Abstract
Currently, the urgency of balancing rice production and environmental risk from nitrogen (N) fertilization is gaining scientific and public attention. As such, a field experiment was conducted to investigate the rice yield and the fate of applied-15N for Yangliangyou 6 (a two-line hybrid cultivar) and Lvdaoq 7 (an inbred cultivar) using 10 combinations of N rates and splitting ratios in the middle reaches of the Yangtze River. The results showed that N application primarily affected fertilizer N loss to the environment, followed by plant N absorption, but had little effect on grain yield. Generally, there was no significant increase in grain yield and N accumulation in the aboveground plant when N inputs surpassed 130 or 170 kg ha−1. Fertilizer N residue in soil peaked at approximately 48 kg ha−1 at an N rate of 170 kg ha−1 for both varieties; however, a sharp increase of fertilizer N loss occurred with further incrementally increasing N rates. Although a higher ratio of panicle-N fertilizer together with a lower ratio of tillering-N fertilizer at rates of 130, 170, and 210 kg ha−1 had no grain yield benefit, it promoted aboveground N accumulation and plant N accumulation derived from fertilizer, and it reduced the amount of N residue in soil and N loss to the environment. Overall, reducing tillering-N ratios and increasing panicle-N ratios at an N rate between 130 and 170 kg ha−1 using fertilizer rates of 90–0–40 kg ha−1 and 90–40–40 kg ha−1 N at basal-tillering-panicle initiation stages could reduce the adverse environmental risks of chemical N from rice production without sacrificing rice yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) plays a key role in crop yield formation because of a fundamental role in biomass accumulation (Salvagiotti and Miralles 2008). Hence, excess N, often as an extra “insurance” component to prevent yield loss, is frequently applied to fields in modern rice production systems (Ju et al. 2009). The N fertilizer rate in paddy fields up to 324 kg ha−1 was monitored in the Yangtze River region (Li et al. 2015a). However, rice grain yield does not increase proportionally with the increasing application of N fertilizer (Huang et al. 2007; Huang et al. 2008; Yan et al. 2009). It was demonstrated that there were no increases in yield for rice cultivars 4007 and Wuyunjing15 at an N rate above 150 kg ha−1 in China (Yan et al. 2009). Han et al. (2012) concluded that the optimum N application rate in the middle Yangtze River basin was approximately 120–180 kg ha−1. Excess N fertilizer application led to low N use efficiency (NUE) and negative environmental impacts such as water and soil pollution (Peng et al. 2002; Li et al. 2015b) and greenhouse gas emission (Chen et al. 2014; Cui et al. 2014). Efficient N fertilizer management is vital to maintaining grain yield and minimizing environmental risk in rice production.
In fact, NUE has been confirmed to be dependent on N management practices, particularly rates, timing, resources, and placement of N fertilizer application (Dobermann et al. 2002; Peng et al. 2006; Zou et al. 2006; Zhong et al. 2007; Ju et al. 2009; Yan et al. 2009). Over fertilization was considered one of the leading causes of low NUE in China (Peng et al. 2002; Ju et al. 2009). In addition, Chen et al. (2015) assumed that the increased NUE was primarily associated with N fertilizer split applications, rather than N rates. Optimizing N fertilizer split applications has been demonstrated to improve the yield and NUE of rice compared to that of farmers practices (Peng et al. 2006; Zhang et al. 2011; Chen et al. 2015). Nitrogen fertilizer applied at the panicle initiation (PI) stage contributed to approximately 43% of the total fertilizer-derived N accumulation in plants at the maturity stage, and N fertilizer at the midtillering (MT) stage contributed only 20% (Yan et al. 2009). Hence, NUE in rice was suggested to be enhanced by reducing the amount of N fertilizer at the MT stage and increasing the amount of N fertilizer at the PI stage (Zhong et al. 2007; Yan et al. 2009; Zhang et al. 2013).
According to previous studies, only 30–40% of applied N fertilizer was used by rice plants and a substantial ratio of more than 45% of applied N was lost from the rice-soil system to the surrounding environment (Ju and Zhang 2003; Ladha et al. 2005; Yan et al. 2008; Pan et al. 2012; Zhang et al. 2012). The heavy agricultural N loss caused mainly by over fertilization and inappropriate timing led to severe environmental consequences, including nitrate pollution of groundwater, eutrophication of surface water, depositions of atmospheric N, and emissions of greenhouse gas, which have become major concerns for scientists, environmental groups, and agricultural policymakers worldwide (Ladha et al. 2005; Ju et al. 2009). As such, some researchers have advocated that environmental consequences should be factored into fertilizer recommendations (Hofmeier et al. 2015; Bell et al. 2016). Ju and Zhang (2003) proposed that the “15N tracer method” was an appropriate way to understand the fate of N fertilizer by distinguishing fertilizer-derived N and soil-derived N. The 15N tracer is often utilized in experiments for determining fertilizer N fate in terms of plant accumulation, soil residue, and loss to the ecosystem through a variety of pathways (Ju and Zhang 2003; Zhu 2008; Zhang et al. 2012).
The urgency of balancing high yields and environmental consequences in agricultural production in China is currently gaining scientific and public attention (Huang et al. 2007; Chen et al. 2014; Cui et al. 2014). To address this urgent issue, some previous studies have attempted to seek the best combination of yield production and negative environmental impacts of N fertilizer on rice farming using a mathematical basis (Ju and Zhang 2003; Huang et al. 2007; Ju et al. 2009). However, few studies have been conducted concerning the effects of N splitting ratios under various N rates with narrow intervals on the environmental consequences of applied 15N in the field, and factored the environmental risks of applied 15N into the N fertilizer recommendation on an agronomic basis.
In the present study, the objectives were as follows: (1) to investigate the effects of different N rates and splitting ratios on grain yield of rice, (2) to determine the effects of different N rates and splitting ratios on N accumulation in rice, (3) to trace the 15N-labeled fertilizer fate in terms of plant accumulation, residue in the soil, and loss to the ecosystem under different N rates and splitting ratios, and (4) to recommend optimum N management for rice cultivation considering both the yield production and the environmental risk of N application in the Yangtze River’s middle reaches.
Materials and methods
Experimental design and cropping establishment of the main plots
The field experiments were conducted in two adjacent fields in Lanjie Village (30°00′N, 115°44′E), Wuxue County, in southeast Hubei Province, central China, from May to October 2013 and 2014. This province stands within the middle reaches of the Yangtze River which is known as one of the largest rice-growing regions in China. The physicochemical properties of the 0–20-cm soil layer are presented in Table 1. The experimental region is classified as humid subtropical with a monsoon climate. Data regarding daily climatic conditions during this study were obtained from an agro-meteorological station nearby. The total radiation, precipitation, and mean daily maximum and minimum temperatures over the growing seasons were 2575 MJ m−2, 507 mm, 30.7 °C, and 22.7 °C, respectively, in 2013 and 2405 MJ m−2, 586 mm, 29.1 °C, and 21.2 °C, respectively, in 2014. In comparison to 2013, 2014 was a cooler year with more rainfall during the period from the panicle initiation stage to the heading stage of rice. The meteorological data including daily radiation, precipitation, and maximum and minimum temperatures during the growth periods are presented in Fig. 1.
The experimental design was a split-plot arrangement with four replications; the main plot had different nitrogen (N) rates, the subplot had different rice varieties. Nitrogen in the form of urea was split-applied at basal, mid tillering and panicle initiation stages. Ten N treatments with N rates ranging from 90 to 250 kg ha−1, including a 0 kg ha−1 control (CK), were implemented (Table 2). There were two to three kinds of N splitting patterns at the mid tillering and panicle initiation stages, with total N rates of 130 kg ha−1, 170 kg ha−1, and 210 kg ha−1. Nitrogen rates of the basal fertilizer, tillering fertilizer, and panicle fertilizer were 90–40–0 kg ha−1 and 90–0–40 kg ha−1; 90–80–0 kg ha−1, 90–40–40 kg ha−1, and 90–0–80 kg ha−1; and 90–80–40 kg ha−1 and 90–40–80 kg ha−1, respectively. All plots received recommended 90 kg P2O5 ha−1 and 75 kg ZnSO4·7H2O ha−1 as basal dressing, and 225 kg K2O ha−1 with split doses at 50% each as basal dressing and topdressing at the panicle initiation stage. A two-line hybrid rice cultivar, Yangliangyou 6 (YLY6) and an inbred rice cultivar, Lvdaoq 7 (LDQ7) were planted both years. The seeds were sown on May 12, 2013, and 2014, and transplantation was performed 30 days after sowing. Two and four seedlings per hill of YLY6 and LDQ7, respectively, were transplanted with consideration of the poor tillering capacity of the inbred cultivar. The hills were 30 cm × 13.3 cm with 25 hills m−2. The subplot sizes were 6.5 m × 5.5 m and 6.3 m × 5.7 m in 2013 and 2014, respectively. Each subplot contained 21 lines, and each line contained 41 hills. Each main plot was separated by a 30-cm ridge, which was covered by plastic film. The plastic film was installed to a depth of 20 cm below the soil surface before transplanting. Crop management, including weeds, insects, and disease, was intensively controlled to avoid biomass and yield loss. The plots were flooded immediately after transplantation, and a 4–10-cm water depth was maintained until 7 days before harvest at maturity.
Field management details in 15N fertilizer microplots
Micro plots as rectangular frames with a length × width of 90 cm × 40 cm were demarcated in the experimental subplots, which excluded 5 hills and 5 rows from the bridge to avoid a border effect. Each micro plot contained 9 hills of plants. The micro plots consisted of four polyethylene plastic boards and some welding rods that were assembled using a plastic welder. All micro plots were inserted 20-cm deep into the soil, given that the arable depth was only 15 cm, and retained a 15-cm height above the soil prior to the basal N fertilizer application to avoid lateral contamination and surface runoff between the micro plots and the subplots. 15N-labeled urea for the micro plots was prepared as follows: the 2.5 atom% (Shanghai Chemical Industry Institute, China) was dissolved in distilled water, transferred to a 2-l volumetric flask, diluted to a predetermined volume, and kept standing for at least one night, then fertilizer solutions were sub packaged into 50-ml centrifuge tubes according to the N dose of each micro plot. When nonlabeled urea was spread over the main plots, the adjacent micro plots were covered with plastic films to prevent cross-contamination. The plastic films were removed, and 15N labeled urea was applied to the micro plots. The applications of N, P, K, and Zn fertilizers and crop management practices in the micro plots were the same as the corresponding main plots. To avoid the exchange of N between subplots and microplots, the microplots were irrigated with water carried by buckets. The water level in the microplots was checked every other day and was replenished as needed.
Plant and soil sampling and measurements
Plants in subplots were sampled from 0.48 m2 (12 hills) at the maturity stage. The plants were divided into straws (including culm, sheath, and leaf) and panicles. After recording the panicle number, the panicle was hand-threshed, and the filled spikelets were separated from half-filled and unfilled spikelets by submerging them in tap water. Further screening was completed by a seed blower to separate the half-filled spikelets from the unfilled spikelets. Three subsamples of 30 g of air-dried filled spikelets, 2 g of unfilled spikelets, and all half-filled spikelets were collected to count the number of spikelets. The panicle number m−2, spikelet number panicle−1, spikelet number m−2, and grain filling percentage were calculated. The 1000-grain weight, aboveground biomass, and harvest index were determined after oven-drying at 85 °C to a constant weight. The aboveground biomass was the total dry matter of straw, rachis, and filled, half-filled, and unfilled spikelets ha−1. Plant samples were then ground into powder for N determination. Grain yield (t ha−1) was determined from a 5 m2 sampling area within each plot, and the value was adjusted to 14% moisture.
To measure the 15N-labeled fertilizer fate, three out of nine hills in microplots were chosen based on the average panicle number hill−1 and the growth status. Their aboveground parts were cut, and roots were collected and carefully preserved for estimation. The plant samples were prepared for N determination using the same methods as in the subplot. Soil samples were taken from 3 representative points with a 5-cm diameter circle soil sampler. After air drying, soil samples were weighed and sifted through a 100 mesh sieve and stored in valve bags for subsequent measurements. The N concentration (%) and 15N abundance (atom% 15N) in plant and soil samples were determined by a combined Isoprime 100 stable isotope ratio mass spectrometer with an Elementar vario EL cube analyzer (Elementar Trading Co., Ltd., Germany) (Wang et al. 2017 and Li et al. 2018).
The following equations were used for the calculation of N accumulation and 15N fertilizer fate:
where Ngrain, Nstraw, Nrs, Nroot, and Nsoil are the N content (kg ha−1) of grain, straw, rachis, half-filled spikelets, unfilled spikelets, root, and soil, respectively; Wgrain, Wstraw, Wrs, Wroot, and Wsoil are weight (kg ha−1) of grain, straw, rachis, half-filled spikelets, unfilled spikelets, and soil, respectively; N% is N concentration in plant and soil samples; and NA is N accumulation in the aboveground plant.
where %Ndff is the percentage of N derived from fertilizer in plant and soil samples; a is 15N abundance in plant and soil samples; b is 15N abundance of 15N fertilizer; c is natural 15N abundance (0.3664%); Ndffgrain, Ndffstraw, Ndffrs, Ndffroot, and Ndffsoil are N accumulation derived from fertilizer of grain, straw, rachis, half-filled spikelets, unfilled spikelets, root, and soil, respectively; Ndff is N accumulation derived from fertilizer in the aboveground plant; N residue and N loss are the residual fertilizer N in soil and the fertilizer N loss, respectively.
The N accumulation and 15N fate calculation methods are taken from Peng et al. (2002), Leon et al. (1995), Wang et al. (2017), and Li et al. (2018). Data were analyzed using analysis of variance (ANOVA), and means were compared based on the least significant difference (LSD) test at the 0.05 probability level using Statistix 9 (Analytical Software, Tallahassee, FL, USA). The figures were generated by SigmaPlot 10.0 (SPSS Inc., Point Richmond, CA, USA).
Results
The effects of N treatments on grain yield
N fertilizer enhanced grain yield by 0.9–3.1 t ha−1 compared to that of the CK depending on rice variety, year, and split pattern (Table 3). Significant influences of N fertilizer rate were observed on rice grain yield. The highest yields (9.74 and 10.21 t ha−1 for YLY6 and 8.55 and 9.76 t ha−1 for LDQ7 in 2013 and 2014, respectively) were achieved with an approximate N rate of 210 kg ha−1. Additionally, the lowest N rates of 130 kg ha−1 and 170 kg ha−1 obtained comparable yields with the highest yields, which were statistically no significant. In addition, there was no significant influence of N split patterns on grain yield under given N rates of 130, 170, and 210 kg ha−1. The N fertilizer application rate impacted almost all the yield components (Table 4). Biomass, productive panicle number m−2, and grain weight increased with incremental N rates but then stagnated and even declined. Grain filling percentage and harvest index declined in both varieties. However, the spikelet number panicle−1 of the two varieties responded differently to the N rate, which declined with the incremental N rate in LDQ7 but showed no obvious response in YLY6.
The effects of N treatments on plant N accumulation
Plant N accumulation increased with the N fertilizer rate and then achieved stagnation (Table 5). The highest plant N accumulations were 166.8 kg ha−1 and 193.7 kg ha−1 for YLY6 and 157.5 kg ha−1 and 195.1 kg ha−1 for LDQ7 in 2013 and 2014, respectively. The lowest N rates of 130 kg ha−1 or 170 kg ha−1 elicited accumulations comparable to the highest plant N accumulations. Under a given N rate of 130, 170, and 210 kg ha−1, N split patterns with an increasing N ratio of panicle fertilizer and a decreasing N ratio of tillering fertilizer remarkably promoted plant N accumulation.
The effects of N treatments on fertilizer N fate
The plant N accumulation derived from fertilizer (Ndff), the residual fertilizer N in the soil (N residue), and the fertilizer N loss (N loss) at the maturity stage determined from 15N tracing showed a substantial variation across N rates and cultivars (Fig. 2). In 2013, increasing the N fertilizer rate gradually increased the Ndff and increased the integral amount of the N residue and the N loss (N residue + loss) in a sharper slope than that of Ndff in both varieties, especially at an N rate higher than 210 kg ha−1 (Fig. 2). To further investigate the contributions of the N residue in the soil and the N loss to the ecosystem to N fate, we measured the N residue and N loss separately in 2014. In 2014, both the Ndff and N residue + loss increased with similar slopes with increasing N fertilizer rate. Notably, the amount of N residue stagnated at an N rate of 170 kg ha−1 in both YLY6 and LDQ7, but the N loss sharpened by further incremental increases in N rates (Fig. 2). The plant N accumulation derived from fertilizer in YLY6 was slightly higher than that in LDQ7, which were 42.4 kg ha−1 in YLY6 and 38.0 kg ha−1 in LDQ7 in 2013 and 59.9 kg ha−1 in YLY6 and 53.5 kg ha−1 in LDQ7 in 2014, calculated by pooling all N treatments. In contrast, the N residue + loss in YLY6 was slightly lower than that in LDQ7, at 127.6 kg ha−1 in YLY6 and 132.0 kg ha−1 in LDQ7 in 2013 and 110.1 kg ha−1 in YLY6 and 116.6 kg ha−1 in LDQ7 in 2014, calculated by pooling all N treatments (Fig. 2).
The effects of N rate on the plant N accumulation derived from fertilizer (Ndff), the residual fertilizer N in soil (N residue), and the fertilizer N loss (N loss) at the maturity stage: (a) Yangliangyou 6 in 2013, (b) Lvdaoq 7 in 2013, (c) Yangliangyou 6 in 2014, and (d) Lvdaoq 7 in 2014, as determined from the 15N tracer method. The solid circle represents the Ndff, and the solid triangle represents the integral amount of the N residue and the N loss. The hollow square and the solid square represent the N residue and the N loss, respectively. The N residue and the N loss were separately determined in 2014 by taking the root and soil samples
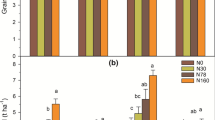
The effects of the different N splitting patterns at N rates of 130, 170, and 210 kg ha−1 on Ndff and N residue + loss are shown in Fig. 3. Increasing the ratio of panicle-N fertilizer significantly increased the Ndff for both YLY6 and LDQ7 at all three N rates, which demonstrated higher 15N-labeled fertilizer recovery efficiency, except for the 90–0–80 kg N ha−1 in LDQ7 in 2014 because of the bias of the N supply at the PI stage. Additionally, N residue + loss was lower when the N ratio of PI fertilizer was increased, and the difference was statistically significant in 2013. The respective influences of adjusting N splitting patterns on fertilizer N residue and fertilizer N loss were unclear.
The effects of N splitting patterns on the plant N accumulation derived from fertilizer (Ndff), the integral amount of the residual fertilizer N in soil, and the fertilizer N loss (N residue + loss) at the maturity stage: (a) Yangliangyou 6 in 2013, (b) Lvdaoq 7 in 2013, (c) Yangliangyou 6 in 2014, and (d) Lvdaoq 7 in 2014, as determined from the 15N tracer method. The solid symbols represent the Ndff, and the hollow symbols represent the N residue + loss. From pattern 1 to pattern 2, and to pattern 3 under certain N rates represent decreasing N fertilizer ratios at the midtillering stage and increasing N fertilizer ratios at the panicle initiation stage, gradually. In detail, pattern 1 with circle symbols at 130 kg N ha−1, 170 kg N ha−1, and 210 kg N ha−1 represent 90–40–0, 90–80–0, and 90–80–40, respectively; pattern 2 with triangle symbols at 130 kg N ha−1, 170 kg N ha−1, and 210 kg N ha−1 represent 90–0–40, 90–40–40, and 90–40–80, respectively; and pattern 3 with square symbols at 170 kg N ha−1 represents 90–0–80, respectively, corresponding to the N application rate at basal-midtillering-panicle initiation stages
The normalized effects of N treatments on grain yield, plant N accumulation, and fertilizer N loss
Figure 4 shows the natural logarithm (ln) normalized values for the N treatment at 90 kg ha−1 that exhibited the influence of N rate on grain yield, plant N accumulation, and N loss to the environment. The dominant effect of the incremental N rate increase was increasing N loss, followed by promotion of plant N accumulation, but there was no obvious impact on grain yield. The effect of the incremental N rate on plant N accumulation was greater in 2014 than that in 2013. YLY6 showed a moderate increase in grain yield and a sharp change in N accumulation at N rates between 0 and 90 kg ha−1 in 2013, while LDQ7 showed a moderate increase in N accumulation at N rates between 0 and 90 kg ha−1 in 2014.
The effects of N rate on the normalized values of grain yield, plant N accumulation, and fertilizer N loss at the maturity stage: (a) Yangliangyou 6 in 2013, (b) Lvdaoq 7 in 2013, (c) Yangliangyou 6 in 2014, and (d) Lvdaoq 7 in 2014. The normalization was carried out by calculating the natural logarithm (ln) of the ratio of values under each N rate relative to that value under 90 kg N ha−1
Discussion
Grain yield influenced by N rates and N splitting patterns
The responses of rice grain yield to N fertilizer application have been widely studied in recent decades. In general, rice grain yield increases and then stagnates or even decreases with increasing N rates (Huang et al. 2008; Wang et al. 2009; Yan et al. 2009); a similar result was confirmed in the present study. The highest grain yield was achieved at the N rate of approximately 210 kg ha−1 for LDQ7 and YLY6 (Tables 3 and 4). The influences of N rates on grain yield were related to multiple yield components (Table 4). The increase in grain yield at relatively low N application rates was mainly influenced by the increase in panicle numbers, grain weight, and aboveground biomass accumulation. However, the plateau and decline of grain yield at relatively high N application rates were attributed to declining grain filling percentage as well as a lower harvest index. Relatively high N application rates can lead to poor canopy photosynthesis due to mutual shading, high respiratory loss due to high tissue N concentration, and poor carbohydrate translocation from straw to grain, thus causing poor grain filling percentage and a lower harvest index (Ying et al. 1998; Zhang et al. 2011; Sui et al. 2013). The plateau and decline of grain yield at relatively high N application rates were not related to soil chemical properties. Although the soil pH of experimental fields was relatively low (5.49–5.67), it is still within the optimum range (5.00–6.50) for rice growth and development (Uchida and Hue 2000). Furthermore, soil micronutrients were sufficient or within the optimum range for rice production in most paddy fields of Wuxue County (data not shown). Compared to YLY6, LDQ7 had a larger spikelet number m−2 due to its large spikelet number panicle−1 and lighter grain weight. Furthermore, the spikelet number panicle−1 of LDQ7 showed a decreasing trend with N rate. Sui et al. (2013) reported that greater competition for nutrients, space, and carbohydrates along with an increase in panicle number at high N application rates might lead to less differentiated spikelet panicle−1 and more severe spikelet degeneration.
The N split application method has been suggested to increase yield in many crops by many previous studies (Chen et al. 2015). However, the N split application had little effect on rice grain yield, partly because of the sufficient soil background N in this area (Peng et al. 2002).
N accumulation influenced by N rates and N splitting patterns
Here, we showed that plant N accumulation in both varieties was significantly affected by the N rate and by the corresponding ratios of tillering-N and panicle-N fertilization (Table 5). Nitrogen accumulation first increased with the incremental rate increase of N fertilizer and then stagnated with a further increased N rate, although the highest N accumulations were attained at high N inputs of 210 or 250 kg N ha−1 for YLY6 and LDQ7 in 2013 and 2014. This result agrees with previous studies demonstrating that N fertilizer application increases N accumulation and therefore grain yield (Zhang et al. 2012; Chen et al. 2015). In addition to the N rate, the timing of N fertilizer significantly affected the N accumulation efficiency. Our results demonstrated that an increased panicle-N ratio and a reduced tillering-N ratio markedly promoted plant N accumulation, which agrees with previous studies (Peng and Cassman 1998; Peng et al. 2002, 2006; Zhang et al. 2011; Pan et al. 2012). Although the largest amount of N absorption occurs at the tillering and booting stages (Lin et al. 2014; Wang et al. 2017), the tillering-N fertilizer experienced a low recovery efficiency caused by high ammonia presence due to surface application (Bell et al. 2016) and the subsequent effects of basal fertilizer (Yan et al. 2009; Lin et al. 2014). The well-known “three controls” nutrient management methodology for irrigated rice in China proposed by Zhong et al. (2007) suggests that a slight increase in basal N fertilizer with no or a very little tillering fertilizer, together with more top-dressed N fertilizer during panicle initiation, would improve N accumulation in rice. However, the results of N accumulation driven by increasing the panicle-N fertilizer ratio and reducing the tillering-N fertilizer ratio at a certain rate of N fertilizer did not show an obvious yield increase (Tables 3, 4, and 5).
Fate of fertilizer 15N influenced by N rates and splitting patterns
The 15N tracing method is a valuable tool for distinguishing fertilizer N and soil N. The fertilizer N fate in flooded soil-rice plant ecosystems has been extensively investigated using this method (Mikkelsen 1987; Wilson et al. 1989; Takahashi and Yagi 2002; Ju et al. 2009; Norman et al. 2013). In the present study, the 15N tracing reflects that the N accumulation derived from fertilizer in plant shoots (Ndff) increased steadily along with the increased N rates. The uncounted fertilizer N loss increased sharply when the N rate exceeded 170 kg ha−1 due to the stagnation of fertilizer N residue in soil (Fig. 2). An average of 31.0% and 28.7% of plant N was derived from fertilizer, with 69.0% and 71.3% of plant N derived from the soil in YLY6 and LDQ7, respectively. This finding is consistent with those of previous studies (Yan et al. 2009; Pan et al. 2012). The 15N residue in soil has always been reported to increase with the rate of fertilizer application (Han et al. 2003; Ju et al. 2009; Zhao et al. 2009; Zhang et al. 2010; Pan et al. 2012; Zhang et al. 2012; Yang et al. 2013; Kadiyala et al. 2014; Cao and Yin 2015). However, our present study observed a residual plateau of fertilizer-derived N in soil at approximately 48 kg N ha−1 based on much narrower N rate intervals of 40 kg ha−1 than previous studies (Han et al. 2003; Zhao et al. 2009; Tian et al. 2009; Zhang et al. 2010, 2012; Cao and Yin 2015), and comprehensive coverage of N ranged from a low input of 90 kg ha−1 to an excessive input of 250 kg ha−1. The 15N not counted in plant and soil was considered the fertilizer N loss. NH3 volatilization and denitrification were previously reported as the dominant pathways of N loss from flooded rice which would exert adverse environmental consequences such as air pollution, soil acidification, and water eutrophication (Peng et al. 2002; Zhao et al. 2009; Chen et al. 2014). Zhao et al. (2009) reported that NH3 volatilization in the rice season increased proportionally more than the fertilizer increases. Pan et al. (2012) also reported a much greater increase of fertilizer N loss than fertilizer-derived N in rice plants and in soil. The lower average rate of 4.0 kg ha−1 fertilizer N residue in soil and the lower average rate of 2.5 kg ha−1 fertilizer N loss in YLY6 cultivation could be partly explained by the approximately 6.5 kg ha−1 higher Ndff than LDQ7 (Fig. 2). Lin et al. (2004) demonstrated that higher plant N accumulation would accelerate and promote remobilization of recently fixed NH4+, which then diminished the pool size of extractable NH4+ in soil. Zhu (1997) reported that the dominant effect of plant growth is the reduction of N loss, which means that the greater the amount of N uptake, the smaller the fertilizer N loss will be.
In addition to the N rate, the timing of N fertilizer application significantly affected the N fate. An increased panicle-N ratio and a reduced tillering-N ratio at an N rate of 130, 170, and 210 kg ha−1 increased the Ndff while decreasing the corresponding N residue plus N loss (Fig. 3) because it promoted the synchrony between inorganic N fertilization and crop N demand. Yan et al. (2009) reported that the contribution of 15N-labeled fertilizer at basal, tillering, and panicle initiation stages accounted for 10.6%, 5.9%, and 12.4% of the total N accumulation at the maturity stage, respectively, with the total fertilizer-derived N contributing 28.9% in rice plants. Nitrogen top dressing at vigorous growth stages when plant N uptake is great (such as panicle initiation or elongation) could consistently reduce uncounted N loss and NH3 volatilization (Zhu 1997; Han et al. 2003; Cao and Yin 2015). In general, the fertilizer fate observed by the 15N tracer method in the present study revealed that 14–40%, 18–26%, and 41–67% of fertilizer was absorbed by plant shoots, remained in the soil, and was lost to the ecosystem, respectively, until the harvest stage across all N supplements and varieties.
Comprehensive influences of N management on grain yield, plant N accumulation, and fertilizer N loss
Taking a whole look at the influence of N fertilization on grain yield, plant N accumulation, and fertilizer N loss (Fig. 4), the sharp increase in N accumulation in YLY6 and the moderate increase in LDQ7 coincided with previous findings that hybrids have a more extensive root system and a superior N accumulation capacity that allow them to take up native soil N and other soil nutrients more effectively than pure-line rice cultivars, which are especially stimulated by low N rates (Jenkinson et al. 1985; Norman et al. 2013). Moreover, the incremental N application had a dominant influence on fertilizer N loss, a much smaller influence on plant N accumulation, and no obvious influence on grain yield (Fig. 4). We supposed this finding would be tenable and even more remarkable in 2013 due to the sharper increases in the integral amounts of fertilizer N residue and fertilizer N loss and the smaller increases in plant N accumulation and grain yield with incremental application of N fertilizer compared to that in 2014 (Tables 3 and 5, and Fig. 2). This finding also reveals the severe loss and “luxury absorption” of N caused by N fertilizer over application. In fact, any further increase of uncounted fertilizer N loss caused by incremental N input would have great environmental consequences to water and air. It is worth applying a proper N rate with an optimized splitting pattern that enhances ecological production of rice. As our results show, an N rate between 130 and 170 kg ha−1 with optimized N splitting ratios of reduced tillering-N fertilizer and increased PI-N fertilizer could not only maintain high grain yield but also control the fertilizer N loss to environment substantially.
Conclusion
In the present study, there was no further significant increase in grain yield when the N rate exceeded 130 or 170 kg ha−1. The plant aboveground N accumulation at the lowest N rates of 130 or 170 kg ha−1 was comparable to the highest N accumulation rates. Although optimizing N splitting patterns at N rates of 130, 170, and 210 kg ha−1 by reducing the N ratio at the tillering stage and increasing the N ratio at the panicle initiation stage did not exert clear effects on grain yield, it apparently promoted greater N accumulation in the plant. The 15N tracing showed the dominant effect of N application as N loss rather than plant N accumulation and grain yield. Additionally, despite the fact that plant N accumulation derived from fertilizer increased gradually with the incremental N rate increases, the uncounted fertilizer N loss increased sharply when N input exceeded 170 kg ha−1 because the fertilizer N residue in the soil peaked at this N input rate. The threshold for soil accumulation of fertilizer N was approximately 48 kg ha−1 in both YLY6 and LDQ7 cultivation. Increasing the N fertilizer ratio at the panicle initiation stage promoted plant N accumulation derived from fertilizer, while it decreased the integral amount of the fertilizer N retention in soil and the uncounted fertilizer N loss to the ecosystem. In conclusion, an N rate of 130 to 170 kg ha−1 with an optimum splitting ratio is recommended to maintain the amount of fertilizer N residue in soil and significantly reducing fertilizer N loss from the paddy field while maintaining rice yield due to optimal synchrony between peak fertilizer N availability and peak crop N demand in similar conditions in the middle reaches of the Yangtze River.
References
Bell M, Schwenke G, Lester D (2016) Understanding and managing N loss pathways. Australian Government, Grains Research and Development Corporation. https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2016/02/understanding-and-managing-n-loss-pathways. Accessed 23 Feb 2016
Cao Y-S, Yin B (2015) Effects of integrated high-efficiency practice versus conventional practice on rice yield and N fate. Agric Ecosyst Environ 202:1–7
Chen X-P et al (2014) Producing more grain with lower environmental costs. Nature 514:486–489. https://doi.org/10.1038/nature13609
Chen Y-T et al (2015) Crop management based on multi-split topdressing enhances grain yield and nitrogen use efficiency in irrigated rice in China. Field Crop Res 184:50–57. https://doi.org/10.1016/j.fcr.2015.09.006
Cui Z-L et al (2014) Closing the N-use efficiency gap to achieve food and environmental security. Environ Sci Technol 48:5780–5787. https://doi.org/10.1021/es5007127
Dobermann A et al (2002) Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crop Res 74:37–66. https://doi.org/10.1016/S0378-4290(01)00197-6
Han X-Z, Wang S-Y, Song C-Y, Qiao Y-F (2003) Fate of fertilizer nitrogen in paddy field of black soil region. Chin J Appl Ecol 14(11):1859–1862
Han B-J et al (2012) Evaluation and present situation of fertilization for rice in Hubei province. Hubei Agric Sci 51:2430–2435 (in Chinese with English abstract)
Hofmeier M et al (2015) Nitrogen management in a rice-wheat system in the Taihu Region: recommendations based on field experiments and surveys. Agric Ecosyst Environ 209:60–73. https://doi.org/10.1016/j.agee.2015.03.032
Huang J-B et al (2007) Investigation on the economically-ecologically appropriate amount of nitrogen fertilizer applied in rice production in Fe-leaching-Stagnic Anthrosols of the Taihu Lake region. Acta Ecol Sin 27(2):0588–0595
Huang J-L et al (2008) Determination of optimal nitrogen rate for rice varieties using a chlorophyll meter. Field Crop Res 105:70–80. https://doi.org/10.1016/j.fcr.2007.07.006
Jenkinson DS et al (1985) Interactions between fertilizer nitrogen and soil nitrogen—the so-called ‘priming’ effect. J Soil Sci 36(3):425–444. https://doi.org/10.1111/j.1365-2389.1985.tb00348.x
Ju X-T, Zhang F-S (2003) Thinking about nitrogen recovery rate. Ecol Environ 12:192–197 (in Chinese with English abstract). https://doi.org/10.3969/j.issn.1674-5906.2003.02.018
Ju X-T et al (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. PNAS 106(9):3041–3046. https://doi.org/10.1073/pnas.0813417106
Kadiyala MDM et al (2014) Uptake efficiency of 15N-urea in flooded and aerobic rice fields under semi-arid conditions. Paddy Water Environ. https://doi.org/10.1007/s10333-014-0473-8
Ladha JK, Pathak H, Krupnik TJ, Six J, van Kessel C (2005) Efficiency of fertilizer nitrogen in cereal production: retrospect and prospects. Adv Agron 87:85–156
Leon M et al (1995) Increased uptake of native soil nitrogen by roots of Lolium multiflorum Lam. after nitrogen fertilization is explained by a stimulation of the uptake process itself. Plant Soil 173:197–203
Li J-J et al (2015a) Evolution characteristics of soil nutrients in the main rice production regions, the middle-lower reach of Yangtze River of China. J Plant Nutr Fert 21(1):92–103 (in Chinese with English abstract). https://doi.org/10.11674/zwyf.2015.0110
Li Y et al (2015b) Evaluation of nitrogen balance in a direct-seeded-rice field experiment using Hydrus-1D. Agric Water Manag 148:213–222. https://doi.org/10.1016/j.agwat.2014.10.010
Li P-F, Li X-K, Hou W-F, Ren T, Cong R-H, Du C-W, Xing L-H, Wang S-H, Lu J-W (2018) Studying the fate and recovery efficiency of controlled release urea in paddy soil using 15N tracer technique. Sci Agric Sin 51(20):3961–3971 http://manu71.magtech.com.cn/zgnykx/CN/10.3864/j.issn.0578-1752.2018.20.014
Lin S et al (2004) Added nitrogen interaction as affected by soil nitrogen pool size and fertilization-significance of displacement of fixed ammonium. J Plant Nutr Soil Sci 167:138–146
Lin J, Li G, Xue L, Zhang W, Xu H, Wang S, Yang L, Ding Y (2014) Subdivision of nitrogen use efficiency of rice based on 15N tracer. Acta Agron Sin 40:1424–1434 (in Chinese with English abstract). https://doi.org/10.3724/SP.J.1006.2014.01424
Mikkelsen DS (1987) Nitrogen budgets in flooded soils used for rice production. Plant Soil 100:71–97. https://doi.org/10.1007/BF02370933
Norman R et al (2013) Nitrogen uptake efficiency of a hybrid compared with a conventional, pure-line rice cultivar. Soil Sci Soc Am J 77:1235–1240. https://doi.org/10.2136/sssaj2013.01.0015
Pan S-G et al (2012) Effects of N management on yield and N uptake of rice in central China. J Integr Agric 11(12):1993–2000
Peng S, Cassman KG (1998) Upper thresholds of nitrogen uptake rates and associated nitrogen fertilizer efficiencies in irrigated rice. Agron J 90:178–185. https://doi.org/10.2134/agronj1998.00021962009000020010x
Peng S et al (2002) Research strategy in improving fertilizer-nitrogen use efficiency of irrigated rice in China. Sci Agric Sin 35:1095–1103 (in Chinese with English abstract). https://doi.org/10.3321/j.issn:0578-1752.2002.09.012
Peng S et al (2006) Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop Res 96:37–47. https://doi.org/10.1016/j.fcr.2005.05.004
Salvagiotti, Miralles (2008) Radiation interception, biomass production and grain yield as affected by the interaction of nitrogen and sulfur fertilization in wheat. Eur J Agron 28(3):282–290. https://doi.org/10.1016/j.eja.2007.08.002
Sui B et al (2013) Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crop Res 150:99–107. https://doi.org/10.1016/j.fcr.2013.06.012
Takahashi S, Yagi A (2002) Losses of fertilizer-derived N from transplanted rice after heading. Plant Soil 242:245–250. https://doi.org/10.1023/A:1016262122991
Tian Y-H, Yin B, He F-Y, Zhu Z-L (2009) Recovery by crop and loss of nitrogen fertilizer applied in rice season in Taihu Lake region. J Plant Nutr Fert 15(1):55–61 (in Chinese with English abstract). https://doi.org/10.3321/j.issn:1008-505X.2009.01.008
Uchida R, Hue N V (2000) Soil acidity and liming. In: Silva JA, Uchida R (eds) Plant nutrient management in Hawaiian soils, approaches for tropical and subtropical agriculture, College of Tropical Agriculture and Human Resources, University of Hawaii, Manoa, pp 101–111. http://hdl.handle.net/10125/1908
Wang D-Y et al (2017) 15N tracer-based analysis of genotypic differences in the uptake and partitioning of N applied at different growth stages in transplanted rice. Field Crop Res 211:27–36
Wang M, Yang J-P, Xv W, Wang H, Sun J-H (2009) Influence of nitrogen rates with split application on N use efficiency and its eco-economic suitable amount analysis in rice. J Zhejiang Univ, Agric Life Sci 35(1):71–76 (in Chinese with English abstract). https://doi.org/10.3785/j.issn.1008-9209.2009.01.010
Wilson CE, Norman RJ, Wells BR (1989) Seasonal uptake patterns of fertilizer nitrogen applied in split applications to rice. Soil Sci Soc Am J 53:1884–1887. https://doi.org/10.2136/sssaj1989.03615995005300060045x
Yan J, Yin B, Zhang S-L, Shen Q-R, Zhu Z-L (2008) Effect of nitrogen application rate on nitrogen uptake and distribution in rice. J Plant Nutr Fert 14(5):835–839. https://doi.org/10.3321/j.issn:1008-505X.2008.05.003
Yan J, Shen Q-R, Yin B, Wan X-J (2009) Fertilizer-N uptake and distribution in rice plants using 15N tracer technique. J Nucl Agric Sci 23:487–491 (in Chinese with English abstract). http://d.oldg.wanfangdata.com.cn/Periodical_hnxb200903026.aspx
Yang L et al (2013) Effects of reducing N application on crop N uptake, utilization and soil N balance under rice-wheat rotation system on middle and lower reaches of Yangtze River Region. Southwest China J Agric Sci 26(1):195–202 (in Chinese with English abstract). https://doi.org/10.16213/j.cnki.scjas.2013.01.007
Ying J-F, Peng S-B, He Q-G, Yang H, Yang C-D, Visperas RM, Cassman KG (1998) Comparison of high-yield rice in tropical and subtropical environments. I Determinants of grain and dry matter yields. Field Crop Res 57:71–84. https://doi.org/10.1016/s0378-4290(98)00077-x
Zhang Q-W et al (2010) The fate of fertilizer-derived nitrogen in a rice field in the Qingtongxia irrigation area. Acta Sci Circumst 30(8):1707–1714 (in Chinese with English abstract. https://doi.org/10.13671/j.hjkxxb.2010.08.023
Zhang H-C et al (2011) Precise postponing nitrogen application and its mechanism in rice. Acta Agron Sin 37(10):1837–1851 (in Chinese with English abstract). https://doi.org/10.3724/SP.J.1006.2011.01837
Zhang Q-W, Yang Z-L, Zhang H, Yi J (2012) Recovery efficiency and loss of 15N-labelled urea in a rice-soil system in the upper reaches of the Yellow River basin. Agric Ecosyst Environ 158:118–126. https://doi.org/10.1016/j.agee.2012.06.003
Zhang Z et al (2013) Mid-season nitrogen application strategies for rice varieties differing in panicle size. Field Crop Res 150:9–18. https://doi.org/10.1016/j.fcr.2013.06.002
Zhao X et al (2009) Nitrogen fate and environmental consequence in paddy soil under rice-wheat rotation in the Taihu lake region, China. Plant Soil 319:225–234. https://doi.org/10.1007/s11104-008-9865-0
Zhong X-H, Huang N-R, Zheng H-B (2007) Some principles for the “three controls” nutrient management technology for irrigated rice. Guangdong Agric Sci (5):19–22. https://doi.org/10.3969/j.issn.1004-874X.2007.05.005
Zhu Z-L (1997) Fate and management of fertilizer nitrogen in agro-ecosystems. In: Zhu Z-L, Wen Q-X, Freney JR (eds) Nitrogen in Soils of China. Developments in Plant and Soil Sciences 74:239–279. https://doi.org/10.1007/978-94-011-5636-3
Zhu Z-L (2008) Research on soil nitrogen in China. Acta Pedol Sin 45(5):778–783 (in Chinese with English abstract). https://doi.org/10.3321/j.issn:0564-3929.2008.05.003
Zou Y-B, Ao H-J, Wang S-H, Tang Q-Y (2006) Studies on San-Ding Cultivation Method for super rice: the concept and the principle. Chin Agric Sci Bull 22(5):158–162 (in Chinese with English abstract). https://doi.org/10.3969/j.issn.1000-6850.2006.05.043
Acknowledgments
Valuable suggestions given by Dr. Xiong D-L of College of Plant Science and Technology of Huazhong Agricultural University are also acknowledged.
Funding
This work was supported by a grant from the National Key Research and Development Program of China (No. 2016YFD0300210) and a grant from the National Natural Science Foundation of China (No. 31671620).
Author information
Authors and Affiliations
Contributions
Jianliang Huang and Jing Wang initiated and designed the research, Jing Wang, Penghao Fu, Yutiao Chen, and Congde Zhang performed the experiments, Jing Wang, Fei Wang, and Shah Fahad analyzed the data and wrote the manuscript, and Fei Wang, Shah Fahad, Pravat K. Mohapatra, Jianliang Huang, Shaobing Peng, Kehui Cui, Lixiao Nie, and Dongliang Xiong revised and edited the manuscript and also provided advices on the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Competing financial interests
The corresponding author on behalf of all coauthors, declare no competing financial interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, J., Fu, P., Wang, F. et al. Optimizing nitrogen management to balance rice yield and environmental risk in the Yangtze River’s middle reaches. Environ Sci Pollut Res 26, 4901–4912 (2019). https://doi.org/10.1007/s11356-018-3943-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3943-5