical industrial complex. Benzene and tetrachloroethylene, which are considered as industrial emission markers (Zhang et al. 2015), showed higher concentrations at Site I6 than at the other sites, and they were highly correlated in summer (r = 0.818) and spring (r = 0.708). In spring, vinyl chloride was negatively (r < − 0.5) correlated with most other compounds, indicating that vinyl chloride might have different sources and emission patterns in Ulsan. In fall and spring, strong correlations (r = 0.742–0.848) were found between cis-1,2-dichloroethylene and trichloroethylene. Their concentrations were somewhat higher at Site I4 than at the other sampling sites, indicating that they could have been influenced by the use of metal degreasers (US-EPA 2001) in the non-ferrous industrial complex.
Conclusion
In this study, spatial and temporal variations of VOCs in Ulsan, Korea were examined using passive air samplers. The temporal variations of VOCs were not significantly different probably due to the continuous emissions from major industrial facilities. The spatial distributions of VOCs indicated that major industries, especially the petrochemical and shipbuilding industries, were the main VOC sources. In addition to the industrial sites, some urban sites were also found to be significantly affected by VOCs emitted from the industrial complexes. Therefore, periodic monitoring of VOCs is recommended in Ulsan. It can be concluded that passive air sampling is a powerful tool for identifying major VOC sources in multi-industrial cities. In addition, the PRTR emission database developed by the Korea Ministry of Environment was found to be useful for source tracking and could also be used for the prediction of a source-receptor relationship without the need for monitoring and for input data of air dispersion models.
References
An J, Zhu B, Wang H, Li Y, Lin X, Yang H (2014) Characteristics and source apportionment of VOCs measured in an industrial area of Nanjing, Yangtze River Delta, China. Atmos Environ 97:206–214
Bhattacharya SS, Kim K-H, Ullah MA, Goswami L, Sahariah B, Bhattacharyya P, Cho S-B, Hwang O-H (2015) The effects of composting approaches on the emissions of anthropogenic volatile organic compounds: a comparison between vermicomposting and general aerobic composting. Environ Pollut 208:600–607
Bruno P, Caselli M, Gennaro G, Scolletta L, Trizio L, Tutino M (2008) Assessment of the impact produced by the traffic source on VOC level in the urban area of Canosa di Puglia (Italy). Water Air Soil Pollut 193:37–50
Chen X, Luo Q, Wang D, Gao J, Wei Z, Wang Z, Zhou H, Mazumder A (2015) Simultaneous assessments of occurrence, ecological, human health, and organoleptic hazards for 77 VOCs in typical drinking water sources from 5 major river basins, China. Environ Pollut 206:64–72
Chen J, Huang Y, Li G, An T, Hu Y, Li Y (2016) VOCs elimination and health risk reduction in e-waste dismantlingworkshop using integrated techniques of electrostatic precipitation with advanced oxidation technologies. J Hazard Mater 302:395–403
Choi S-D, Kwon H-O, Lee Y-S, Park E-J, Oh J-Y (2012) Improving the spatial resolution of atmospheric polycyclic aromatic hydrocarbons using passive air samplers in a multi-industrial city. J Hazard Mater 241-242:252–258
Civan MY, Elbir T, Seyfioglu R, Kuntasal ÖO, Bayram A, Doğan G, Yurdakul S, Andiç Ö, Müezzinoğlu A, Sofuoglu SC, Pekey H, Pekey B, Bozlaker A, Odabasi M, Tuncel G (2015) Spatial and temporal variations in atmospheric VOCs, NO2, SO2, and O3 concentrations at a heavily industrialized region in Western Turkey, and assessment of the carcinogenic risk levels of benzene. Atmos Environ 103:102–113
Clarke K, Kwon H-O, Choi S-D (2014) Fast and reliable source identification of criteria air pollutants in an industrial city. Atmos Environ 95:239–248
Demirel G, Özden Ö, Döğeroğlu T, Gaga EO (2014) Personal exposure of primary school children to BTEX, NO2 and ozone in Eskişehir, Turkey: relationship with indoor/outdoor concentrations and risk assessment. Sci Total Environ 473-474:537–548
Dumanoglu Y, Kara M, Altiok H, Odabasi M, Elbir T, Bayram A (2014) Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmos Environ 98:168–178
Fanizza C, Incoronato F, Baiguera S, Schiro R, Brocco D (2014) Volatile organic compound levels at one site in Rome urban air. Atmos Pollut Res 5:303–314
Gallego E, Roca FJ, Perales JF, Guardino X (2011) Comparative study of the adsorption performance of an active multi-sorbent bed tube (Carbotrap, Carbopack X, carboxen 569) and a Radiello® diffusive sampler for the analysis of VOCs. Talanta 85:662–672
Geiss O, Giannopoulos G, Tirendi S, Barrero-Moreno J, Larsen BR, Kotzias D (2011) The AIRMEX study - VOC measurements in public buildings and schools/kindergartens in eleven European cities: statistical analysis of the data. Atmos Environ 45:3676–3684
Ho KF, Lee SC, Guo H, Tsai WY (2004) Seasonal and diurnal variations of volatile organic compounds (VOCs) in the atmosphere of Hong Kong. Sci Total Environ 322:155–166
Hoque RR, Khillare PS, Agarwal T, Shridhar V, Balachandran S (2008) Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Sci Total Environ 392:30–40
Hsieh L-T, Yang H-H, Chen H-W (2006) Ambient BTEX and MTBE in the neighborhoods of different industrial parks in Southern Taiwan. J Hazard Mater 123:106–115
IARC (2016) Agents classified by the IARC monographs, volumes 1–120, International Agency for Research on Cancer (IARC). http://monographs.iarc.fr/ENG/Classification/List_of_Classifications.pdf, Accessed 15 Dec 2016
Jaars K, Beukes JP, PGv Z, Venter AD, Josipovic M, Pienaar JJ, Vakkari V, Aaltonen H, Laakso H, Kulmala M, Tiitta P, Guenther A, Hellén H, Laakso L, Hakola H (2014) Ambient aromatic hydrocarbon measurements at Welgegund, South Africa. Atmos Chem Phys 14:7075–7089
Jo W-K, Chun H-H, Lee S-O (2012) Evaluation of atmospheric volatile organic compound characteristics in specific areas in Korea using long-term monitoring data. Environ Eng Res 17:103–110
Kerchich Y, Kerbachi R (2013) Measurement of BTEX (benzene, toluene, ethybenzene, and xylene) levels at urban and semirural areas of Algiers city using passive air samplers. J Air Waste Manage Assoc 62:1370–1379
Kim K-H, Ho DX, Park CG, Ma C-J, Pandey SK, Lee SC, Jeong HJ, Lee SH (2012) Volatile organic compounds in ambient air at four residential locations in Seoul, Korea. Environ Eng Sci 29:875–889
Lan TTN, Binh NTT (2012) Daily roadside BTEX concentrations in East Asia measured by the Lanwatsu, Radiello and ultra I SKS passive samplers. Sci Total Environ 441:248–257
Lee B-K, Jun N-Y, Lee HK (2004) Comparison of particulate matter characteristics before, during, and after Asian dust events in Incheon and Ulsan, Korea. Atmos Environ 38:1535–1545
Lee S-H, Kim Y-K, Kim H-S, Lee H-W (2007) Influence of dense surface meteorological data assimilation on the prediction accuracy of ozone pollution in the southeastern coastal area of the Korean Peninsula. Atmos Environ 41:4451–4465
Li J, RongrongWu LY, Hao Y, Xie S, Zeng L (2016) Effects of rigorous emission controls on reducing ambient volatile organic compounds in Beijing, China. Sci Total Environ 557-558:531–541
Liu P-WG, Yao Y-C, Tsai J-H, Hsu Y-C, Chang L-P, Chang K-H (2008) Source impacts by volatile organic compounds in an industrial city of southern Taiwan. Sci Total Environ 398:154–163
Maugeri-IRCCS FS (2006) Manual full version, Fondazione Salvatore Maugeri-IRCCS. http://www.radiello.com/english/Radiello%27s%20manual%2001-06.pdf, Accessed 1 Jan 2016
Miller L, Xu X, Luginaah I (2009) Spatial variability of volatile organic compound concentrations in Sarnia, Ontario, Canada. J Toxicol Environ Health A 72:610–624
Miller L, Xu X, Wheeler A, Atari DO, Grgicak-Mannion A, Luginaah I (2011) Spatial variability and application of ratios between BTEX in two Canadian cities. ScientificWorldJournal 11:2536–2549
Miller L, Xu X, Grgicak-Mannion A, Brook J, Wheeler A (2012) Multi-season, multi-year concentrations and correlations amongst the BTEX group of VOCs in an urbanized industrial city. Atmos Environ 61:305–315
MOE (2014a) Monthly report of air quality, July 2014, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=19481, Accessed 13 June 2016
MOE (2014b) Monthyl report of air quality, August 2014, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=19483, Accessed 13 June 2016
MOE (2014c) Monthyl report of air quality, November 2014, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=19490, Accessed 13 June 2016
MOE (2014d) Monthyl report of air quality, October 2014, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=19488, Accessed 13 June 2016
MOE (2014e) Pollutant release and transfer registers (PRTR), Ministry of Environment (MOE). http://ncis.nier.go.kr/triopen, Accessed 7 Oct 2015
MOE (2015a) Monthyl report of air quality, Feburuary 2015, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=42446, Accessed 13 June 2016
MOE (2015b) Monthyl report of air quality, April 2015, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=22274, Accessed 13 June 2016
MOE (2015c) Monthyl report of air quality, January 2015, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=19649, Accessed 13 June 2016
MOE (2015d) Monthyl report of air quality, May 2015, Ministry of Environment (MOE). http://www.airkorea.or.kr/file/download/?atch_id=22276, Accessed 13 June 2016
Na K, Kim YP, Moon K-C, Moon I, Fung K (2001) Concentrations of volatile organic compounds in an industrial area of Korea. Atmos Environ 35:2747–2756
Nelson PF, Quigley SM (1983) The m,p-xylenes:ethylbenzene ratio. A technique to estimating hydrocarbon age in ambient atmospheres. Atmos Environ 17:659–662
Pennequin-Cardinal A, Plaisance H, Locoge N, Ramalho O, Sv K, Galloo J-C (2005) Performances of the Radiellos diffusive sampler for BTEX measurements: influence of environmental conditions and determination of modelled sampling rates. Atmos Environ 39:2535–2544
Pugliese SC, Murphy JG, Geddes JA, Wang JM (2014) The impacts of precursor reduction and meteorology on ground-level ozone in the Greater Toronto Area. Atmos Chem Phys 14:8197–8207
Roukos J, Riffault V, Locoge N, Plaisance H (2009) VOC in an urban and industrial harbor on the French North Sea coast during two contrasted meteorological situations. Environ Pollut 157:3001–3009
Saalberg Y, Wolff M (2016) VOC breath biomarkers in lung cancer. Clin Chim Acta 459:5–9
Sarkar C, Chatterjee A, Majumdar D, Ghosh SK, Srivastava A, Raha S (2014) Volatile organic compounds over eastern Himalaya, India: temporal variation and source characterization using positive matrix factorization. Atmos Chem Phys 14:32133–32175
Shi J, Deng H, Bai Z, Kong S, Wang X, Hao J, Han X, Ning P (2015) Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci Total Environ 515-516:101–108
Shin SH, Jo WK (2012) Volatile organic compound concentrations, emission rates, and source apportionment in newly-built apartments at pre-occupancy stage. Chemosphere 89:569–578
Susaya J, Kim K-H, Shon Z-H, Brown RJC (2013) Demonstration of long-term increases in tropospheric O3 levels: causes and potential impacts. Chemosphere 92:1520–1528
Tassi F, Capecchiacci F, Giannini L, Vougioukalakis GE, Vaselli O (2013) Volatile organic compounds (VOCs) in air from Nisyros Island (Dodecanese archipelago, Greece): natural versus anthropogenic sources. Environ Pollut 180:111–121
Terrés IMM, Miñarro MD, Ferradas EG, Caracena AB, Rico JB (2010) Assessing the impact of petrol stations on their immediate surroundings. J Environ Manag 91:2754–2762
Tiwari V, Hanai Y, Masunaga S (2010) Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual Atmos Health 3:65–75
Tovalin-Ahumada H, Whitehead L (2007) Personal exposures to volatile organic compounds among outdoor and indoor workers in two Mexican cities. Sci Total Environ 376:60–71
US-EPA (1999) National Air Toxics Program: the integrated urban strategy; notice, United States Environmental Protection Agency. https://www3.epa.gov/airtoxics/area/fr19jy99.pdf, Accessed 20 Apr 2018
US-EPA (2001) Sources, emission, and exposure for trichloroethylene and related chemicals, United States Environmental Protection Agency. http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=4824, Accessed 20 Apr 2018
Valach AC, Langford B, Nemitz E, MacKenzie AR, Hewitt CN (2014) Concentrations of selected volatile organic compounds at kerbside and background sites in Central London. Atmos Environ 95:456–467
Vega E, Sánchez-Reyna G, Mora-Perdomo V, Iglesias GS, Arriaga JL, Limón-Sánchez T, Escalona-Segura S, Gonzalez-Avalos E (2011) Air quality assessment in a highly industrialized area of Mexico: concentrations and sources of volatile organic compounds. Fuel 90:3509–3520
Villanueva F, Tapia A, Notario A, Albaladejo J, Martínez E (2014) Ambient levels and temporal trends of VOCs, including carbonyl compounds, and ozone at Cabañeros National Park border, Spain. Atmos Environ 85:256–265
Warneke C, Geiger F, Edwards PM, Dube W, Pétron G, Kofler J, Zahn A, Brown SS, Graus M, Gilman JB, Lerner BM, Peischl J, Ryerson TB, JAd G, Roberts JM (2014) Volatile organic compound emissions from the oil and natural gas industry in the Uintah Basin, Utah: oil and gas well pad emissions compared to ambient air composition. Atmos Chem Phys 14:10977–10988
Yurdakul S, Civan M, Tuncel G (2013) Volatile organic compounds in suburban Ankara atmosphere, Turkey: sources and variability. Atmos Res 120-121:298–311
Zhang J, Sun Y, Wu F, Sun J, Wang Y (2014) The characteristics, seasonal variation and source apportionment of VOCs at Gongga Mountain, China. Atmos Environ 88:297–305
Zhang Z, Wang X, Zhang Y, Lü S, Huang Z, Huang X, Wang Y (2015) Ambient air benzene at background sites in China's most developed coastal regions: exposure levels, source implications and health risks. Sci Total Environ 511:792–800
Funding
This work was supported by the 2018 Research Fund (1.180015.01) of UNIST (Ulsan National Institute of Science and Technology), by the Korea Ministry of Environment (MOE) as “Public Technology Program based on Environmental Policy (2016000160002)”, and by a grant [KCG-01-2017-01] through the Disaster and Safety Management Institute funded by Korea Coast Guard of Korean government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1779 kb)
Rights and permissions
About this article
Cite this article
Kim, SJ., Kwon, HO., Lee, MI. et al. Spatial and temporal variations of volatile organic compounds using passive air samplers in the multi-industrial city of Ulsan, Korea. Environ Sci Pollut Res 26, 5831–5841 (2019). https://doi.org/10.1007/s11356-018-4032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-4032-5