Abstract
In the present study, the influence of cadmium, copper, and lead on two enzymes often used as biomarkers in toxicological analysis was investigated. Bees were fed with 1 M sucrose solution containing 10-fold serial dilutions of CuCl2 (1000 mg L−1, 100 mg L−1, and 10 mg L−1), CdCl2 (0.1 mg L−1, 0.01 mg L−1, and 0.001 mg L−1), or PbCl2 (10 mg L−1, 1 mg L−1, and 0.1 mg L−1) during 48 h. Our results showed that the total glutathione S-transferase activity was not changed under the influence of cadmium and lead, and it was decreased with the highest concentration of copper. The level of gene expression of the three analyzed classes of glutathione S-transferase was significantly increased with increasing concentrations of copper and cadmium. Lead did not cause significant changes in glutathione S-transferase activity and gene expression, while it showed biphasic effect on acetylcholinesterase activity: lower concentration of lead, 0.1 mg L−1 inhibited and higher dose, 10 mg L−1 induced acetylcholinesterase activity in honey bees. Furthermore, our results showed a significant decrease of the acetylcholinesterase activity in honey bees treated with 0.001 and 0.01 mg L−1 CdCl2. Our results indicate the influence of cadmium, copper, and lead on GST and AChE in the honey bees. These results form the basis for future research on the impact of metallic trace element pollution on honey bees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is a global problem that leads to the emergence of many diseases in organisms, as well as to the decline in numbers of many species (Ahmad 1995). Metallic trace elements are among the most significant potentially harmful pollutants, and their presence can affect a variety of physiological and metabolic processes. Given that they do not decompose, metallic trace elements are constantly present in the environment and enter the biological cycles.
Metals in biological systems are very briefly found in the ionic form, because they are very reactive and easily interact with numerous macromolecules. In case that an organism is chronically exposed to toxic metals or excessive concentrations of some essential metals, they can accumulate and directly or indirectly affect the integrity of biomolecules and consequently disrupt the homeostasis of the organism. Organisms have developed various, mostly energy-intensive detoxification strategies to neutralize undesired inorganic substances. Most often, metal ions bind to sulfhydryl, carboxyl, and phosphate groups of proteins, and also to amines and various anions, such as the chloride anion, which could affect numerous biochemical pathways (Buchwalter 2008). Metallic trace elements affect cellular components such as cell membranes, mitochondria, lysosomes, endoplasmic reticulum, nucleus, and enzymes involved in metabolism, detoxification, and reparation (Wang and Shi 2001). Numerous studies indicate that heavy metals can increase the production of highly reactive particles, such as hydroxyl radical, superoxide anion radical, and hydrogen peroxide, leading to oxidative stress (Ercal et al. 2001; Stone et al. 2002; Kim et al. 2011). Redox-active metals, such as Fe, Cu, and Cr, cause these types of toxicity by Fenton-like reactions, while the depletion of thiol reserves of the organism is considered to be the way in which redox-inactive metals, such as Pb, Cd, Hg, and As, achieve toxic oxidative effects. Additionally, redox-inactive metals bind to thiols that represent the main reserves of sulfur in the body and are often important elements of the non-enzymatic antioxidative system. The onset of oxidative stress further leads to lipid peroxidation, DNA damage, reduction in the number of free thiol groups in proteins, and other changes (Stohs and Bagchi 1995).
Because of the foraging activity, the European honey bee (Apis mellifera, L.) is particularly affected by environmental pollution (Johnson 2015). It is a widely distributed species with a vital role in the pollination of flowering plants and the most dominant worldwide crop pollinator (vanEngelsdorp and Meixner 2010). Bees make about 12–15 trips a day (Perugini et al. 2011), and during the foraging, the body of the bees retain airborne contaminants from the atmosphere (Negri et al. 2015). Furthermore, contaminants are brought to the hive together with pollen and nectar, and through diet come in contact with other castes and brood in the colony. Therefore, the worker bees, honey, and bee bread are often used as bioindicators of environmental pollution by various organic and inorganic compounds (Conti and Botre 2001; Celli and Maccagnani 2003; Lambert et al. 2012). In the last few decades, bees have been exposed to increased levels of environmental xenobiotics, which together with pathogens and poor nutrition contribute to the weakening of bee populations in Europe and worldwide (Aufauvre et al. 2014; Schmehl et al. 2014). Compared to other insect species, honey bees have a reduced number of genes for enzymes involved in the detoxification, which could be a compensation for larger number of detected genes important for their ecology and social organization (The Honeybee Genome Sequencing Consortium 2006). Namely, honey bees have 80 genes for glutathione S-transferase, cytochrome P450, and carboxyl/cholinesterase, while Drosophila melanogaster and Anopheles gambiae have 157 and 188 genes for these enzymes, respectively (Claudianos et al. 2006), which may result in greater susceptibility of bees to xenobiotics.
Glutathione S-transferase (GST) isoenzymes are a complex group of ubiquitously distributed multifunctional enzymes (Sherratt and Hayes 2001). Glutathione S-transferases are the major detoxifying enzymes, and their most important reaction is the conjugation of glutathione (GSH) with various electrophilic substrates resulting in their lower reactivity and higher solubility in water. Besides detoxification of various exogenous compounds, conjugation reactions with GSH are also crucial for the metabolism of endogenous reactive intermediates and metabolites formed during oxidative stress (Hayes et al. 2005). In addition to conjugation reactions, some GST possess various catalytic and non-catalytic functions, including GSH peroxidase activity (Sherratt and Hayes 2001). Eight different classes of GSTs have been identified in insects: Epsilon, Delta, Theta, Zeta, Omega, Sigma, microsomal class, and the denominated unclassified class (u) (Ding et al. 2003). In A. mellifera genome, only 10 putatively functional genes for GST were identified, which is considerably less compared to D. melanogaster (38 genes) and A. gambiae (28 genes) (Claudianos et al. 2006; Corona and Robinson 2006). Interestingly, the distribution of representatives by classes differs, so honey bee genome is characterized by one ortholog of Delta class compared to 11 in D. melanogaster and 12 in A. gambiae, complete absence of Epsilon class and a larger number of representatives of Sigma class compared to dipteran species—4 in A. mellifera and 1 in both D. melanogaster and A. gambiae (Claudianos et al. 2006).
Carboxyl/cholinesterases are a multi-gene family of enzymes that hydrolyze a diverse range of carboxylesters. The enzymes from the class of carboxyl/cholinesterase involved in the metabolism of xenobiotics are less represented in the genome of the honey bee: 8 genes compared to 13 in D. melanogaster and 16 in A. gambiae (Claudianos et al. 2006). Acetylcholinesterase (AChE), a member of this family, catalyzes the hydrolysis of the neurotransmitter acetylcholine into choline and acetate. AChE is found at neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. The enzyme inactivation, induced by various inhibitors, leads to acetylcholine accumulation, hyperstimulation of receptors, and disrupted neurotransmission (Čolović et al. 2013), which cause alterations in locomotor behavior in insects (Jensen et al. 1997). Measurement of AChE inhibition has been used as a biomarker of neurotoxicity in honey bees (Badiou and Belzunces 2008). Specific inhibitors of acetylcholinesterase activity are organophosphorus and carbamate pesticides, but several other chemical species as well, including metals, such as Hg2+, Cd2+, Cu2+, and Pb2+ (Costa et al. 2007; Frasco et al. 2007; De Lima et al. 2013). The inhibition is exerted presumably by binding to protein-SH residues, and not by interacting with the organophosphate-sensitive active site (Lionetto et al. 2013).
Previous studies have shown the effect of cadmium, copper, and lead on development and survival of honey bee larvae and foragers (Di et al. 2016; Hladun et al. 2016). Copper and cadmium had adverse effect on pupal survival, indicating that long-term exposure to these metals in contaminated habitats could lead to a reduction of honey bee number and weakening of the colony. On the other hand, minimal effect on colony performance was observed for lead (Hladun et al. 2016). Furthermore, the same authors have deepened their research using laboratory bioassays and gave another confirmation of the negative effect of these metals on larvae and adult honey bees (Di et al. 2016). In our previous work, we analyzed the impact of sublethal concentrations of copper, cadmium, and lead on honey bee redox status and the activity of the main antioxidative enzymes in laboratory conditions (Nikolić et al. 2016). Here, we investigate the influence of the same metals on GST and AChE activity and gene expression of three isoforms of GST: Delta (Gstd1), Sigma (Gsts1), and microsomal (Gstmic1). These enzymes are often used as biomarkers in toxicological analysis in different organisms (Sarkar et al. 2006; Li 2009; Badiou-Beneteau et al. 2012).
Materials and methods
Honey bee workers were collected from a beehive of the Faculty of Sciences at the University of Novi Sad in July 2015. The bee colony was strong, with no clinical signs of infectious diseases. Adult worker bees were collected from the top super, from frames containing honey and pollen in order to ensure sampling of older bees. The total number of captured bees was 240 for each tested metal. Bees were placed in a 1000-mL jar and fed with 1 M sucrose solution containing 10-fold serial dilutions of CuCl2 (1000 mg L−1, 100 mg L−1, and 10 mg L−1), CdCl2 (0.1 mg L−1, 0.01 mg L−1, and 0.001 mg L−1), or PbCl2 (10 mg L−1, 1 mg L−1, and 0.1 mg L−1) during 48 h. Control group was fed with the same sucrose solution without addition of metals. Each metal salt concentration, as well as control, was tested with three replicates of 20 randomly selected bees. Metal concentrations were chosen in order to cover a wide range of values, and also to include the concentrations that bees ingest in their natural habitats. Hladun et al. (2016) gave an overview of metal concentrations in honey collected from hives in contaminated environments located worldwide. Values for copper were in range 0.01–25 mg kg−1, for cadmium 0.016–0.41 mg kg−1, and for lead 0.01–23.5 mg kg−1. Furthermore, Roman (2007) measured trace elements in pollen from stationary apiary during 2 years, 2005 and 2006, and average values for Pb were 0.9 and 0.49 mg kg−1 and for Cd 0.24 and 0.26 mg kg−1, respectively. Formicki et al. (2013) measured metal concentrations in honey and pollen from apiaries located in southern Poland, and ranges for Cd were 0.001–0.0065 mg kg−1 in honey and 0.026–0.092 mg kg−1 in pollen, and for Pb, 0.06–0.13 mg kg−1 in honey and 1.24–2.49 mg kg−1 in pollen. Therefore, the tested concentrations were within the range of concentrations of these metals occurring in honey bee’s food from natural habitats.
Bees had ad libitum access to sucrose solution. The experiment was maintained for 48 h, in controlled conditions at 25 °C, 65% RH, and in the dark. After 48 h, bee mortality was estimated; the amount of metal salt consumed per bee was calculated (published in Nikolić et al. 2016), and survived bees were collected and transferred into plastic vials which were immediately frozen in dry ice and stored for subsequent analysis. The total RNA from five abdomens (three replicates for every experimental group) was extracted using TRIzol protocol for Apis mellifera (Evans et al. 2013), and cDNA synthesis was carried out using The QuantiTect Reverse Transcription Kit (Qiagen) according to manufacturer’s protocol starting with 1 μg of total RNA. Relative expression was measured for three genes for GST enzymes: GstD1, GstS1, and Gstmic1, using gene for β-actin (ActB) as an endogenous control. The suitability of this gene as an endogenous control in A. mellifera qPCR assays has previously been confirmed (Lourenço et al. 2008). PCR primer sequences are shown in Table 1. The reaction included 7 μL of 2X SYBR® Green PCR Master Mix (Applied Biosystems), 200 nM of each primer, and 50 ng cDNA in total volume of 14 μL. Quantitative PCR on the cDNA products was carried out using MasterCycler RealPlex4 (Eppendorf). Amplification program consisted of pre-incubation step at 95 °C (10 min) and 40 cycles of 95 °C (15 s) and 60 °C (1 min) with additional melt-curve analysis to confirm the specificity of amplification. The difference in the analyzed gene expression was calculated by REST 2009 software (Qiagen), where relative up- or downregulations were calculated and tested for statistical significance by the integrated Bootstrap randomization test (2000 iterations) between control and treated groups for p < 0.05 confidence interval.
Enzyme activities were measured in homogenates of whole body of honey bee workers. Whole bees were homogenized in ice-cold Tris-HCl buffer, pH 7.4 (10% w/v). Crude homogenates were centrifuged 10 min at 10000g (4 °C), and the supernatants were further used in enzymatic assays.
Glutathione S-transferase activity was determined spectrophotometrically following the formation of the product of the reaction between reduced glutathione and 1-chloro-2,4-dinitrobenzene (CDNB) at 340 nm, described by Habig et al. (1974). The reaction consisted of 0.4 M phosphate buffer pH 6.5, 1 mM CDNB, 0.1 mM GSH, and 5 μl of 10% homogenate in the final volume of 0.7 mL. Unit of GST activity is determined as nanometer GSH oxidized in 1 min. Glutathione S-transferase activity was calculated using 9.6 mM−1 cm−1 as the absorption coefficient and was expressed as units of enzyme activity per mg of protein.
Acetylcholinesterase enzymatic activity was measured spectrophotometrically using the Ellman method (Ellman et al. 1961), optimized for microplate assay. The Ellman method is based on acetylcholine hydrolysis by acetylcholinesterase to give tiocholine and acetate. Tiocholine further reacts with DTNB (5,5′-dithiobis(2-nitrobenzoic acid)) giving yellow color nitrobenzoate, which an increase in intensity is measured on 405 nm during 10 min. The reaction mixture contained 100 mM phosphate buffer pH 8.0, 75 mM acetylthiocholine iodide and 10 mM DTNB in 150:2:5 ratio and 50 μl of 10% homogenate in the final volume of 250 μL. The activity was calculated on the basis of an extinction coefficient of 13.6 mM−1 cm−1, in which one unit of AChE is the amount of enzyme that catalyzes the production of 1 μmole of thiocholine per minute, expressed per milligram of protein.
Protein concentration was determined using the Bradford method, with bovine serum albumin (BSA) as a protein standard (Bradford 1976). A significant difference in enzyme activities was estimated using one-way ANOVA with the post hoc Dunnett t test in order to compare experimental groups against the control group (Statistica v.13).
Results
The effect of ingestion of copper, cadmium, and lead on the activity of glutathione S-transferase and acetylcholinesterase is shown on Fig. 1. There was no change in the activity of GST after treatment with metals in the duration of 48 h (Fig. 1a), with an exception of the highest concentration of CuCl2 (1000 mg L−1) that caused the decrease in activity. The activity of acetylcholinesterase was decreased by cadmium in concentrations of 0.001 and 0.01 mg L−1 CdCl2 (Fig. 1b), with lead in the concentration of 0.1 mg L−1 PbCl2, while increased in the group fed with 10 mg L−1 PbCl2 (Fig. 1c). There was no change detected under the influence of copper.
Specific activity of GST (U mg protein−1) and AChE (mU mg protein−1) in whole body of honey bees treated with copper (a), cadmium (b), and lead (c) for 48 h. Experimental groups are named after each metal and concentration of its salt in 1 M sucrose solution in mg L−1. Results are expressed as mean ± SE and analyzed by the post hoc Dunnett t test. Statistically significant difference comparing to control with significance level p < 0.05 is indicated with an asterisk (*p < 0.05)
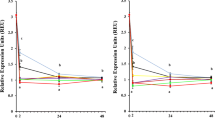
Results for gene expression of three isoforms of glutathione S-transferase are presented in Fig. 2. Expression of the gene for glutathione S-transferase class Delta, GstD1, was changed in the presence of copper and cadmium. All the tested concentrations of copper and the highest applied dose of cadmium (0.1 mg L−1) induced the expression of this gene (Fig. 2a, b). Gene for Sigma class GST, GstS1, showed increased expression after exposure to copper at a concentration of 100 mg L−1 and 1000 mg L−1 CuCl2 and cadmium at the concentration of 0.01 mg L−1 and 0.1 mg L−1 CdCl2 (Fig. 2a, b). The expression of microsomal GST gene, Gstmic1, was also induced under the influence of copper at the maximum applied concentration (1000 mg L−1) and of cadmium in the concentration of 0.01 mg L−1 and 0.1 mg L−1 CdCl2 (Fig. 2a, b). Lead in tested concentrations did not cause significant changes in the expression of GST genes (Fig. 2c).
The mean fold changes of gene expression for GstD1, GstS1, and Gstmic1 genes in honey bees treated with copper (a), cadmium (b), and lead (c) compared with the control fed with 1 M sucrose solution. Experimental groups are named after each metal and concentration of its salt in 1 M sucrose solution in mg L−1. The difference in the analyzed gene expression was calculated by REST 2009 and tested for statistical significance by the integrated Bootstrap randomization test between control and target groups. The range of values for a confidence interval of 68% is presented above the columns. Statistically significant difference compared to the control group (= 1) is indicated with an asterisk (*p < 0.05; **p < 0.01; ***p < 0.001)
Discussion
In this study, the response of two enzymes often used as biomarkers for environmental pollution, glutathione S-transferase and acetylcholinesterase, to the oral exposure to copper, cadmium, and lead was investigated.
Our previously published results (Nikolić et al. 2016) showed that concentrations of copper, cadmium, and lead used in this experiment are sublethal, since the only increase in mortality was observed in the group fed with the highest dose of CuCl2 (1000 mg L−1). However, it is unlikely that honey bees come into contact with this amount of Cu in nature, considering published data about concentration in pollen and honey (Hladun et al. 2016). The same study by Nikolić et al. (2016) showed that exposure of 48 h to given metals led to significant changes of catalase and superoxide dismutase gene expression, the levels of their enzyme activity and redox status, but the effects were metal and dose dependent. More profound effect was observed at the level of gene expression in comparison with the enzyme activity and redox parameters, as glutathione and thiol groups were affected only by lead.
The total activity of GST and the expression of the three genes which belong to various classes, Gstd1 (Delta), Gsts1 (Sigma), and Gstmic1 (microsomal), was monitored after the oral exposure of bees to copper, cadmium, and lead. Delta class is specific to insects, and it is involved in resistance to the pesticides (Udomsinprasert et al. 2005); Sigma GST classes have a protective effect against oxidative stress and elimination of lipid peroxides (Sigh et al. 2001), while the microsomal enzyme membrane proteins are involved in the metabolism of eicosanoids, but they also have detoxifying role which involves peroxidase activity and can protect the membrane from the lipid peroxides (Zimniak and Singh 2006). The response of different classes of GST could be independently regulated, and there are also differences among species (Yan et al. 2013a, b). Our results showed that the total GST activity was not changed under the influence of cadmium and lead, and it was decreased with the highest concentration of copper. The inhibitory effect of copper to GST activity was observed in other animals as well. In grasshopper Locusta migratoria, sigma GST appeared to be the most sensitive to inhibition by Cu2+ and Cd2+ (Qin et al. 2013). The activity of mu-class GST decreased in the presence of micromolar concentrations of Cu2+ in a dose-dependent manner in shrimp Litopenaeus vannamei (Salazar-Medina et al. 2010) and Cu2+-inhibited GST concentrations in the milligram per liter range in two marine gastropods, Monodonta lineata and Nucella lapillus (Cunha et al. 2007). In addition to the enzyme activity, relative expression of the GST genes belonging to different classes was measured. The level of gene expression of the three analyzed classes was significantly increased with increasing concentrations of copper and cadmium (Fig. 1a, b). Changes in the level of gene expression are also present in groups in which enzyme activity was unchanged, suggesting that gene expression is more appropriate for determining acute effect of metals. Similar results were obtained in other species, as mercury, as one of the redox-inactive toxic metals, induced the expression of genes in AccGSTS1 in Asian honey bee A. cerana after 2 h in a dose of 3 μg per bee (Yan et al. 2013b). In copepods Tigriopus japonicus, ten isoforms of GST genes and their response to the presence of toxic metals were examined. After treatment with copper or cadmium for a period of 96 h, there was an increased expression of Sigma and microsomal classes while the Delta, as well as Theta and Omega classes, was unchanged (Lee et al. 2008). Nair and Choi (2011) examined the influence of cadmium on Sigma and Delta class GST genes in Chironomus riparius. Expression of all isoforms, three from the class of Delta and four from the class of Sigma, was increased following exposure, with the largest difference measured after 24 h or 48 h, after which it began to decrease. Our results are in accordance with results on other species and suggest that upregulation of Sigma, Delta, and microsomal classes of GST is involved in response against cadmium and excessive concentrations of copper. Upregulation of gene expression and unchanged enzyme activity after exposure to cadmium point to higher sensitivity and faster response in gene expression level compared to protein level. The highest concentration of copper (1000 mg L−1) inhibited GST enzyme activity, while gene expression was upregulated. This could be explained by the induction of gene expression in order to compensate lower activity caused by inhibition of the enzyme. On the other side, Pb in our experiment did not induce upregulation of GST genes (Fig. 1c). According to our previous results (Nikolić et al. 2016), chelation of Pb might be the first level of defense from lead intoxication in honey bee, so maybe the treatment period of 48 h was too short to cause an increase in expression of GST genes.
Acetylcholinesterase (AChE) is a crucial enzyme in the nervous system of vertebrates and invertebrates, which function could be affected by several xenobiotics including drugs, pesticides, and metallic trace elements (Forget et al. 1999). Mostly, the influence of metals on AChE activity is determined in the brain, since it is the main organ in which this enzyme occurs, but its expression is also proven in the thorax and abdomen of the honey bee (Kim et al. 2012). It is assumed that metals inhibit AChE activity through binding to its thiol groups (Lionetto et al. 2011). In another study, it is suggested that low concentrations of metals in vivo do not interact with enzyme directly, but rather interfere with brain metabolism (de Lima et al. 2013). Nevertheless, the response of AChE to the presence of different metals is species-specific (Brown et al. 2004). In our study, the activity of AChE in whole body of honey bees had shown changes that were related to Cd and Pb exposure, but was not affected by copper. Lead showed biphasic effect on AChE activity: lower concentration of lead, 0.1 mg L−1 PbCl2 inhibited, while higher dose, 10 mg L−1 induced AChE activity in honey bees, in comparison with control. Although metals are considered to be inhibitors of AChE, recent findings indicate that this enzyme could have a role in detoxification, and its protein expression and activity could be increased under the influences of different xenobiotics, including metals (Kang et al. 2011; Kim et al. 2014; Perić-Mataruga et al. 2017), which is in accordance with our results. Reason for bi-phase response may be found in different Pb concentrations. Some low concentrations could have been more effective in inhibition of AChE activity, while intermediary concentration could initiate some other mechanisms of detoxification (such as GSH – chelating (as shown Nikolić et al. 2016), metallothionein, etc.). High concentration probably breaks this mechanism and could provoke an untypical response. However, additional analyses are necessary for understanding this biphasic response.
Cadmium could alter AChE activity in insects by interfering with calcium metabolism, as shown in Lymantria dispar (Wang and Du 2013; Perić-Mataruga et al. 2017) and in skeletal muscle fibers of honey bee, where Cd2+ modified action potential by blocking voltage-dependent calcium channels (Collet and Belzunces 2007). Cadmium in the diet of L. dispar caterpillars induced activity of AChE (Perić-Mataruga et al. 2017) and inhibited activity of this enzyme in zebrafish Danio rerio (de Lima et al. 2013) and earthworm Lumbricus terrestris (Calisi et al. 2013). Our results showed a significant decrease of the enzyme activity in honey bees treated with 0.001 and 0.01 mg L−1 CdCl2 in comparison with the control group. The obtained results suggest that honey bees exposed to cadmium could be more susceptible to intoxication with pesticides. Namely, exposure to different xenobiotics with a similar mode of action could exhibit additive toxicity in bees (Johnson 2015). Acetylcholinesterase is the primary target of inhibition by organophosphates and carbamates such as nerve agents and pesticides. Therefore, doses of pesticides lower than LD50 might be lethal, considering additional inhibition of enzyme activity by cadmium. Overall, among the three analyzed metals in this experiment, copper did not change AChE activity; lead induced biphasic response, so AChE could be considered as a potential biomarker in honey bee populations only for cadmium pollution monitoring.
Conclusions
Our results indicate the influence of cadmium, copper, and lead on GST and AChE in the honey bee. Further research in this field could indicate a concrete mechanism of this response. These results form the basis for future research on the impact of metallic trace element pollution on honey bees and represent an important step in the comprehensive assessment of the impact of stress factors from the environment on honey bees.
References
Ahmad S (1995) Oxidative stress from environmental pollutants. Arch Insect Biochem Physiol 29:135–157
Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290
Aufauvre J, Misme-Aucouturier VB, Texier C, Delbac F, Blot N (2014) Transcriptome analyses of the honey bee response to Nosema ceranae and insecticides. PLoS One 9(3):e91686
Badiou A, Belzunces LP (2008) Is acetylcholinesterase a pertinent biomarker to detect exposure of pyrethroids? A study case with deltamethrin. Chem Biol Interact 175:406–409
Badiou-Beneteau A, Carvalho SM, Brunet J, Carvalho GA, Bulete A, Giroud B, Belzunces LP (2012) Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: application to the systemic insecticide thiamethoxam. Ecotoxicol Environ Saf 82:22–31
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66:267–278
Buchwalter DB (2008) Metals. In: Smart RC, Hodgson E (eds) Molecular and biochemical toxicology. John Wiley & Sons, Inc., Hoboken, pp 413–439
Calisi A, Zaccarelli N, Lionetto MG, Schettino T (2013) Integrated biomarker analysis in the earthworm Lumbricus terrestris: application to the monitoring of soil heavy metal pollution. Chemosphere 904:2637–2644
Celli G, Maccagnani B (2003) Honey bees as bioindicators of environmental pollution. Bull Insectology 56:137–139
Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Feyereisen R, Oakeshott JG (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15:615–636
Collet C, Belzunces L (2007) Excitable properties of adult skeletal muscle fibres from the honeybee Apis mellifera. J Exp Biol 210:454–464
Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11:315–335
Conti ME, Botre F (2001) Honeybees and their products as potential bioindicators of heavy metals contamination. Environ Monit Assess 69:267–282
Corona M, Robinson GE (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol 15:687–701
Costa JR, Mela M, de Assis HC, Pelletier É, Randi MA, de Oliveira Ribeiro CA (2007) Enzymatic inhibition and morphological changes in Hoplias malabaricus from dietary exposure to lead(II) or methylmercury. Ecotoxicol Environ Saf 67:82–88
Cunha I, Mangas-Ramirez E, Guilhermino L (2007) Effects of copper and cadmium on cholinesterase and glutathione S-transferase activities of two marine gastropods (Monodonta lineata and Nucella lapillus). Comp Biochem Physiol C 145:648–657
de Lima D, Roque GM, de Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51
Di N, Hladun KR, Zhang K, Liu T, Trumble JT (2016) Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honeybee (Apis mellifera L.) larvae and foragers. Chemosphere 152:530–538
Ding Y, Ortelli F, Rossiter LC, Hemingway J, Ranson H (2003) The Anopheles gambiae glutathione transferase supergene family: annotation, phylogeny and expression profiles. BMC Genomics 4:35
Ellman GL, Courtney KD, Andres JV, Featherstone RM (1961) A new rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal induced oxidative damage. Curr Top Med Chem 1:529–539
Evans JD, Schwartz RS, Chen YP, Budge G, Cornman RS, DeLaRua P, DeMiranda JR, Foret S, Foster L, Gauthier L, Genersch E, Gisder S, Jarosch A, Kucharski R, Lopez D, Lun CM, Moritz RFA, Maleszka R, Muñoz I, Pinto MA (2013) Standard methodologies for molecular research in Apis mellifera. In: Dietemann, V, Ellis JD, Neumann P (eds), The COLOSS BEEBOOK, Volume I: standard methods for Apis mellifera research. J Apic Res 52. https://doi.org/10.3896/IBRA.1.52.4.11
Forget J, Pavillon J, Beliaeff B, Bocquene G (1999) Joint action of pollutant combinations (pesticides and metals) on survival (LC50 values) and acetylcholinesterase activity of Tigriopus brevicornis (Copepoda, Harpacticoida). Environ Toxicol Chem 18:912–918
Formicki G, Greń A, Stawarz R, Zyśk B, Gał A (2013) Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Pol J Environ Stud 22:99–106
Frasco MF, Colletier J, Weik M, Carvalho F, Guilhermino L, Stojan J, Fournier D (2007) Mechanisms of cholinesterase inhibition by inorganic mercury. FEBS J 274:1849–1861
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. J Biol Chem 29:7130–7139
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol 45:51–88
Hladun KR, Di N, Liu TX, Trumble JT (2016) Metal contaminant accumulation in the hive: consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.). Environ Toxicol Chem 35:322–329
Jensen CS, Garsdal L, Baatrup E (1997) Acetylcholinesterase inhibition and altered locomotor behavior in the carabid beetle Pterostichus cupreus. A linkage between biomarkers at two levels of biological complexity. Environ Toxicol Chem 16:1727–1732
Johnson RM (2015) Honey bee toxicology. Annu Rev Entomol 60:415–434
Kang JS, Lee DW, Koh YH, Lee SH (2011) A soluble acetylcholinesterase provides chemical defense against xenobiotics in the pinewood nematode. PLoS One 6:e19063
Kim BY, Hui WL, Lee KS, Wan H, Yoon HJ, Gui ZZ, Chen S, Jin BR (2011) Molecular cloning and oxidative stress response of a sigma-class glutathione S-transferase of the bumblebee Bombus ignitus. Comp Biochem Physiol B 158:83–89
Kim YH, Cha DJ, Jung JW, Kwon HW, Lee SH (2012) Molecular and kinetic properties of two acetylcholinesterases from the western honey bee, Apis mellifera. PLoS One 7:e48838
Kim YH, Kwon DH, Ahn HM, Koh YH, Lee SH (2014) Induction of soluble AChE expression via alternative splicing by chemical stress in Drosophila melanogaster. Insect Biochem Mol Biol 48:75e82
Lambert O, Piroux M, Puyo S, Thorin C, Larhantec M, Delbac F, Pouliquen H (2012) Bees, honey and pollen as sentinels for lead environmental contamination. Environ Pollut 170:254–259
Lee KW, Raisuddin S, Rhee JS, Hwang DS, Yu IT, Lee YM, Park HG, Lee JS (2008) Expression of glutathione S-transferase (GST) genes in the marine copepod Tigriopus japonicus exposed to trace metals. Aquat Toxicol 89:158–166
Li X (2009) Glutathione and glutathione-S-transferase in detoxification mechanisms. In: Ballantyne B, Marrs TC, Syversen T (eds) General and applied toxicology, third edn, vol 2009. John Wiley & Sons ltd, Chichester, pp 411–423
Lionetto MG, Caricato R, Calisi A, Schettino T (2011) Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: new insights and perspectives. In: Visser JE (ed) Ecotoxicology around the globe. Nova Science Publishers, New York, pp 87–115
Lionetto MG, Caricato R, Calisi A, Giordano ME, Schettino T (2013) Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. Biomed Res Int 2013:1–8
Lourenço AP, Mackert A, Cristino AD, Simões ZLP (2008) Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 39:372–385
Morimoto T, Kojima Y, Toki T, Komeda Y, Yoshiyama M, Kimura K, Nirasawa K, Kadowaki T (2011) The habitat disruption induces immune-suppression and oxidative stress in honey bees. Ecol Evol 1:201–217
Nair PMG, Choi J (2011) Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat Toxicol 101:550–560
Negri I, Mavris C, Di Prisco G, Caprio E, Pellecchia M (2015) Honey bees (Apis mellifera, L.) as active samplers of airborne particulate matter. PLoS One 10:e0132491
Nikolić TV, Kojić D, Orčić S, Batinić D, Vukašinović E, Blagojević DP, Purać J (2016) The impact of sublethal concentrations of copper, lead and cadmium on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 164:98–105
Perić-Mataruga V, Petković B, Ilijin L, Mrdaković M, Dronjak Čučaković S, Todorović D, Vlahović M (2017) Cadmium and high temperature effects on brain and behaviour of Lymantria dispar L. caterpillars originating from polluted and less polluted forests. Chemosphere 185:628–636
Perugini M, Manera M, Grotta L, Abete MC, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: honey bees as bioindicators. Biol Trace Elem Res 140:170–176
Qin G, Jia M, Liu T, Zhang X, Guo Y, Zhu KY, Ma E, Zhang J (2013) Characterization and functional analysis of four glutathione S-transferases from the migratory locust, Locusta migratoria. PLoS One 8(3):e58410
Roman A (2007) Content of some trace elements in fresh honeybee pollen. Pol J Food Nutr Sci 57:475–478
Salazar-Medina AJ, García-Rico L, García-Orozco KD, Valenzuela-Soto E, Contreras-Vergara CA, Arreola R, Arvizu-Flores A, Sotelo-Mundo RR (2010) Inhibition by Cu2+ and Cd2+ of a mu-class glutathione S-transferase from shrimp Litopenaeus vannamei. J Biochem Mol Toxicol 24:218–222
Sarkar A, Ray D, Shrivastava AN, Sarker S (2006) Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology 15:333–340
Schmehl DR, Teal PEA, Frazier JL, Grozinger CM (2014) Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J Insect Physiol 71:177–190
Sherratt PJ, Hayes JD (2001) Glutathione S-transferases. In: Ioannides C (ed) Enzyme systems that metabolize drugs and other xenobiotics. John Wiley & Sons, Ltd, Chichester, pp 319–352
Sigh SP, Coronella JA, Beneš H, Cochrane BJ, Zimniak P (2001) Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur J Biochem 268:2912–2923
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Stone D, Jepson P, Laskowski R (2002) Trends in detoxification enzymes and heavy metal accumulation in ground beetles (Coleoptera: Carabidae) inhabiting a gradient of pollution. Comp Biochem Physiol C 132:105–112
The Honeybee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443:931–949
Udomsinprasert R, Pongjaroenkit S, Wongsantichon J, Oakley AJ, Prapanthadara L, Wilce MCJ, Ketterman AJ (2005) Identification, characterization and structure of a new Delta class glutathione transferase isoenzyme. Biochem J 388:763–771
vanEngelsdorp D, Meixner MD (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103:S80–S95
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034
Wang S, Shi X (2001) Molecular mechanisms of metal toxicity and carcinogenesis. Mol Cell Biochem 222:3–9
Yan H, Jia H, Wang Z, Gao H, Guo X, Xu B (2013a) Identification and characterization of an Apis cerana cerana Delta class glutathione S-transferase gene (AccGSTD) in response to thermal stress. Naturwissenschaften 100:153–163
Yan H, Jia H, Gao H, Guo X, Xu B (2013b) Identification, genomic organization, and oxidative stress response of a sigma class glutathione S-transferase gene (AccGSTS1) in the honey bee, Apis cerana cerana. Cell Stress Chaperones 18:415–426
Zimniak P, Singh SP (2006) Families of glutathione transferases. In: Awasthi YC (ed) Toxicology of glutathione transferases. CRC press LLC, Boca Raton, pp 777–780
Funding
This work was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant no. 173014, project entitled “Molecular mechanisms of redox signalling in homeostasis: adaptation and pathology.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikolić, T.V., Kojić, D., Orčić, S. et al. Laboratory bioassays on the response of honey bee (Apis mellifera L.) glutathione S-transferase and acetylcholinesterase to the oral exposure to copper, cadmium, and lead. Environ Sci Pollut Res 26, 6890–6897 (2019). https://doi.org/10.1007/s11356-018-3950-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3950-6