Abstract
Toxic metal contamination in food products and the environment is a public health concern. Therefore, understanding human exposure to cadmium (Cd), lead (Pb), cobalt (Co), and copper (Cu) levels in the general population of Taiwan is necessary and urgent. We aimed to establish the human biomonitoring data of urine toxic metals, exposure profile changes, and factors associated with metal levels in the general population of Taiwan. We randomly selected 1601 participants older than 7 years of age (36.9 ± 18.7 years (7–84 years)) from the Nutrition and Health Survey in Taiwan (NAHSIT) conducted during 1993–1996 (93–96) and 2005–2008 (05–08) periods and measured the levels of four metals in the participants’ urine samples using inductively coupled plasma-mass spectrometry. The median (range) levels of urinary Cd, Pb, Co, and Cu in participants from the NAHSIT 93–96 (N = 821)/05–08 (N = 780) were 0.60 (ND–13.90)/0.72 (ND–7.44), 2.28 (ND–63.60)/1.09 (0.04–48.88), 0.91 (0.08–17.30)/1.05 (0.05–22.43), and 16.87 (2.62–158.28)/13.66 (1.67–189.70) μg/L, respectively. We found that the urinary median levels of Pb and Cu in our participants were significantly lower in the NAHSIT 05–08 (Pb 1.09 μg/L, Cu 13.66 μg/L) than in the NAHSIT 93–96 (Pb 2.28 μg/L, Cu 16.87 μg/L; P < 0.01), whereas those of Cd and Co were significantly higher in the NAHSIT 05–08 (Cd 0.72 μg/L, Co 1.05 μg/L; P < 0.01). Youths had higher exposure levels of Pb, Co, and Cu than adults. Participants with alcohol consumption, betel quid chewing, or cigarette smoking had significantly higher median levels of urinary Pb or Cu (P < 0.01) than those without. Principal components and cluster analysis revealed that sex had different exposure profiles of metals. We concluded that levels of urinary Cd, Pb, Co, and Cu exposure in the general Taiwanese varied by age, sex, and lifestyles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Taiwan is one of the most important countries producing commercial electronic products, including mobile phone, printed circuit board, and computers, which may release toxic metals (Pb, Cd, etc.) during the process of production and in waste. In Taiwan, some studies revealed high exposure levels of certain petrol-related metals in residents residing near industrial complexes (Chang et al. 2006; Wang et al. 2006; Yuan et al. 2016). Toxic metal contamination may occur because of the increasing use of these metals for different land use, such as industrial, agricultural, and domestic purposes, as well as the improper treatment of e-wastes in Asian countries, especially a restriction or ban of e-waste treatment in China (Doong et al. 2008; Song and Li 2014). In addition, cadmium (Cd) and lead (Pb) in rice (Chen 1991; Watanabe et al. 1989) and mercury (Hg) in fish (Hsu et al. 2007) have raised concerns regarding environmental pollution. Toxic metal contamination concerns in agricultural and seafood products, such as the accumulation of high level of copper (Cu) in oysters due to water contamination, has caused public health concern in Taiwan (Han and Hung 1990; Han et al. 1993). Therefore, exposure to heavy metals in the general population should be assessed and sub-populations with high exposure should be identified.
Cd has been classified as a group 1A human carcinogen by the International Agency for Research on Cancer (IARC) (IARC 2012). It is associated with an increased risk of itai-itai disease in Japanese people (Nogawa and Kido 1993) and cardiovascular diseases (Tellez-Plaza et al. 2013), blood pressure elevation (Tellez-Plaza et al. 2008), and renal dysfunction in general population (Chung et al. 2014). Rice is identified as a major source of Cd exposure in Asian populations (Uraguchi and Fujiwara 2012). Some studies have reported that the high levels of blood Pb, Cd, and Hg in the Asian-American and East Asian immigrated population, which indicated the exposure sources, may link to the characteristic of exposure profile in Asian population (Awata et al. 2017; Dix-Cooper and Kosatsky 2018). However, limited information is available for those toxic metals in a national-wide scale in the Chinese population.
Pb is neurotoxic and has been classified as a group 2A human carcinogen by the IARC (IARC 2006a). It is associated with several human diseases, such as osteopenia or osteoporosis in adult women (Tsai et al. 2015; Tsai et al. 2016). Neurotoxicity that inhibits early cognitive and intellectual development in children may occur after Pb exposure (Bellinger et al. 1987; Huang et al. 2012). Some paints, other materials, and toys may contain Pb; however, no study was systematically conducted to assess the impact of unleaded gasoline use (since 2000) on urinary Pb in the general Taiwanese population (Wu et al. 2012).
Cobalt (Co) is classified as a group 2B human carcinogen by the IARC (IARC 2006b). It is usually mixed with other metals, and its compounds are often used as trace element additives in medicine and agriculture (ATSDR 2004a). Co-exposure episodes result from long-term occupational exposure, which causes a reduction of erythropoietic variables (median 70 μg/g cre.) (Swennen et al. 1993). Indeed, Co is widely used in electroplating industry and a side pollutant or waste of the electronics industry, which may be released into groundwater. Co is also an essential component of vitamin B12 in humans, but exposure to high Co level can result in adverse lung and heart conditions, asthma, dermatitis, and a higher risk of post-operative fibroid diagnoses and potentially deleterious effects to the mother or fetus (ATSDR 2004a; Fort et al. 2015; Johnstone et al. 2014; Rehfisch et al. 2012; Walters et al. 2014). However, the level of urinary Co in the general population of Taiwan is limited.
Cu can enter the environment through release from factories that use Cu or Cu compounds. A previous study reported unusual green oysters in the downstream close to the scientific park of Northern Taiwan, which could possibly be contaminated by wastewater of certain industry, like the printed circuit board, in the early 90s in Southern Taiwan (Han and Hung 1990). Besides, Cu is an essential metal for the human body; however, beyond its tolerable limit, Cu can be toxic. The adverse effects of exposure to high doses of Cu include nausea, vomiting, stomach cramps, and diarrhea (ATSDR 2004b). Several studies address the association between high Cu exposure and health issues, including acute myocardial infarction (Singh et al. 1985), cognitive decline (Zatta et al. 2008), and effects in the immune function (Turnlund et al. 2004). Even within the nutritional range, uncertainties remain regarding Cu reference values for humans, and reliable biomarkers of Cu status are needed.
In this study, we aimed to assess the exposure levels of urinary Cd, Pb, Co, and Cu as well as associated factors and temporal changes in the general population of Taiwan using the Nutrition and Health Survey in Taiwan (NAHSIT) in a two-period survey at 1993–1996 (NAHSIT 93–96) and 2005–2008 (NAHSIT 05–08).
Materials and methods
Ethics statement
This study was approved by the Research Ethics Committee of the National Health Research Institutes (NHRI, No. EC0990103) in Taiwan. Written informed consent from each participant was obtained prior to study enrollment. This study follows the guidance of the research ethics committee.
Study participants
NAHSIT is a nationally representative cross-sectional survey of the health and nutrition in Taiwan, and all detailed sampling methods have been described in previous studies (Pan et al. 2011; Tu et al. 2011; Yeh et al. 2011). In order to obtain an adequate participation from the general population of Taiwan, the sample number in each age and gender was according to the population density and urbanization of each city in Taiwan. The survey divided whole townships and city districts in Taiwan and was divided into seven and eight strata in the NAHSIT 93–96 and NAHSIT 05–08, respectively. After the strata were defined, three townships or city districts were selected from each stratum with probability proportional to size (PPS) sampling methods. The survey excluded participants who were pregnant or breastfeeding women, had severe diseases, were foreigners, or were citizens in military units, nursing homes, schools, hospitals, or jails. Participants had to be Taiwanese and ≧ 7 years old. We recruited 892 and 870 participants from the NAHSIT 93–96 and NAHSIT 05–08, respectively. We excluded 71 and 90 participants from the NAHSIT 93–96 and NAHSIT 05–08, respectively, for whom sufficient urine sample or questionnaire data were not available. From the NAHSIT 93–96, we included 632 adults and 189 youths, yielding a total of 821 participants. From the NAHSIT 05–08, we included 677 adults and 103 youths, yielding a total of 780 participants. The total of 1601 participants from the NAHSIT studies consists of 821 (response rate 92%) and 780 (response rate 90%) participants from the NAHSIT 93–96 and NAHSIT 05–08 sampling periods, respectively (Fig. S1).
The participants were categorized into groups according to NAHSIT period, sex, age, regions [northern, central, southern, eastern, or remote island (Penghu)], and urbanization (urban, suburban, rural, harbor, or industrial). Participants were classified as youths [children (7 to 11 years), adolescents (12 to 17 years)] or adults [young adults (18 to 39 years), middle-aged adults (40 to 64 years), elderly adults (≥ 65 years)]. The definition of urbanization was categorized by previous studies in Taiwan (Liu et al. 2006; Lin et al. 2013). All townships were classified based on the population density, population ratio of education, population ratio of elderly people over 65 years, population ratio of people of agriculture workers, and the number of physicians per 100,000 people (Liu et al. 2006; Lin et al. 2013). Townships with harbors or industrial areas classified by the Taiwan Fisheries Agency and the Industrial Development Bureau were categorized as “harbor” and “industrial” areas, respectively.
Data and specimen collection
All participants were interviewed by well-trained interviewers. We assessed the recorded data and specimens of each participant from the NAHSIT 93–96 and NAHSIT 05–08 and obtained basic demographic information (i.e., age, sex, urbanization, and region). The first void spot urine specimens were collected and stored at 4 °C during transport to the laboratory and then frozen at − 80 °C until analysis, when they were analyzed for levels of the major ubiquitous heavy metals, namely Cd, Pb, Co, and Cu.
Measurement of urinary metals
We used a published method to analyze certain metals in the urine sample (Chen et al. 2011; Tsai et al. 2017). Briefly, after thawing the samples, 1 mL samples were diluted 10-fold with 9 mL of 1% (v/v) nitric acid (J.T. Baker Chemical Company, Phillipsburg, New Jersey, USA) in 15 mL polypropylene tubes. Inductively coupled plasma-mass spectrometry (ICP-MS, 7700 Series; Agilent Technologies, Inc., Santa Clara, CA, USA) was used to determine the concentrations of urinary Cd, Pb, Co, and Cu. In addition, a guideline of quality control and method detection limit (MDL) was modified from the National Institute of Environmental Analysis (NIEA PA-103, PA-104, PA-107) and the Taiwan Environmental Protection Agency (NIEA PA-103, PA-104, PA-107, 2005), which are based on the standard of the National Environmental Laboratory Accreditation (NELAC 2001). The calibration curves were ranged from 0.01 to 50 μg/L for Cd, Co, Pb, and Cu [correlation coefficient (R2) > 0.995], and one check sample for the calibration curve was performed. To ensure the quality of the analysis results, one blank, spike, duplicate, and standard reference material (SRM) were checked in every 10 samples (each batch). The blank sample (1% (v/v) nitric acid solution) of all metals was less than 2-fold MDL. The spike recoveries using 5 μg/L urinary matrix for every 10 urine samples were 92.6 ± 5.9, 98.6 ± 6.0, 114.1 ± 7.9, and 93.3 ± 5.3 for Cd, Pb, Co, and Cu, respectively. Additionally, the duplicate sample mean relative error of each metal was within ± 15%. The SRM (Seronorm™ Trace Elements Urine, Billingstad, Norway) for Cd, Pb, and Co was tested for every 10 samples, with relative errors of 10.0, 1.65, and 5.65%, respectively. The data qualifying our quality control criteria were used for subsequent analyses. The limit of detection (LOD) of Cd, Pb, Co, and Cu was 0.008, 0.005, 0.003, and 0.003 μg/L, respectively. In both periods, the detectable rate of urinary Cd and Pb levels in the general population of Taiwan was higher than 98.8% and the detectable rate of urinary Co and Cu levels was 100%. Notably, when the levels of the metals were below their MDL (Cd 0.02 μg/L, Pb 0.012 μg/L, Co 0.019 μg/L, Cu 0.16 μg/L), they were recalculated as MDL/√2 (Paschal et al. 1998).
Statistical analysis
Demographic statistics of the participants in NAHSIT 93–96 and NAHSIT 05–08 are presented. The geometric mean (GM), geometric standard deviation (GSD), 95% confidence interval (95% CI), mean, standard deviation (SD), and selected percentiles (25th, 50th, 75th, and 95th) of the metal levels were also calculated. The correlations between each urinary level of the metals were determined using Pearson’s correlation coefficient. Non-parametric statistics (Mann–Whitney U test and Kruskal–Wallis test) were used to assess the differences in the levels of each metal according to characteristics, such as the participants’ NAHSIT period, sex, age, urbanization, and regions. Finally, a principal component analysis (PCA) was applied to extract the exposure profiles of various metals. PCA was used for eigenvalues > 1, and we set independent interpretable factors according to a factor loading > 0.5 (or <− 0.5) with specific factors considering its major constituents. Hierarchical clustering analysis (HCA) was performed to illustrate the relationships of metals with each group (Chang et al. 2017; Liao et al. 2018). The significance level was set at P < 0.05, and all statistical analyses were performed using SPSS statistical software (version 22.0, IBM Corporation).
Results
Characteristics of the study participants
Table 1 illustrates the demographic characteristics of all participants. The distribution of sex was approximately equal for the entire population across both time periods. The majority of the participants were adults in NAHSIT 93–96 (77.0%) and NAHSIT 05–08 (86.8%). There were 72.9 and 48.9% with alcohol consumption in NAHSIT 93–96 and NAHSIT 05–08, respectively. Around 18% of the participants have betel quid chewing in NAHSIT 05–08; however, data were not available in NAHSIT 93–96. In both periods, more than 30% of the participants have cigarette smoking. Additionally, regarding regional and urbanization distributions, most of the participants were from Northern Taiwan (n = 542, 33.8%) and urban (n = 391, 24.4%) area (Table 1).
Urinary Cd, Pb, Co, and Cu levels in different periods and sexes
The selected percentiles and GM of urinary Cd, Pb, Co, and Cu levels in all participants were classified by youths and adults in the periods of NAHSIT 93–96 and NAHSIT 05–08 (Table 2). In the two periods, the median (range) levels of urinary Cd [0.72 (ND–7.44) μg/L] and Co [1.05 (0.05–22.43) μg/L)] in participants from the NAHSIT 05–08 period were significantly higher than in those from the NAHSIT 93–96 [Cd: 0.60 (ND–13.90) μg/L; Co: 0.91 (0.08–17.30) μg/L; P < 0.001]. However, the median (range) levels of urinary Pb [1.09 (0.04–48.88) μg/L] and Cu [13.66 (1.67–189.70) μg/L] in participants from the NAHSIT 05–08 were significantly lower than in those from the NAHSIT 93–96 [Pb: 2.28 (ND–63.60) μg/L, Cu: 16.87 (2.62–158.28) μg/L; P < 0.001].
In urinary Cd, the median (range) level in boys [0.49 (ND–3.07) μg/L] was significantly higher than that in girls [0.34 (ND–5.11) μg/L; P = 0.014] in the NAHSIT 93–96. For females, the median levels of urinary Cd in girls and women in the NAHSIT 05–08 were 1.9- and 1.2-fold higher than those in girls and women in the NAHSIT 93–96, respectively, and these differences were significant (both P < 0.01). In urinary Pb, the median levels in adults and youths were lower overall in participants from the NAHSIT 05–08 than in those from the NAHSIT 93–96. For the adult participants, we found that the median urinary Pb level in men was significantly higher than that in women in both periods (both P < 0.01). In urinary Co, the median (range) level was significantly higher in girls [1.59 (0.57–10.82) μg/L] than that in boys [1.31 (0.55–3.74) μg/L] in the NAHSIT 05–08 (P = 0.028) and the median level in women was significantly higher than that in men in both periods (both P < 0.01). In urinary Cu, we found that the median level in men was significantly higher than that in women in both periods (93–96: 17.40 vs. 15.28 μg/L; 05–08: 14.53 vs. 12.45; both P < 0.01). For the adult participants, the urinary Cu level in the NAHSIT 05–08 was significantly lower than that in the NAHSIT 93–96 (both men and women; P < 0.01) (Table 2).
Urinary Cd, Pb, Co, and Cu levels in different age groups
Table 3 depicts the selected percentiles and GM of urinary metal levels in our samples in the general population of Taiwan, classified by age, from the NAHSIT 93–96 and NAHSIT 05–08. We noted that participants’ exposure to Cd, Pb, Co, and Cu varied significantly according to their age. For example, the GM of urinary Cd was high in all adults from both sampling periods, and the median level of urinary Co in all participants increased, regardless of age, from the NAHSIT 93–96 to the NAHSIT 05–08. The median level of urinary Pb in youths and adults in the NAHSIT 05–08 was significantly lower than that in the NAHSIT 93–96 (both P < 0.01). Furthermore, the median level of urinary Cu in adults in the NAHSIT 05–08 was lower than that in the 93–96, particularly significant in the 18–39 and 40–64 age groups (both P < 0.01) (Table 3, Fig. S2).
Urinary Cd, Pb, Co, and Cu levels in different regions
We presented the distribution of urinary metal levels by different regions (Fig. 1a–d, Table S1). Our data revealed that the median level of urinary Cd in participants from the NAHSIT 93–96 varied by region; however, a significantly higher urinary Cd level was observed in central (P < 0.001) and eastern (P = 0.001) Taiwan between the two sampling periods. The median level of urinary Pb was lower among the participants across all regions from the NAHSIT 93–96 to the NAHSIT 05–08. The median level of urinary Co was significantly higher among the participants from the NAHSIT 05–08 who lived in northern (P < 0.001) and eastern (P = 0.044) Taiwan, compared with the participants from the NAHSIT 93–96 who lived in those same regions. Our data also revealed that the median level of urinary Cu indicated significant regional differences (P = 0.019), but in overall, it was lower in the participants from the NAHSIT 05–08 compared with that in the NAHSIT 93–96, except in central Taiwan (Fig. 1a–d, Table S1).
Urinary Cd, Pb, Co, and Cu levels in different urbanization
We also categorized our participants from both sampling periods into the following urbanization: urban, suburban, rural, harbor, and industrial areas. The median level of urinary Pb was lower, regardless of urbanization, from the NAHSIT 93–96 to the NAHSIT 05–08. Moreover, the participants in the NAHSIT 05–08 who lived in suburban, harbor, and industrial areas had a lower median level of urinary Cu than those in the NAHSIT 93–96 who lived in those areas, whereas higher Cd and Co exposure was observed in urban and rural areas between the two sampling periods (Fig. 1e–h, Table S1).
Urinary Cd, Pb, Co, and Cu levels in different lifestyles
We analyzed the urinary metal levels in the two study periods in relation to the characteristics of alcohol consumption, betel quid chewing, and cigarette smoking. In both periods, the median levels of urinary Cu and Pb in participants with alcohol consumption and cigarette smoking were significantly higher than those without (93–96: Cu: 17.51 vs. 15.36 μg/L, P = 0.014, Pb: 2.63 vs. 2.07 μg/L, P < 0.001; 05–08: Cu: 13.55 vs. 12.65 μg/L, P = 0.031, Pb: 1.34 vs. 0.88 μg/L, P < 0.001, respectively). By contrast, the Co levels were significantly higher in the non-smoking participants than in the smoking participants (93–96: 0.93 vs. 0.87 μg/L, P = 0.039; 05–08: 1.01 vs. 0.90 μg/L, P = 0.036) in the two periods. We assessed betel quid chewing in the NAHSIT 05–08 participants only, and we found that participants with betel quid chewing had significantly higher median levels of urinary Pb and Cu than those without (Pb: 1.67 vs. 0.91 μg/L, P < 0.001; Cu: 14.54 vs. 12.74 μg/L, P = 0.004). Considering the co-exposure by different lifestyles, we evaluated the urinary levels in both lifestyles. We found that the median levels of urinary Pb and Cu in participants with alcohol consumption, betel quid chewing, and cigarette smoking were significantly higher than those without (Pb: 1.67 vs. 0.86 μg/L, P < 0.001; Cu: 14.45 vs. 12.64 μg/L, P = 0.004) (Table 4).
The percentage differences of the four metal levels among the five age groups in the NAHSIT 05–08 compared with the NAHSIT 93–96
The percentage differences in the NAHSIT 05–08 were higher at 15–37% and 15–55% for urinary Cd and Co and were lower at 29–58% and 1–3% for urinary Pb and Cu than those in the NAHSIT 93–96, respectively. The adolescent group exhibited the most substantial lowest percentage difference in Pb exposure and the highest percentage difference in Cd exposure, whereas the middle-aged group exhibited the highest percentage difference in Co exposure and the most substantial lowest percentage difference in Cu exposure (Fig. 2).
The correlation between urinary Cd, Pb, Co, and Cu levels
We used the Pearson’s correlation to investigate the correlations between urinary metal levels in the NAHSIT 93–96 and NAHSIT 05–08 periods. The correlation coefficients of the four metals were 0.349 to 0.563 in the NAHSIT 93–96 and 0.361 to 0.556 in the NAHSIT 05–08 (Table S2). A weak to moderate correlation between metals in this study represented the route of simultaneous exposure of Cd, Pb, Co, and Cu during the two periods.
Principal component and hierarchical clustering analysis of urinary heavy metal exposure in the general population of Taiwan
We performed a PCA on the individual values of the urinary levels of the four metals in the participants from the NAHSIT 93–96 and NAHSIT 05–08 and analyzed the participants’ exposure to metals according to age and sex; the results are summarized in Fig. 3. Notably, two factors were extracted from the urinary metals which explained the total variance in the participants, regardless of age and sampling period. For the youths, the following two principal components (PCs; PC1 and PC2) explained 62.3% of the total variance in the NAHSIT 93–96: PC1 correlated with all four metals in girls, whereas PC2 correlated with all four metals in boys (Fig. 3a). In the NAHSIT 05–08, PC1 and PC2 explained 62.0% of the total variance in youths: PC1 correlated with all four metals in girls, whereas PC2 correlated with all four metals in boys (Fig. 3b). For adults, two PCs explained 49.1% of the total variance in the NAHSIT 93–96: PC1 correlated with all four metals in men, whereas PC2 correlated with Cd and Cu in women (Fig. 3c). In the NAHSIT 05–08, PC1 and PC2 explained 61.5% of the total variance in adults: PC1 correlated with all four metals in men, whereas PC2 correlated all four metals in women (Fig. 3d). Moreover, in each group, hierarchical clustering analysis identified two major clusters by sex (Fig. 3e–h).
Comparison of metal exposure in different countries
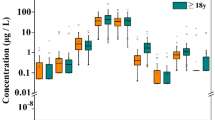
We compared the four metal GM levels in the population of various countries for similar periods. A comparison of the GM levels of the four metals in the general population of Taiwan (based on the NAHSIT 93–96 and NAHSIT 05–08), the United States (based on the National Health and Nutrition Examination Survey (NHANES) periods 1999–2000 and 2007–2008), Germany (based on the German Environmental Survey (GerES) II period 1990–1992 and GerES III period 1998), the Czech Republic (based on the Human Biomonitoring (HBM) project periods 2001–2003 and 2005–2009), Canada (based on the Canadian Health Measures Survey (CHMS) 2007–2013) (Haines et al. 2017), Spain (based on 2009 data) (Castano et al. 2012), China (based on 2008 data) (Wang et al. 2011), Korea (based on the Korea National Survey for Environmental Pollutants in the Human Body (KorSEP-III), 2008) (Lee et al. 2012), and Japan (based on 2000–2005 data) (Ohashi et al. 2006) is shown in Fig. 4. The GM levels of Cd in the Taiwanese population according to the NAHSIT 93–96 (0.54 μg/L) and NAHSIT 05–08 (0.69 μg/L) were 2.8- and 3.6-fold higher, respectively, than in the U.S. population according to the NHANES 2007–2008 (0.19 μg/L), 1.9- and 2.4-fold higher than in the Germany population according to the GerES II (0.29 μg/L), and 1.6- and 2.1-fold higher in the Canada population according to the CHMS 2007–2009 (0.34 μg/L), respectively. A 2.5-fold decrease in the GM of urinary Pb in the Taiwanese population was observed between the NAHSIT 93–96 and NAHSIT 05–08. Nevertheless, the GM of urinary Pb in the Taiwanese population according to the NAHSIT 05–08 (0.9 μg/L) was 1.8-fold higher than that in the U.S. population according to the NHANES 2007–2008 (0.49 μg/L), 1.9-fold higher than that in the Canada population according to the CHMS 2007–2009 (0.48 μg/L), and 3.1-fold higher than that in the Czech Republic population (0.29 μg/L). Conversely, the GM of urinary Pb in the Taiwanese population according to the NAHSIT 05–08 was lower than that in Spain (1.11 μg/L) and China (1.90 μg/L) population. Except for the Czech Republic population, according to the 2005–2009 survey period, the GM of urinary Cd in the Taiwanese population was higher than all of the other countries. In addition, the GM of urinary Co in the Taiwanese population according to the NAHSIT 05–08 (1.07 μg/L) was 1.6-, 2.9-, and 4.7-fold higher than that of Japan population (0.68 μg/L) and the U.S. population according to the NHANES 2007–2008 (0.37 μg/L) and the Canada population according to the CHMS 2009–2011 (0.23 μg/L), respectively. Finally, the GM of urinary Cu decreased in the Taiwanese population from the NAHSIT 93–96 to the NAHSIT 05–08 (16.67 vs. 13.81 μg/L), it but was still 8- and 6-fold higher, respectively, than in the Chinese population (2.07 μg/L). In addition, the GM of urinary Co in the Taiwanese population according to the NAHSIT 05–08 was 1.5-fold higher than that of the Canada population according to the CHMS 2007–2009 (9.00 μg/L) (Fig. 4).
Comparison of urinary levels of Cd (a), Pb (b), Co (c), and Cu (d) (μg/L) in the following: ■, general population of Taiwan [≧ 7 years, NAHSIT 93–96 (n = 821) and 05–08 (n = 780)]; ▲, America [≧ 6 years, NHANES 99–00 (n = 1007) and 07–08 (n = 2627)]; ◆, Czech Republic [15–58 years, HBM 2001–03 (n = 657) and 2005–09 (n = 896)]; ◎, Germany [18–69 years, GerES II 1998 (n = 4740) and GerES III 2003–06 (n = 1734)]; ●, Spain [23–66 years, 2009 (n = 165)]; ━, China [25–64 years, 2008 (n = 117)]; ★, Korea [≧ 20 years, KorSEP-III, 2008 (n = 4702)]; ☆, Japan [20–81 years, 2000–05 (n = 13,000); and ◇, Canada [3–79 years, CHMS 2007–09 (n = 5319) and 09–11 (n = 6070)]
Discussion
This is the first study that systematically evaluated exposure to Cd, Pb, Co, and Cu in the general population of Taiwan over the past decade. We observed significant differences in Cd, Pb, Co, and Cu exposure levels according to sex, age, urbanization, regions, and lifestyles. We found that metal exposure in youths was higher than in adults and exposure in men was higher than in women. In addition, the urinary levels of Pb and Cu in the Taiwanese participants in the NAHSIT 05–08 were lower than the corresponding levels in the NAHSIT 93–96, whereas the levels of Cd and Co were higher than those in the NAHSIT 93–96. However, between those same time periods, the urinary levels of Cd and Co in the participants were significantly higher in eastern Taiwan and in the urbanization of urban and rural. Differences in lifestyle may have contributed to differences in urinary metal levels.
Different lifestyles may cause different human metal exposure. Our data found that the median level of urinary Cu in the participants with alcohol consumption was significantly higher than that without (P < 0.05). Navarro-Alarcon et al. indicated that consuming alcoholic beverages is an important human exposure source (Navarro-Alarcon et al. 2007). We observed that the participants with cigarette smoking have a higher urinary Pb level (P < 0.01) and lower urinary Co level (P < 0.05) in both periods, which is consistent with the previous report from the NHANES 1999–2004 (Richter et al. 2009). Cigarette smoking is an important source of Pb exposure and is increasingly relevant as leaded gasoline has declined (Bernhard et al. 2005; Richter et al. 2009). Co is a component of vitamin B12, and the previous study indicated that cigarette smoking interferes with absorption of vitamin B12 (Cogswell et al. 2003). This may be the reason why the urinary Co levels were significantly lower in the cigarette-smoking participants. Besides, our data showed that the median levels of urinary Pb and Cu in the participants with betel quid chewing were significantly higher than those without (Cd: P = 0.067; Pb and Cu: P < 0.01). One study has reported that the betel quid chewing components have higher arsenic, Cd, and Pb (Al-Rmalli et al. 2011). Considering that betel quid may contain higher levels of heavy metals, the possible health impacts associated with exposure to these metals should be a concern. Although some studies have revealed that human activities like alcohol consumption and cigarette smoking can affect urinary metal levels, further researches are needed for other lifestyles. We suggested that the implementation of public health education or intervention strategies of alcohol consumption, betel quid chewing, and cigarette smoking could be beneficial for the reduction of certain metal exposure in the general population of Taiwan.
The exposure to Cd in Taiwan is considerably higher than that in other countries. The main exposure routes of cadmium are through foods, especially in rice. In Asian countries like Japan and Taiwan, rice is the main staple food, which is known as the major source of exposure to Cd. In this study, around 23–29% of the participants have over the threshold Cd level (1 μg/L), which might cause renal, bone, hematological, etc., problems that are well-documented (Nordberg et al. 2007). One previous meta-analysis indicated that even at low levels of Cd exposure, urinary Cd levels (≥ 0.48 μg/g cre.) are associated with increased cardiovascular disease mortality in the general population (hazard ratio 1.33–1.87) (Larsson and Wolk 2016). As we discussed previously, the prevalence of cigarette smoking in Taiwan from 2001 to 2013 significantly decreased (Chiang and Chang 2016); however, in the present study, the Cd level in the NAHSIT 05–08 was significantly higher than that in the NAHSIT 93–96 period (P < 0.01). Although these results contradict the reduced smoking rate in Taiwan, the consumption of foods, such as fish, meat, fruit, and rice, is a Cd exposure pathway for non-smokers; according to a previous NAHSIT study, Taiwan’s intake frequency of sea fish, shellfish, poultry, vegetables, and fruit increased from the NAHSIT 93–96 to NAHSIT 05–08 (Yeh et al. 2011). This may be one reason for the increased Cd exposure between our studied sampling periods; however, these other sources of Cd exposure must be examined to confirm our results.
Exposure to Pb in the general population of Taiwan in the NAHSIT 05–08 (0.90 μg/L) was significantly lower than that in the NAHSIT 93–96 (2.21 μg/L, P < 0.01), regardless of age, sex, or regions. Martinez et al. (2013) showed that banning leaded gasoline in Argentina resulted in a 66.5% reduction in blood lead levels (Martinez et al. 2013). This phenomenon is possibly attributable to Taiwan’s ban on leaded gasoline and implementation of unleaded gasoline in 2000. In the present study, we found that children (age group 7 to 11 years) exhibited the highest levels of urinary Pb. This observation may be because children of this age tend to ingest Pb via soil and be exposed in lead-contaminated dust (Lanphear et al. 2002) and have higher absorption from the gastrointestinal tract than adults (Ziegler et al. 1978). Smoking habit is also a critical factor leading to Pb exposure (Castano et al. 2012; Richter et al. 2009). A recent study showed that the prevalence of cigarette smoking in Taiwan from 2001 to 2013 significantly decreased from 44.4 to 34.2% (Chiang and Chang 2016); this may be another reason to explain the decrease in Pb levels between the sampling periods. Moreover, although Pb levels decreased, our previous study indicated that exposure to even low levels of Pb may have lagging effects on the intellectual development of children (Huang et al. 2012) and that women with higher Pb exposure may have increased risk of osteopenia and osteoporosis (Tsai et al. 2016). Thus, strategies to decrease Pb exposure in Taiwan should continue, such as the elimination of Pb pipelines, restrictions on the use of Pb in the paint, and the prohibition of Pb additives in products for children.
Data regarding urinary Co levels in the general population of Taiwan are limited. Co and some of its components may be toxicants (Domingo 1989). One study conducted in Taiwan indicated that urinary Co levels in steel production workers (n = 63, mean ± SD 8.18 ± 2.73 μg/L) and quality control workers (n = 63, mean ± SD: 7.39 ± 1.26 μg/L) were significantly higher than those in the general population (n = 63, mean ± SD: 3.18 ± 1.59 μg/L) (both P < 0.01) (Horng et al. 2003). All participants in the aforementioned study were men, and their mean age was approximately 45 years. In the present study, the mean (± SD) urinary Co levels of male participants were 1.06 (±0.95) and 1.19 (±1.36) μg/L in the NAHSIT 93–96 and NAHSIT 05–08, respectively. Thus, urinary Co levels identified in the present study were lower than those reported in the previous study in Taiwan. However, the exposure levels of Co in the two periods examined in the present study were still 1.6- to 4.7-fold higher than those in the U.S. NHANES, Japan, and Canada for the corresponding periods.
Cu is an essential nutritional element required in small amounts for the well-being of humans; by contrast, high levels of Cu can be harmful, particularly in children (ATSDR 2004a). Maintaining adequate Cu levels in the human body is crucial. A study indicated that a basic relationship exists between the serum Cu level and atherosclerosis (Bagheri et al. 2015). Cu is also a crucial trace element in the brain; when a deficiency occurs, Menkes disease, Wilson’s disease, and other serious neurological disorders may develop (de Bie et al. 2007). In this study, we found that the urinary Cu levels in the NAHSIT 05–08 in young and middle-aged adults were significantly lower than those in the NAHSIT 93–96 period (P < 0.01) and that participants from northern Taiwan exhibited the highest urinary Cu levels across both sampling periods (Table 3). Furthermore, the median levels of urinary Cu in youths were approximately 1.2- to 1.5-fold higher than those of adults in both sampling periods. Hence, monitoring and reducing the exposure of children to heavy metal is necessary.
Gender could be a factor dominating the exposure profile of metal exposure. Results of PCA and HCA revealed that two components and clusters of metal exposure were classified by sex regardless of different age or periods. Furthermore, HCA results showed that exposure profile in youth was changed for both sexes between the two periods whereas no similar phenomena were found in adults. It is indicated that youths could be more sensitive to environmental metal exposure. Meanwhile, we observed that 2 PCs for NAHSIT 93–96 adults only accounted for nearly 50%, which is much lower than others. HCA result showed an obviously different similarity for the exposure of the four metals between sexes in NAHSIT 93–96. The change of similarity for metal exposure between the two periods could be possibly influenced by the dramatic environmental change in the population, like the ban of leaded gasoline (Huang et al. 2012; Hwang et al. 2004).
The strengths of this study include its large sample size, evaluation of levels of urinary metal content over time, and survey of different regions in Taiwan. With a large sample size of 1601 participants, we found significant differences in urinary metal content in the Taiwanese population according to sex, age, urbanization, regions, and sampling period. A PCA was also performed to identify the PCs of the different age- and sex-exposure profiles. The PCA provided an overview of the urinary Pb, Cd, Co, and Cu content found in the participants from the NAHSIT 93–96 and NAHSIT 05–08 and revealed the participants’ exposure to metals according to age and sex. Notably, we analyzed urinary heavy metal levels, rather than blood heavy metal levels. This strategy enabled us to avoid invasive extraction methods for all the age groups (which is a particular concern for children) and mitigate participants’ refusal to allow blood collection. Urinary metal levels are suitable for representing recent human exposure (ATSDR 2007; Ince et al. 2008; Ishizaki et al. 2015; Linder 1984). We collected over 90% of the participants’ specimens to establish an exposure database in Taiwan. To the best of our knowledge, this is the first study to examine the urinary levels of heavy metals in the general population and discuss sampling period-based differences on a national scale in Taiwan. Our data provide useful exposure reference values for Cd, Pb, Co, and Cu in the general population of Taiwan.
This study also has some limitations in data interpretation. First, this was a cross-sectional, one-time measurement study examining survey data from 1993 to 1996 and from 2005 to 2008. The exposure data only reflected the participants’ exposure immediately before the survey and, thus, may not be representative of the long-term exposure. Second, we presented only the basic demographic data (e.g., sex, age, urbanization, and region) and partly lifestyles (alcohol consumption, betel quid chewing, and cigarette smoking) of the participants. The detailed information regarding exposure, lifestyles, and other associated factors, which is required for a comprehensive assessment of potential exposure sources, was not presented. Third, we could not exclude the possibility of residual confounding factors associated with the co-exposure to other metals and metal concentration in soil that is potentially related to personal metal levels. Finally, because of the limited volume of specimens collected, we could not conduct a creatinine-based estimation of metal levels in this study. However, we have compared the correlation between unadjusted and adjusted urine creatinine of the urinary metal levels (n = 377) in the NAHSIT 05–08, and we found the significant correlation coefficients of urine creatinine adjusted and unadjusted of Cd, Pb, Co, and Cu levels which were 0.642, 0.790, 0.516, and 0.288 (both P < 0.01), respectively (Table S3). Despite the lack in creatinine, the results are suitably comparable to those of other nation-wide studies, namely the NHANES in the U.S., the GerES in Germany, and the CHMS in Canada.
In conclusion, the levels of Cd, Pb, Co, and Cu exposure among the general population of Taiwan varied with age, sex, and lifestyles. Although the overall exposure to Pb and Cu in the NAHSIT 05–08 was lower than that in the NAHSIT 93–96 period, youths are determined to be exposed to higher levels of Pb, Co, and Cu metals. In both periods, the metal exposure profiles in youths were changed for sex. Metal exposure was possibly affected by distinct lifestyles. Related heavy metal exposure sources and the influence of lifestyle in health warrant further investigation. Our findings also indicated that the urinary levels of metals in Taiwan were higher than those in other developed countries. We suggest that the implementation of public health education or intervention strategies of relative risk factors shall be taken for the reduction of metal exposure in the general population of Taiwan.
Abbreviations
- Cd :
-
Cadmium
- CHMS :
-
Canadian Health Measures Survey
- Co :
-
Cobalt
- Cu :
-
Copper
- GerES :
-
German Environmental Survey
- GM :
-
Geometric mean
- GSD :
-
Geometric standard deviation
- HBM :
-
Human biomonitoring
- HCA :
-
Hierarchical clustering analysis
- IACR :
-
International Agency for Research on Cancer
- ICP-MS :
-
Inductively coupled plasma-mass spectrometry
- KorSEP :
-
Korea National Survey for Environmental Pollutants in the Human Body
- LOD :
-
Limit of detection
- MDL :
-
Method detection limit
- NAHSIT :
-
Nutrition and Health Survey in Taiwan
- NHANES :
-
National Health and Nutrition Examination Survey
- NHRI :
-
National Health Research Institutes
- NIEA :
-
National Institute of Environmental Analysis
- Pb :
-
Lead
- PCA :
-
Principal component analysis
- SRM :
-
Standard reference material
References
Al-Rmalli SW, Jenkins RO, Haris PI (2011) Betel quid chewing elevates human exposure to arsenic, cadmium and lead. J Hazard Mater 190:69–74
ATSDR (2004a) Toxicological profile for cobalt. U.S. Department of Health and Human Services
ATSDR (2004b) Toxicological profile for copper. U.S. Department of Health and Human Services
ATSDR (2007) Toxicological profile for lead. U.S. Department of Health and Human Services
Awata H, Linder S, Mitchell LE, Delclos GL (2017) Biomarker levels of toxic metals among Asian populations in the United States: NHANES 2011-2012. Environ Health Perspect 125:306–313
Bagheri B, Akbari N, Tabiban S, Habibi V, Mokhberi V (2015) Serum level of copper in patients with coronary artery disease. Niger Med J 56:39–42
Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M (1987) Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med 316:1037–1043
Bernhard D, Rossmann A, Wick G (2005) Metals in cigarette smoke. IUBMB Life 57:805–809
Castano A, Sanchez-Rodriguez JE, Canas A, Esteban M, Navarro C, Rodriguez-Garcia AC, Arribas M, Diaz G, Jimenez-Guerrero JA (2012) Mercury, lead and cadmium levels in the urine of 170 Spanish adults: a pilot human biomonitoring study. Int J Hyg Environ Health 215:191–195
Chang FH, Wang SL, Huang YL, Tsai MH, Yu ST, Chang LW (2006) Biomonitoring of chromium for residents of areas with a high density of electroplating factories. J Expo Sci Environ Epidemiol 16:138–146
Chang JW, Lee CC, Pan WH, Chou WC, Huang HB, Chiang HC, Huang PC (2017) Estimated daily intake and cumulative risk assessment of phthalates in the general Taiwanese after the 2011 DEHP food scandal. Sci Rep 7:45009
Chen JW, Chen HY, Li WF, Liou SH, Chen CJ, Wu JH, Wang SL (2011) The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere 84:17–24
Chen ZS (1991) Cadmium and lead contamination of soils near plastic stabilizing materials producing plants in Northern Taiwan. Water Air Soil Pollut 57:745–754
Chiang CY, Chang HY (2016) A population study on the time trend of cigarette smoking, cessation, and exposure to secondhand smoking from 2001 to 2013 in Taiwan. Popul Health Metrics 14:38
Chung S, Chung JH, Kim SJ, Koh ES, Yoon HE, Park CW, Chang YS, Shin SJ (2014) Blood lead and cadmium levels and renal function in Korean adults. Clin Exp Nephrol 18:726–734
Cogswell ME, Weisberg P, Spong C (2003) Cigarette smoking, alcohol use and adverse pregnancy outcomes: implications for micronutrient supplementation. J Nutr 133:1722S–1731S
de Bie P, Muller P, Wijmenga C, Klomp LW (2007) Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet 44:673–688
Dix-Cooper L, Kosatsky T (2018) Blood mercury, lead and cadmium levels and determinants of exposure among newcomer South and East Asian women of reproductive age living in Vancouver, Canada. Sci Total Environ 619-620:1409–1419
Domingo JL (1989) Cobalt in the environment and its toxicological implications. Rev Environ Contam Toxicol 108:105–132
Doong RA, Lee SH, Lee CC, Sun YC, Wu SC (2008) Characterization and composition of heavy metals and persistent organic pollutants in water and estuarine sediments from Gao-ping River, Taiwan. Mar Pollut Bull 57:846–857
Fort M, Grimalt JO, Casas M, Sunyer J (2015) Interdependence between urinary cobalt concentrations and hemoglobin levels in pregnant women. Environ Res 136:148–154
Haines DA, Saravanabhavan G, Werry K, Khoury C (2017) An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007-2019. Int J Hyg Environ Health 220:13–28
Han BC, Hung TC (1990) Green oysters caused by copper pollution on the Taiwan coast. Environ Pollut 65:347–362
Han BC, Jeng WL, Tsai YN, Jeng MS (1993) Depuration of copper and zinc by green oysters and blue mussels of Taiwan. Environ Pollut 82:93–97
Horng CJ, Horng PH, Hsu JW, Tsai JL (2003) Simultaneous determination of urinary cadmium, cobalt, lead, and nickel concentrations in steel production workers by differential pulse stripping voltammetry. Arch Environ Health 58:104–110
Hsu CS, Liu PL, Chien LC, Chou SY, Han BC (2007) Mercury concentration and fish consumption in Taiwanese pregnant women. BJOG 114:81–85
Huang PC, Su PH, Chen HY, Huang HB, Tsai JL, Huang HI, Wang SL (2012) Childhood blood lead levels and intellectual development after ban of leaded gasoline in Taiwan: a 9-year prospective study. Environ Int 40:88–96
Hwang YH, Ko Y, Chiang CD, Hsu SP, Lee YH, Yu CH, Chiou CH, Wang JD, Chuang HY (2004) Transition of cord blood lead level, 1985-2002, in the Taipei area and its determinants after the cease of leaded gasoline use. Environ Res 96:274–282
IARC (2006a) Inorganic and organic lead compounds. IARC Monogr Eval Carcinog Risks Hum 87
IARC (2006b) Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr Eval Carcinog Risks Hum 86
IARC (2012) Evaluation of carcinogenic risks to humans—cadmium and cadmium compounds. IARC (International Agency for Research on Cancer) 58
Ince AT, Kayadibi H, Soylu A, Ovunc O, Gultepe M, Toros AB, Yasar B, Kendir T, Abut E (2008) Serum copper, ceruloplasmin and 24-h urine copper evaluations in celiac patients. Dig Dis Sci 53:1564–1572
Ishizaki M, Suwazono Y, Kido T, Nishijo M, Honda R, Kobayashi E, Nogawa K, Nakagawa H (2015) Estimation of biological half-life of urinary cadmium in inhabitants after cessation of environmental cadmium pollution using a mixed linear model. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:1273–1276
Johnstone EB, Louis GM, Parsons PJ, Steuerwald AJ, Palmer CD, Chen Z, Sun L, Hammoud AO, Dorais J, Peterson CM (2014) Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: findings from the ENDO study. Reprod Toxicol 49:27–32
Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, Knauf K (2002) Environmental lead exposure during early childhood. J Pediatr 140:40–47
Larsson SC, Wolk A (2016) Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: systematic review and meta-analysis of cohort studies. Int J Epidemiol 45:782–791
Lee JW, Lee CK, Moon CS, Choi IJ, Lee KJ, Yi SM, Jang BK, Yoon B, Kim DS, Peak D, Sul D, Oh E, Im H, Kang HS, Kim JH, Lee JT, Kim K, Park KL, Ahn R, Park SH, Kim SC, Park CH, Lee JH (2012) Korea National Survey for environmental pollutants in the human body 2008: heavy metals in the blood or urine of the Korean population. Int J Hyg Environ Health 215:449–457
Liao KW, Kuo PL, Huang HB, Chang JW, Chiang HC, Huang PC (2018) Increased risk of phthalates exposure for recurrent pregnancy loss in reproductive-aged women. Environ Pollut 241:969–977
Lin YH, Tseng YH, Chen YC, Lin MH, Chou LF, Chen TJ, Hwang SJ (2013) The rural-urban divide in ambulatory care of gastrointestinal diseases in Taiwan. BMC Int Health Hum Rights 13:15
Linder MC (1984) Other trace elements and the liver. Semin Liver Dis 4:264–276
Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS, Liang KY (2006) Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag 4:1–22
Martinez SA, Simonella L, Hansen C, Rivolta S, Cancela LM, Virgolini MB (2013) Blood lead levels and enzymatic biomarkers of environmental lead exposure in children in Cordoba, Argentina, after the ban of leaded gasoline. Hum Exp Toxicol 32:449–463
Navarro-Alarcon M, Velasco C, Jodral A, Terres C, Olalla M, Lopez H, Lopez MC (2007) Copper, zinc, calcium and magnesium content of alcoholic beverages and by-products from Spain: nutritional supply. Food Addit Contam 24:685–694
NELAC (2001) National Environmental Laboratory Accreditation Conference, NELAC Standards Chapter 1 Program Policy and Structure, Appendix A - Glossary
Nogawa K, Kido T (1993) Biological monitoring of cadmium exposure in itai-itai disease epidemiology. Int Arch Occup Environ Health 65:S43–S46
Nordberg GF, Nogawa K, Nordberg M, Friberg LT (2007) Chapter 23—cadmium, handbook on the toxicology of metals, third edn. Academic Press, Burlington, pp 445–486
Ohashi F, Fukui Y, Takada S, Moriguchi J, Ezaki T, Ikeda M (2006) Reference values for cobalt, copper, manganese, and nickel in urine among women of the general population in Japan. Int Arch Occup Environ Health 80:117–126
Pan WH, Wu HJ, Yeh CJ, Chuang SY, Chang HY, Yeh NH, Hsieh YT (2011) Diet and health trends in Taiwan: comparison of two nutrition and health surveys from 1993-1996 and 2005-2008. Asia Pac J Clin Nutr 20:238–250
Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ, Miller DT, Caldwell KL (1998) Trace metals in urine of United States residents: reference range concentrations. Environ Res 76:53–59
Rehfisch P, Anderson M, Berg P, Lampa E, Nordling Y, Svartengren M, Westberg H, Gunnarsson LG (2012) Lung function and respiratory symptoms in hard metal workers exposed to cobalt. J Occup Environ Med 54:409–413
Richter PA, Bishop EE, Wang J, Swahn MH (2009) Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health 6:1930–1946
Singh MM, Singh R, Khare A, Gupta MC, Patney NL, Jain VK, Goyal SP, Prakash V, Pandey DN (1985) Serum copper in myocardial infarction—diagnostic and prognostic significance. Angiology 36:504–510
Song Q, Li J (2014) Environmental effects of heavy metals derived from the e-waste recycling activities in China: a systematic review. Waste Manag 34:2587–2594
Swennen B, Buchet JP, Stanescu D, Lison D, Lauwerys R (1993) Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Br J Ind Med 50:835–842
Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E (2008) Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect 116:51–56
Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A (2013) Cadmium exposure and incident cardiovascular disease. Epidemiology 24:421–429
Tsai MS, Lin CL, Tu YK, Lee PH, Kao CH (2015) Risks and predictors of osteoporosis in patients with inflammatory bowel diseases in an Asian population: a nationwide population-based cohort study. Int J Clin Pract 69:235–241
Tsai TL, Pan WH, Chung YT, Wu TN, Tseng YC, Liou SH, Wang SL (2016) Association between urinary lead and bone health in a general population from Taiwan. J Expo Sci Environ Epidemiol 26:481–487
Tsai TL, Kuo CC, Pan WH, Chung YT, Chen CY, Wu TN, Wang SL (2017) The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int 92:710–720
Tu SH, Chen C, Hsieh YT, Chang HY, Yeh CJ, Lin YC, Pan WH (2011) Design and sample characteristics of the 2005-2008 Nutrition and Health Survey in Taiwan. Asia Pac J Clin Nutr 20:225–237
Turnlund JR, Jacob RA, Keen CL, Strain JJ, Kelley DS, Domek JM, Keyes WR, Ensunsa JL, Lykkesfeldt J, Coulter J (2004) Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am J Clin Nutr 79:1037–1044
Uraguchi S, Fujiwara T (2012) Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice (N Y) 5:5
Walters GI, Robertson AS, Moore VC, Burge PS (2014) Cobalt asthma in metalworkers from an automotive engine valve manufacturer. Occup Med (Lond) 64:358–364
Wang H, Han M, Yang S, Chen Y, Liu Q, Ke S (2011) Urinary heavy metal levels and relevant factors among people exposed to e-waste dismantling. Environ Int 37:80–85
Wang SL, Yu ST, Chang FH, Liou SH, Wang YC (2006) Body burden of cadmium and its relations to exposure factors and erythrocyte parameters in an industrial area of Taiwan. Epidemiology 17:S495
Watanabe T, Nakatsuka H, Ikeda M (1989) Cadmium and lead contents in rice available in various areas of Asia. Sci Total Environ 80:175–184
Wu WT, Lin YJ, Liou SH, Yang CY, Cheng KF, Tsai PJ, Wu TN (2012) Brain cancer associated with environmental lead exposure: evidence from implementation of a National Petrol-Lead Phase-Out Program (PLPOP) in Taiwan between 1979 and 2007. Environ Int 40:97–101
Yeh CJ, Chang HY, Pan WH (2011) Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pac J Clin Nutr 20:292–300
Yuan TH, Chio CP, Shie RH, Pien WH, Chan CC (2016) The distance-to-source trend in vanadium and arsenic exposures for residents living near a petrochemical complex. J Expo Sci Environ Epidemiol 26:270–276
Zatta P, Drago D, Zambenedetti P, Bolognin S, Nogara E, Peruffo A, Cozzi B (2008) Accumulation of copper and other metal ions, and metallothionein I/II expression in the bovine brain as a function of aging. J Chem Neuroanat 36:1–5
Ziegler EE, Edwards BB, Jensen RL, Mahaffey KR, Fomon SJ (1978) Absorption and retention of lead by infants. Pediatr Res 12:29–34
Acknowledgments
We thank Dr. Jein-Wen Chen, Dr. Ya-Tang Liao, Dr. Yu-Te Chung, Dr. Hsiu-Ying Ku, and Mr. Yun-Hsiang Liu from the NHRI in Taiwan for their assistance with data and specimen collection.
Funding
This study was funded by the NHRI (EO-99-SP-02, EM-105-PP-05, EH-102-PP-05, EH-103-PP-05, EM-105-SP-16, EM-105-PP-15, EM-106-PP12, EM-107-PP12).
Author information
Authors and Affiliations
Contributions
PCH, SLW, SHL, and WHP conceived and designed the experiments. CWS performed the experiments. KWL analyzed the data. PCH and SLW contributed the reagents, materials, and analysis tools. KWL, PCH, and SLW wrote the paper. WHP directed the specimens and questionnaire collection. PCH and SLW contributed to the critical revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 466 kb)
Rights and permissions
About this article
Cite this article
Liao, KW., Pan, WH., Liou, SH. et al. Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ Sci Pollut Res 26, 6048–6064 (2019). https://doi.org/10.1007/s11356-018-3911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3911-0