Abstract
Shale gas flowback fluid (SGF) is generated during shale gas extraction and typically contains a variety of toxic and refractory organic compounds. In this work, a microwave-activated persulfate process (MW-PS process) was developed to pretreat SGF. The major factors influencing the treatment efficiency of the MW-PS process (PS dose, initial pH, MW power, and reaction time) were optimized, and the synergetic effect (SE), degradation of recalcitrant matter, and energy consumption were systematically investigated. Results showed that the SE of the process reached a high index (i.e., 9.85), suggesting a significant synergetic effect of MW and PS. In addition, under the optimal MW-PS condition (PS dose of 2.5 g/L, MW power of 900 W, and initial pH of 2), chemical oxygen demand removal reached 66.40% in a short reaction time of 10 min. Other analyses demonstrated that benzene series compounds, organic acids, lipid substances, alkanes, antioxidants, and fluorescent dissolved organic matter in SGF were decomposed to smaller-molecule organic matter, suggesting that refractory and toxic organic matter was removed by the MW-PS treatment process. Overall, the results of this study showed that MW-PS technology is an effective and promising method to treat SGF once the operation parameters are optimized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural gas is an efficient and clean energy source as well as a source of raw chemical materials (Koh et al. 2016; Mackenzie et al. 1983). The exploitation and utilization of natural gas not only can improve the energy structure of a region or country, but also is of great significance to improving the ecological environment (He et al. 2016). Shale gas is a natural gas that is extracted from rammell (shale bedding) and mainly contains methane (Zou et al. 2017). Particularly, the shale is dense, and thus during the drilling process, pressurized cleaning water must be injected into the stratum to facilitate drilling to the target layer. The cleaning water and suspended particulate matter (sludge) from the stratum will return to the surface from the shaft bottom as flowback fluid during the drainage test stage (Butkovskyi et al. 2018). Consequently, a large amount of shale gas flowback fluid (SGF) is generated. SGF is rich in a variety of more than 750 chemicals, some of which are chemical additives (such as surfactants, antiseptics, antibacterial agents, demulsifiers, corrosion inhibitors, friction reducers, and acid). The other components of SGF originate from natural groundwater, fracturing fluid, and drilling cuttings brought out with the return fluid (Zhang et al. 2017). Overall, SGF is mainly compromised of toxic and carcinogenic aromatics; consequently, the fluid is characterized by high toxicity and extreme-low biodegradability. Untreated SGF that is discharged into a water body or used for re-injection during drilling poses potential risks to the water and soil environment, ecosystem health, and human health.
Methods for treating SGF have been widely reported and include ozone oxidation (Liu et al. 2018), adsorption (Warner et al. 2013), coagulation (Kausley et al. 2017), and others. In actual practice, these methods are limited by relatively high cost, complex operation, and unstable effluent quality. In recent years, advanced oxidation processes (AOPs) have been extensively applied to treat various types of wastewater (Baeza and Knappe 2011; Cortez et al. 2011; Li et al. 2017; Oulego et al. 2016). AOPs include such techniques as catalytic ozonation (Jung et al. 2016; Zhang et al. 2008), the microwave-enhanced Fenton-like method (Homem et al. 2013; Li et al. 2016), ultraviolet irradiation-ozone oxidation (Baeza and Knappe 2011), and the ultrasound-Fenton method (Weng and Huang 2015). Although enhanced (catalyzed) AOPs have shown a satisfactory treatment efficacy for many wastewaters, their application to the treatment of SGF has been rarely reported except by Zhang et al. (Zhang et al. 2017). These researchers developed a combined mFe0/persulfate/O3 process (i.e., mFe0/PS/O3) to degrade and transform the characteristic pollutants in SGF. However, the cost of disposing the metal sludge resulting from treatment constrained full-scale application of this technique. Consequently, there is an urgent need for an economical and operationally convenient treatment method for SGF.
Persulfate (PS) activation technology is widely used in AOPs, and is based on the particular chemical characteristics of the sulfate radical (SO4•−) (Qi et al. 2017; Qi et al. 2015; Tan et al. 2017; Yang et al. 2014). PS ions can be decomposed into SO4•− via heating, ultraviolet (UV) irradiation, transition metals activation (Yang et al. 2014), or other techniques. However, some of these activation techniques pose problems when used with PS to treat SGF. For example, UV irradiation cannot be effectively used to treat SGF because the fluid contains a high concentration of solids that make the liquid resistant to UV light penetration. Furthermore, transition metals (the most commonly used ion is Fe2+) require strict pH conditions (in the range 2 to 4) to be effective, and can form a large amount of undesired metal hydroxide-based sludge combined with macro molecular organics in SGF. Additionally, excessive doses of metal ions scavenge SO4•−, thus reducing the treatment efficacy.
In contrast, heating not only has a positive PS-activation effect, but also can enhance the solubility of slightly soluble organic pollutants and increase the reaction temperature of the process (Qi et al. 2017); consequently, the reaction rate increases and reaction time is shortened. Compared to conventional heating, microwave (MW) irradiation can increase the reaction rate, induce PS to produce more SO4•−, selectively heat certain compounds, and make treatment reactions more controllable (Homem et al. 2013; Horikoshi et al. 2003; Ravera et al. 2009; Zhang et al. 2006). Thus, in comparison with other heating techniques, MW irradiation yields a higher reaction rate (and therefore shorter reaction time) while consuming less energy.
Recently, Qi et al. (2015, 2017) used MW irradiation to induce PS activation for the treatment of Bisphenol A, and concluded that MW irradiation showed a great advantage for the rapid degradation of this synthetic compound. In addition, MW irradiation can not only enhance the treatment efficacy of the Fenton method, but also can considerably reduce the amount of iron-based sludge that is produced. Overall, the operation of a MW reactor is simple, and the quality of MW-treated effluent is stable. However, the application of MW irradiation alone or combined with other processes for the treatment of SGF has been rarely reported.
In this research, a MW-activated PS process was developed to treat SGF. The objectives of the study were (a) to evaluate the oxidation efficiency of the MW-PS process and the synergetic treatment effect of MW and PS; (b) to investigate the effect of influential factors (PS dose, initial pH, MW power and reaction time) on chemical oxygen demand (COD) removal; (c) to determine the transformation of dissolved organic matter in SGF as a result of MS-PS treatment; and (d) to compare the efficacy of different processes in treating SGF and evaluate the energy consumption. The goal of this study was to provide a scientific reference for the rapid and efficient treatment of SGF using MS-PS.
Materials and methods
Reagents
The SGF used in this study was collected from a shale gas field in southwest China. The SGF had a pH of 7.02 and a COD concentration of 229.02 mg/L. The 5-day biochemical oxygen demand (BOD5) was approximately 14 mg/L. Thus, the biodegradability index (BOD5/COD) of the fluid was 0.06. The extremely low biodegradability of the SGF indicated that it was unsuitable for traditional biological treatment.
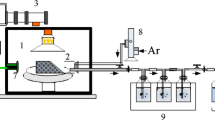
Analytical grade potassium persulfate (K2S2O8), hydrogen peroxide, sodium hydroxide, hydrochloric acid, and other reagents were purchased from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). Ultrapure water was used throughout the experiment. A MW reactor (model M1-211A, Midea Group, Guangdong, China) was used to produce MW radiation and activate PS.
Experimental procedure
MW irradiation was used in combination with PS to treat SGF. The MW irradiation activated PS to produce reactive oxidant species, such as SO4•−. First, 100 mL of pH-adjusted SGF was placed into a 500-mL round-bottom flask equipped with a reflux condenser. Then, the desired dose of PS was added into the flask, which was placed into the microwave reactor. The flask and SGF sample were irradiated for a controlled reaction time. After the reaction, the samples were cooled in ice-water and then passed through a 0.45-μm glass fiber filter. Ultrapure water was used to dilute samples for further analysis. In addition, catalase (Sigma-Aldrich c9322, Merck Life Science (Shanghai) Co., Ltd., Shanghai, China) was added into each sample that was treated using MW combined with hydrogen peroxide (MW-H2O2, as a comparison treatment process) to eliminate the influence of H2O2 on COD determination. The organic composition of each SGF effluent sample was examined using ultraviolet and visible (UV–Vis) spectroscopy, Fourier-transform infrared (FT-IR) spectroscopy, three-dimensional excitation emission matrix (3D-EEM) spectroscopy, and gas chromatography coupled with mass spectrometry (GC-MS) to reveal the transformation of organics during the MW-PS process.
Analytical methods
Basic wastewater quality
COD was determined using the microwave digestion titration method according to the Chinese Standard Method (HJ 828-2017). The contribution of PS (i.e., 1 g/L K2S2O8) to COD concentration was determined to be 29.79 mg/L COD. The pH of each sample was measured using a pH electrode and meter (model Phs-3C+, Chengdu Fangzhou Technology Co., Ltd., Chengdu, China). Residual PS concentration was determined according to the method reported by Liang et al. (2008).
Dissolved organic matter characterization
UV–Vis spectroscopy was used to determine the absorbance of light at wavelengths from 220 to 600 nm using a Lambda 950 spectrometer (PerkinElmer LAS (Germany) GmbH, Rodgau, Germany).
Aromatic organic compounds in samples were identified using 3D-EEM spectroscopy. Synchronized adsorption of 3-D fluorescence spectra was measured with an Aqualog-UV-800-C instrument (HORIBA Scientific, Edison, NJ, USA) (fixed excitation wavelength slit of 5 nm, scan speed of 500 nm/min, excitation wavelength of 200–550 nm, and emission wavelength of 200–650 nm). The 3D spectra were determined using a charge-coupled device detector. Ultrapure water served as a blank, and Rayleigh scattering and Raman scattering were eliminated automatically by the HORIBA Scientific software kit intrinsic to the Aqualog-UV-800-C. Spectra were plotted and the iso-height was set using Origin 9.0 software (OriginLab Corp., Northampton, MA, USA).
FTIR was to characterize the change of functional groups in treated SGF. A VERTEX70 FT-IR spectrometer (Bruker Optik GmbH, Ettlingen, Germany) was used with a test wavenumber range of 4000–400 cm−1, resolution of 2 cm−1, and scan time of 32 s. Cooled and dried samples were mixed with potassium bromide (SP), ground uniformly and placed in the press plate for testing.
GC-MS (Agilent 7890A, Agilent Technologies Co., Santa Clara, CA, USA) was used to analyze the types of organic compounds in the untreated and treated SGF. An AB-InoWax GC column (Abel Industries Canada Ltd., Pitt Meadows, BC, Canada) 30 m × 0.25 mm × 0.25 μm was used and the analytical cycle was 60 °C for 30 min, then an increase of 10 °C/min to 250 °C for 30 min.
Synergetic effect index and energy consumption
-
(i)
To determine the removal rate of COD, a systematic kinetics study of the MW-PS process was conducted using a pseudo-first order model to evaluate the data (Eq. 1).
In Eq. (1), CODt is the COD concentration of SGF at time t; CODi is the initial COD concentration of SGF; k is the pseudo-first order constant (min−1); and t is reaction time (min).
-
(ii)
The synergetic effect (SE) index (Xiong et al. 2016) was estimated on the basis of the kinetics study and calculated using Eq. (2). A high SE value (SE > 1) indicated a strong synergetic effect, and a low SE value (SE < 1) indicated an antagonistic effect. The higher (or lower) was the SE value, the stronger was the synergistic (or antagonistic) effect.
In Eq. (2), SE represents the synergetic effect; k(MW − PS) is the k for COD removal from SGF in the MW-PS process; ∑k(Single process) is the sum of k for COD removal obtained from the MW-single (i.e., MW alone) and PS-single (i.e., PS alone) processes. Notably, when the pseudo-first order constant is k(MW − PS), ∑k(Single process) should be the sum of k obtained from the MW-single and PS-single processes.
-
(iii)
The cost of the MS-PS process mainly arises from electricity consumption for MW irradiation. To study the economic cost of the MW-PS process, E was defined as the amount of electrical energy (kWh) required to reduce an order of magnitude concentration of a pollutant in 1 m3 of SGF (Eq. (3)).
In Eq. (3), P is the power (kW) of the microwave reactor or other energy source; t is the MW irradiation time (min); V is the volume (L) of SGF treated; and CODt is the COD concentration of SGF at time t; and CODi is the initial COD concentration of SGF. The constant “60” converts min to h.
Results and discussion
Comparison of synergetic effect
As shown in Fig. 1a–c, the PS-single process achieved negligible removal of organic matter, and the observed reaction constant (kobs) was only 0.0025 min−1. In addition, the pH change was also slight, suggesting that the reaction was less significant. Similar results were reported by Yang et al. (2014) who observed an extremely low organic matter removal rate using PS without heating. Previous studies have reported that the activation energy of the heat-activated PS process is approximately 140.2 kJ/mol (Xu and Li 2010). At room temperature, organics removal is less significant (Qi et al. 2017) than that at higher temperature because the decomposition of PS into SO4•− is difficult at low temperature. The O–O bond is much easier to rupture at a higher ambient temperature, which facilitates greater concentration of SO4•− and thus improved degradation of organic matter (Qi et al. 2015).
Synergetic effect of different treatment processes treating shale gas flowback fluid as a function of reaction time: a chemical oxygen demand (COD) removal; b pseudo-first order kinetic constant fitting; c variation of effluent pH; d temperature changes in the microwave-persulfate (MW-PS) process. Other processes are microwave-hydrogen peroxide (MW-H2O2), MW alone (MW), and PS alone (PS). Experimental conditions: [oxidant]0 = 7.4 mmol/L, MW power = 600 W, [pH]0 = 6, reaction time = 20 min
Likewise, in the MW-single process, the kobs was small (0.0029 min−1) because there was no reactive oxygen species participating in the degradation reaction. Therefore, hardly any organic matter was removed in the MW-single process.
In the MW-PS process, the COD concentration of treated SGF continuously decreased as reaction time increased. After 20 min, COD removal reached 55.41% and the kobs was 0.0532 min−1. Compared with results from the PS-single and MW-single processes, these results demonstrated that combining MW irradiation with PS can considerably increase the removal efficacy of organic pollutants by decreasing the PS activation energy and accelerating the reaction rate.
Previously, a fourfold increase was reported in the decomposition rate of PS under MW irradiation compared to that under conventional heating (Qi et al. 2017). Furthermore, the SE index (Eq. 2) calculated from reaction dynamics confirmed the superiority of the combined MW-PS process compared to the PS-single and MW-single processes. Because a higher SE indicates a more significant synergetic effect, the SE of 9.85 demonstrated a significant synergetic effect between MW and PS during treatment of organic pollutants in the MW-PS process.
PS is similar to H2O2 in its chemical structure. The effects of PS and H2O2 on treatment efficacy under the same molar dose and other reaction conditions were investigated by comparing the MW-PS process with the MW-H2O2 process. In the MW-H2O2 process, the kobs was 0.0226 min−1, which was 2.45 times lower than the reaction constant in the MW-PS process. Three reasons can explain this difference. First, H2O2 can produce the hydroxyl radical (•OH) under MW irradiation (Homem et al. 2013), but an exorbitant temperature can lead to the self-decomposition of H2O2 (Fig. 1d). Second, •OH and SO4•− in the MW-H2O2 and MW-PS processes, respectively, have a similar oxidation potential; however, SO4•− has a much longer half-life than •OH (Qi et al. 2017). Additionally, SO4•− is able to oxidize some organics that •OH cannot oxidize (Yang et al. 2014). Therefore, the MW-PS process is more suitable than the MW-H2O2 process for the treatment of SGF.
Influential factors
To obtain optimal treatment conditions in the MW-PS process, the effects of PS dose (0.25–3.00 g/L), initial pH (2–12), MW power (300–900 W), and reaction time (0–20 min) as influential factors on COD removal were investigated thoroughly in single-factor experiments. In addition, the effluent pH was observed carefully during the experimental process because the pH of the reaction solution was expected to decrease as a consequence of bisulfate (HSO4−) formation during the decomposition of PS (Qi et al. 2017). Furthermore, organic acid would be generated when the pollutants were decomposed by PS activation (Qi et al. 2015).
Effect of PS dose
When PS dose increased from 0.25 to 2.5 g/L, COD removal greatly increased from 5.44% to 66.40% (Fig. 2a). A further increase of PS dose to 3 g/L resulted in the COD removal decreasing to 48.19%. These results can be explained by noting that the SO4•− generated from the PS under MW irradiation can mineralize most recalcitrant organic pollutants (Qi et al. 2015; Xu and Li 2010; Zhang et al. 2017). Furthermore, excessive PS may react with SO4•− to form S2O8•2− (Eq. (4)). Thus, because the oxidation capacity of S2O8•2− is much lower than that of SO4•− (Deng and Ezyske 2011; Qi et al. 2015; Tan et al. 2017; Xu and Li 2010), COD removal decreased when the PS dose increased from 2.5 to 3 g/L.
Effects of a persulfate (PS) dose, b initial pH, c microwave (MW) power, and d reaction time on chemical oxygen demand (COD) removal, effluent pH and residual PS concentration during treatment of shale gas flowback fluid in the microwave-activated persulfate process. Experimental conditions: [oxidant]0 = 2 g/L, MW power = 750 W, [pH]0 = 6, reaction time = 20 min
The effluent pH decreased from 8.74 to 1.16 as PS dose increased from 0.25 to 3 g/L. This occurred for two reasons: (a) PS itself is a weak acid complex; and (b) some organic acids substances were produced during the treatment process. Notably, the residual PS concentration increased from 0.0013 to 0.0700 g/L with increasing PS dose, showing that an excessive dose of PS not only inhibits the treatment reaction, but also causes unused PS to accumulate in wastewater, thereby reducing the efficiency of PS utilization.
Effect of initial pH
COD removal gradually decreased from 60.44 to 16.29% as the initial pH increased from 2 to 8 (Fig. 2b). When the initial pH exceeded 8, COD removal efficiency increased. For instance, the COD removal reached 47.27% when the initial pH was 12. These results indicated that acidic treatment conditions benefitted the generation of SO4•− (Eq. (5)) (Deng and Ezyske 2011; Shukla et al. 2011; Yang et al. 2014). Increasing the initial pH of samples reduced the concentration of SO4•−; therefore, COD removal efficacy decreased. However, when the initial pH was higher than 8, COD removal continuously increased and residual PS concentration decreased. These results suggested that a base-activated PS process formed, and the dramatic increase of COD removal at high pH was attributed to the coexistence of the MW-activation and base-activation systems.
Notably, under the coexistence of MW-activation and base-activation systems (Furman et al. 2010) and initial pH of 12, the COD removal was lower than that at initial pH of 2 (only MW activation). According to previous studies, it can be reasonably inferred that the dominant reactive oxidant species were SO4•− and singlet-oxygen (initial pH = 12), and under acidic conditions (initial pH = 2) the dominant reactive oxidant species were SO4•− and •OH. Owing to the fact that the oxidation capacity of singlet-oxygen is weaker than that of •OH, the COD removal continuously increased and residual PS concentration significantly decreased as initial pH increased from 8 to 12.
Effect of MW power
Higher levels of MW power resulted in the rapid increase of the process reaction temperature, and the time needed to heat the reaction temperature to 95 °C decreased from 10 to 2 min (Fig. 2c). For instance, the COD removal increased from 16.26% to 56.59% as MW power increased from 300 to 900 W. On the one hand, increasing the MW power decreased the activation energy of the process, which enhanced the degradation of organic pollutants. On the other hand, polar molecules that absorbed MW irradiation rotated at a high rate, and the possibility of molecules colliding increased; therefore, PS decomposed into SO4•− and the COD removal changed remarkably (Li et al. 2016; Qi et al. 2017; Ravera et al. 2009). In addition, the residual PS concentration significantly decreased from 0.0537 to 0.0191 g/L as MW power increased from 300 to 900 W. These results showed that higher MW power favored the activation effect of PS.
Effect of reaction time
Figure 2d shows that COD removal increased from 7.39 to 49.13% as reaction time increased from 1 to 10 min. At reaction times exceeding 10 min, COD removal changed only slightly (for example, increasing by only 6.1% when reaction time increased from 10 to 20 min). On the one hand, more organic pollutants could be degraded by the MW-PS process with a longer reaction time. On the other hand, the PS doses used in the experiments limited the SO4•− concentration available for degrading the organics. Thus, during the reaction time from 12 to 20 min, the consumed PS amount changed only slightly and effluent pH varied negligibly. These results indicated that in the latter stage of the reaction, the production of micromolecular organic acids stabilized and the reaction rate of the process tended to decrease; therefore, the degradation efficacy of organic pollutants did not improve significantly when the reaction time exceeded 10 min.
Dissolved organic matter degradation
GC-MS analysis
As shown in Fig. 3 and Table 1, approximately 13 types of organic substances were identified in raw SGF; these mainly contained benzene ring compounds, organic acids, lipids, alkanes, and others. Among them, some organic acids, trimethylamine, and other organic compounds were added artificially during drilling. For instance, organic acids are used as scale inhibitors, emulsifiers, moisturizers, plasticizers, and fungicides. Furthermore, trimethylamine and other organic compounds played a role in the biological refractories. In short, SGF was characterized by a complex organic constitution.
After treatment of the SGF in the MW-PS process, five organic compounds were completely degraded and the concentrations of other organics decreased significantly. In addition, three new organic compounds (as by-products) were identified in the treated SGF. The results showed that the MW-PS process can efficiently degrade or transform the bio-refractory organic pollutants in SGF and implied an improvement in the biodegradability of SGF following treatment.
3D-EEM analysis
As shown in Fig. 4, two main peaks were observed in SGF. Peak 1 (at an excitation/emission wavelength ratio, λEx/λEm = 240/400) corresponded to fluorescent humic substances in the ultraviolet region (Fida et al. 2016; Lai et al. 2013; Saleem et al. 2018; Song et al. 2017). Peak 2 (λEx/λEm = 335/405) corresponded to fulvic-like acids in the visible region (Lai et al. 2013; Song et al. 2017; Wu et al. 2011), which may have been due to the introduction of humic substances (terrigenous input) during the drilling process. Substances associated with Peak 1 and Peak 2 are generally produced by aromatic organic compounds with large molecular weight and stable molecular structure. Comparing the 3D-EEM spectra of SGF before (Fig. 4a) and after (Fig. 4b) treatment in the MW-PS process shows that the two fluorescence-absorbing compounds were completely removed, which suggested that the SO4•− showed a great ability to remove humic substances.
Characteristic structural transformation of dissolved organic matter
The transformation of dissolved organic matter in SGF after treatment in the MW-PS process was determined by UV–Vis spectroscopy. The characteristic absorbance is depicted in Fig. 5a and listed in Table 2. As shown in Fig. 5a, SGF exhibited no obvious characteristic peak prior to treatment, indicating that the composition of SGF was complex. Strong absorbance was observed in the ultraviolet region, suggesting that SGF contained a large amount of conjugated organic compounds. As reaction time increased, the absorbance gradually decreased, indicating that the organic structure was destroyed in the MW-PS process and that the concentrations of organic substances changed considerably. Therefore, the complexity of organic compounds in the SGF greatly decreased after 20 min reaction in the MW-PS process.
At specific wavelengths, absorbance of light can indicate the humification degree, condensation degree, and molecular weight of dissolved organic matter. The absorbance at wavelengths of 254 and 280 nm (i.e., E254 and E280) indicates the aromatic degree of wastewater (Fuentes et al. 2006; Korshin et al. 1997; Lai et al. 2013). The absorbance at wavelengths of 254 and 280 nm for SGF were 1.578 and 1.044, respectively; after treatment in the MW-PS process, the absorbance at these wavelengths decreased to 0.183 and 0.074, respectively. These notable reductions in absorbance indicated that the aromatic degree of the treated SGF gradually decreased. Similarly, E250/E365 indicates the molecular weight of organics in wastewater (Baeza and Knappe 2011; Guo et al. 2011; Jiang et al. 2011; Kavurmaci and Bekbolet 2014); for SGF, this ratio increased as reaction time increased, suggesting that the molecular weights of organics decreased. E300/E400 represents the molecular weight and condensation degree (Domeizel et al. 2004; Kavurmaci and Bekbolet 2014; Korshin et al. 1997). For SGF, this ratio first increased and then tended to stabilize, indicating that the condensation degree of organics in treated SGF gradually decreased. E226–400 represents changes in benzene ring substances (Fuentes et al. 2006; Horikoshi et al. 2003; Jiang et al. 2011; Rodríguez et al. 2016). This value continuously decreased as reaction time increased, implying that the concentration of benzene ring substances in SGF decreased during treatment.
Figure 5b illustrates the FTIR spectra of SGF before and after treatment in the MW-PS process for 20 min. The peaks at 3468.86 cm−1 (amino group substances), 1636.75 cm−1 (benzene ring substances, and/or carbonyl), 1401.38 cm−1 (COO- of carboxylic acid substances), 1096.35 cm−1 (C–O–C in alcohols) and 605.36 cm−1 (C–O–C in surfactants) can be attributed to the O–H bond of hydroxyl substances (Rodríguez et al. 2016).
After treatment of SGF in the MW-PS process for 20 min, the intensity of peaks at 1401.38 and 1096.35 cm−1 increased, indicating that some aromatic organic compounds containing the amino group and benzene rings were degraded or transformed, or even mineralized to H2O and CO2. The intensity of the peak at 1636.75 cm−1 increased, suggesting that some organic compounds in SGF were oxidized to by-products containing the C–O–H group. A significant increase of peak intensity at 605.36 cm−1 was observed, implying that the surfactant in the SGF was initially decomposed.
Energy consumption
To compare the energy consumption of the MW-PS and MW-H2O2 processes, the energy consumption of the MW-PS process was calculated for the optimum operating conditions (PS dose = 2.0 g/L, initial pH = 6, MW power = 650 W). According to Eq. (3), to reduce a magnitude order of concentration of a pollutant in 1 m3 of contaminated water, the MW-PS process consumed 5082.8 kWh/m3 at a reaction time of 10 min, which was far less than that of the MW-H2O2 process (1368.6kWh/m3). Therefore, based on its reduced energy consumption alone, the MW-PS process exhibited significant economic benefits and decreased the reaction time greatly compared to what would be consumed using conventional heating and the MW-H2O2 process. Thus, considering both its low energy consumption and effective removal of organic contaminants, the MW-PS process is an efficient and economical technique for treating SGF.
Conclusions
In the MW-PS process, a significant synergetic effect occurs between MW and PS during the treatment of SGF. A significant COD removal can be achieved by subjecting SGF to the MW-PS process for 10 min at a PS dose = 2.0 g/L, initial pH = 6, and MW power = 650 W. Furthermore, the MW-PS process not only destroys benzene ring substances, organic acids, lipids, alkanes, and antioxidants typically contained in SGF, but also greatly degrades fluorescent organic substances. Therefore, recalcitrant organic matter is removed and transformed to smaller organics. As a result, the MW-PS process is effective for treating SGF. This study provides references for the practical application of the MW-PS process to rapidly and efficiently treat recalcitrant organic matter in SGF.
References
Baeza C, Knappe DR (2011) Transformation kinetics of biochemically active compounds in low-pressure UV photolysis and UV/H2O2 advanced oxidation processes. Water Res 45:4531–4543
Butkovskyi A, Faber AH, Wang Y, Grolle K, Hofmancaris R, Bruning H, Van AW, Rijnaarts H (2018) Removal of organic compounds from shale gas flowback water. Water Res 100(76)
Cortez S, Teixeira P, Oliveira R, Mota M (2011) Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J Environ Manag 92:749–755
Deng Y, Ezyske CM (2011) Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res 45:6189–6194
Domeizel M, Khalil A, Prudent P (2004) UV spectroscopy: a tool for monitoring humification and for proposing an index of the maturity of compost. Bioresour Technol 94:177–184
Fida H, Zhang G, Guo S, Naeem A (2016) Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst. J Colloid Interface Sci 490:859–868
Fuentes M, González-Gaitano G, García-Mina JMa (2006) The usefulness of UV–visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org Geochem 37:1949–1959
Furman OS, Teel AL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428
Guo XJ, Xi BD, Yu HB, Ma WC, He XS (2011) The structure and origin of dissolved organic matter studied by UV-vis spectroscopy and fluorescence spectroscopy in lake in arid and semi-arid region. Water Sci Technol 63:1010–1017
He C, Zhang T, Vidic RD (2016) Co-treatment of abandoned mine drainage and Marcellus shale flowback water for use in hydraulic fracturing. Water Res 104:425–431
Homem V, Alves A, Santos L (2013) Microwave-assisted Fenton's oxidation of amoxicillin. Chem Eng J 220:35–44
Horikoshi S, Saitou A, Hidaka H, Serpone N (2003) Environmental remediation by an integrated microwave/UV illumination method. V. Thermal and nonthermal effects of microwave radiation on the photocatalyst and on the photodegradation of rhodamine-B under UV/Vis radiation. Environ Sci Technol 37:5813–5822
Jiang J, Yu H, Xi B, Meng F, Zhou Y, Liu H (2011) UV–visible spectroscopic properties of dissolved fulvic acids extracted from salined fluvo-aquic soils in the Hetao Irrigation District, China. Soil Res 49:670–679
Jung C, Deng Y, Zhao R, Torrens K (2016) Chemical oxidation for mitigation of UV-quenching substances (UVQS) from municipal landfill leachate: Fenton process versus ozonation. Water Res 108:260–270
Kausley SB, Malhotra CP, Pandit AB (2017) Treatment and reuse of shale gas wastewater: electrocoagulation system for enhanced removal of organic contamination and scale causing divalent cations. J Water Process Eng 16:149–162
Kavurmaci SS, Bekbolet M (2014) Specific UV–vis absorbance changes of humic acid in the presence of clay particles during photocatalytic oxidation. Desalin Water Treat 52:1903–1910
Koh DY, Kang H, Lee JW, Park Y, Kim SJ, Lee J, Lee JY, Lee H (2016) Energy-efficient natural gas hydrate production using gas exchange. Appl Energy 162:114–130
Korshin GV, Li CW, Benjamin MM (1997) Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Res 31:1787–1795
Lai B, Zhou Y, Wang J, Yang Z, Chen Z (2013) Application of excitation and emission matrix fluorescence (EEM) and UV–vis absorption to monitor the characteristics of Alizarin Red S (ARS) during electro-Fenton degradation process. Chemosphere 93:2805–2813
Li S, Zhang G, Wang P, Zheng H, Zheng Y (2016) Microwave-enhanced Mn-Fenton process for the removal of BPA in water. Chem Eng J 294:371–379
Li X, Zhou M, Pan Y, Xu L, Tang Z (2017) Highly efficient advanced oxidation processes (AOPs) based on pre-magnetization Fe0 for wastewater treatment. Sep Purif Technol 178:49–55
Liang C, Huang CF, Mohanty N, Kurakalva RM (2008) A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 73:1540-1543
Liu P, Ren Y, Ma W, Ma J, Du Y (2018) Degradation of shale gas produced water by magnetic porous MFe2O4 (M = Cu, Ni, Co and Zn) heterogeneous catalyzed ozone. Chem Eng J
Mackenzie AS, Leythaeuser D, Schaefer RG, Bjorøy M (1983) Expulsion of petroleum hydrocarbons from shale source rocks. Nature 301:506–509
Oulego P, Collado S, Laca A, Díaz M (2016) Impact of leachate composition on the advanced oxidation treatment. Water Res 88:389–402
Qi C, Liu X, Zhao W, Lin C, Ma J, Shi W, Sun Q, Xiao H (2015) Degradation and dechlorination of pentachlorophenol by microwave-activated persulfate. Environ Sci Pollut Res Int 22:4670–4679
Qi C, Liu X, Lin C, Zhang H, Li X, Ma J (2017) Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem Eng J 315:201–209
Ravera M, Buico A, Gosetti F, Cassino C, Musso D, Osella D (2009) Oxidative degradation of 1,5-naphthalenedisulfonic acid in aqueous solutions by microwave irradiation in the presence of H2O2. Chemosphere 74:1309–1314
Rodríguez FJ, Schlenger P, Garcíavalverde M (2016) Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and (1) H NMR techniques. Sci Total Environ 541:623–637
Saleem M, Spagni A, Alibardi L, Bertucco A, Lavagnolo MC (2018) Assessment of dynamic membrane filtration for biological treatment of old landfill leachate. J Environ Manag 213:27–35
Shukla P, Sun H, Wang S, Ang HM, Tadé MO (2011) Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment. Catal Today 175:380–385
Song F, Wu F, Guo F, Wang H, Feng W, Zhou M, Deng Y, Bai Y, Xing B, Giesy JP (2017) Interactions between stepwise-eluted sub-fractions of fulvic acids and protons revealed by fluorescence titration combined with EEM-PARAFAC. Sci Total Environ 605-606:58–65
Tan C, Dong Y, Fu D, Gao N, Ma J, Liu X (2017) Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: kinetics and mechanism of radical generation. Chem Eng J 334
Warner NR, Christie CA, Jackson RB, Vengosh A (2013) Impacts of shale gas wastewater disposal on water quality in Western Pennsylvania. Environ Sci Technol 47:11849–11857
Weng CH, Huang V (2015) Application of Fe0 aggregate in ultrasound enhanced advanced Fenton process for decolorization of methylene blue. J Ind Eng Chem 28:153–160
Wu J, Zhang H, He PJ, Shao LM (2011) Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res 45:1711–1719
Xiong Z, Lai B, Yuan Y, Cao J, Yang P, Zhou Y (2016) Degradation of p -nitrophenol (PNP) in aqueous solution by a micro-size Fe0/O3 process (mFe0/O3): optimization, kinetic, performance and mechanism. Chem Eng J 302:137–145
Xu XR, Li XZ (2010) Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep Purif Technol 72:105–111
Yang Y, Pignatello JJ, Ma J, Mitch WA (2014) Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ Sci Technol 48:2344–2351
Zhang X, Wang Y, Li G, Qu J (2006) Oxidative decomposition of azo dye C.I. Acid Orange 7 (AO7) under microwave electrodeless lamp irradiation in the presence of H2O2. J Hazard Mater 134:183–189
Zhang T, Lu J, Ma J, Qiang Z (2008) Comparative study of ozonation and synthetic goethite-catalyzed ozonation of individual NOM fractions isolated and fractionated from a filtered river water. Water Res 42:1563–1570
Zhang H, Xiong Z, Ji F, Lai B, Yang P (2017) Pretreatment of shale gas flowback fluid (SGF) by the microscale Fe0/persulfate/O3 process (mFe0/PS/O3). Chemosphere 176:192–201
Zou J, Rezaee R, Liu K (2017) The effect of temperature on methane adsorption in shale gas reservoirs. Energy Fuel 31:12081–12092
Funding
The authors received financial support from the Key Laboratory of Special Waste Water Treatment (SWWT2015-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Highlights
(1) Reaction parameters and the degradation mechanism were systematically studied.
(2) Typical pollutants in SGF were degraded by the synergistic effects of MW and PS.
(3) MW-PS technology is an effective and promising method to treat SGF.
Rights and permissions
About this article
Cite this article
Chen, W., Luo, Z., Wu, C. et al. Oxidative removal of recalcitrant organics in shale gas flowback fluid by the microwave-activated persulfate process. Environ Sci Pollut Res 26, 684–693 (2019). https://doi.org/10.1007/s11356-018-3668-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3668-5