Abstract
A growing number of studies have highlighted the contamination and the effects towards organisms of diverse microplastics (μPs) in the marine environment. Surprisingly, although the main sources of μPs for marine environments are inland surface waters, the information on the occurrence and the effects of μPs in freshwater ecosystems is still scant. Thus, the aim of the present work is to investigate the ingestion and possible adverse effects due to the exposure to polystyrene μPs (PSμPs; Ø = 3 μm) on tadpoles of the Amphibian Xenopus laevis. Larvae at the developmental stage 36, prior to mouth opening, were exposed under semi-static conditions to 0.125, 1.25, and 12.5 μg mL−1 of PSμPs, and allowed to develop until stage 46. At the end of the exposure, the digestive tract and the gills from exposed and control tadpoles were microscopically examined, as well as changes in body growth and swimming activity. PSμPs were observed in tadpoles’ digestive tract, but not in the gills, from each tested concentration. However, neither body growth nor swimming activity were affected by PSμPs exposure. Our results demonstrated that PSμPs can be ingested by tadpoles, but they did not alter X. laevis development and swimming behavior at least during early-life stages, also at high, unrealistic concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic contamination is a worrisome environmental problem gripping aquatic ecosystems worldwide. Over the past 50 years, an unfathomable amount of plastic debris has reached the marine environment, representing a serious hazard for seas and oceans at all latitudes (Thompson et al. 2004). Although the negative impact of big plastic debris (i.e., macroplastics; > 25 mm in size) on marine ecosystems has been highlighted since the 1980s (Stefatos et al. 1999), a growing scientific interest has recently raised on microplastics. Microplastics (μPs) are small plastic particles (< 5 mm in size) that are produced ex novo to be used in cosmetics, industrial or medical applications, or derive from macroscopic debris after chemical, physical, and biological breakdown (Barnes et al. 2009). A number of studies have identified marine ecosystems as hotspots of μPs pollution (Wright et al. 2013 and references therein), where they have been recorded up to a maximum estimated density of 100,000 particles m−3 in surface waters and in the range of 100,000 items m−2 on shorelines (e.g., Desforges et al. 2014).
In spite of these findings, the contamination of freshwaters cannot be underestimated. In fact, freshwaters are the primary source of μPs entering seas and oceans through household sewage discharge (e.g., Fendall and Sewell 2009), direct input in water run-off or via storm-7water and wastewater treatment plant outlets (Dris et al. 2015), spillage of plastic resin powders or pellets used for airblasting (Gregory 1996), and feedstocks used to manufacture plastic products (Zbyszewski et al. 2014) or, alternatively, from the breakdown of larger plastic items. Microplastic contamination of surface waters that has been reported was in the 0.001–0.1 items m−2 range for lakes and 0.1–1 items m−2 range for rivers, while in the 10–10,000 items m−2 and 1–1000 items m−2 for lake and river sediments, respectively (Dris et al. 2015). The presence of μPs in different environmental matrices and their small size can result in the ingestion by organisms. A wealth of studies has demonstrated the ingestion of different μP items in 160 marine species (see Lusher 2015 and reference therein), including fish (Collard et al. 2017), seabirds (Lavers et al. 2014), mammals (Fossi et al. 2012), and invertebrates (Graham and Thompson 2009; Cole et al. 2013; Messinetti et al. 2018), as well as in 39 freshwater species (Scherer et al. 2017). Experimental studies have also demonstrated that μPs ingestion might negatively affect the health status of aquatic species, including fish (e.g., Lei et al. 2018), molluscs (e.g., Sussarellu et al. 2015), and crustacean (e.g., Frydkjaer et al. 2017). However, such investigations have returned contrasting results mainly depending on μP size and shape, as well as the tested concentration (Lee et al. 2013; Wright et al. 2013; Scherer et al. 2017).
Whilst evidence of strong negative effects, including intestinal damage, inhibition of feeding activity, and reduction of survival rates and body growth have been found (Lei et al. 2018; Murphy and Quinn 2018), some studies have pointed out slight or null adverse effects due to μPs ingestion (Hämer et al. 2014; Imhof et al. 2017; Weber et al. 2018). In spite of these findings, information on the impact of μPs on swimming activity of aquatic organisms are still limited. However, this effect cannot be neglected because ingestion of plastic microparticles could constrain organisms’ movements in water.
To the best of our knowledge, only two studies have been focused on μPs ingestion on amphibian species even though these organisms can be a target of μPs contamination, being exposed both in aquatic and terrestrial ecosystems. Moreover, as amphibians are filter feeders until they complete their metamorphosis, tadpoles are excellent models to investigate the ingestion of μPs and the subsequent effects during early-life periods. A first laboratory study demonstrated the uptake, accumulation, and elimination of polystyrene μPs in Xenopus tropicalis, showing their presence in both the digestive tract and on the gills (Hu et al. 2016). Similarly, a recent field work performed by Hu et al. (2018) confirmed that tadpoles can ingest μPs from their surrounding environment, showing the presence of different μPs typologies in the digestive tract of tadpoles belonging to four different species sampled in small waterbodies of the Yangtze River Delta (China). Despite of these findings, no study was focused on the potential adverse effects induced by μPs ingestion in tadpoles. Thus, the present study was aimed at investigating the ingestion and the possible negative caused by polystyrene spherical microplastics (PμPs; Ø = 3 μm) on Xenopus laevis tadpoles. We exposed X. laevis tadpoles to three increasing concentration of PSμPs (0.125, 1.25, and 12.5 μg mL−1) from stage 36, prior to mouth opening, to stage 46 (Nieuwkoop and Faber 1994). At the end of the exposure, we assessed the ingestion of PSμPs in tadpoles’ digestive tract and gills, as well the effects on survival, body growth, and swimming activity.
Materials and methods
Chemicals and polystyrene microplastic preparation
All analytical grade reagents, l-cysteine, 3-amino-benzoic acid ethyl ester (MS222), salts for FETAX solution, and blue polystyrene microplastics (PSμPs; Ø = 3 μm) were purchased from Sigma-Aldrich, Milano, Italy. Chemical-physical properties of the μP beads were tested. The size of polystyrene μPs was assessed by measuring size of 500 particles on different pictures captured with a scanning electron microscope (SEM) (Fig. S1) using Fiji freeware software (Schindelin et al. 2012), resulting in 2.75 ± 0.09 μm of diameter. Polystyrene μPs were chemically characterized by using a Fourier transformed infrared spectroscope (FT-IR) PerkinElmer Spectrum 100: PSμPs were analyzed as received. Subsequently, 10 mL of FETAX solution was dried at room temperature overnight (16 h) together with the same volume of a FETAX solution containing the PSμP (50 μg mL−1). The two residues were compared with the PSμPs. In Fig. 1, the spectra obtained are overlapped and signals showing the presence of PSμP are indicated. We focused on PSμPs because this polymer is one of the most abundant in both marine and freshwater ecosystems (Li et al. 2016). Moreover, polystyrene has a negligible styrene release in water solution; therefore, we can be reasonably sure that possible effects are due to the physical presence of μPs and not to monomer release (Cohen et al. 2002). The commercial standard was an aqueous suspension (50 mg mL−1) that was diluted in culture medium to obtain a stock solution of 50 μg mL−1 concentration. Three PSμPs concentrations, namely 0.125 (1 × 105 particles mL−1), 1.25 (2.833 × 105 particles mL−1), and 12.5 μg mL−1 (8.666 × 105 particles mL−1), were tested according to previous works on other aquatic organisms (Lee et al. 2013; Messinetti et al. 2018).
Animals and experimental design
Adults of Xenopus laevis were maintained at the University of Milan in aquaria filled with dechlorinated tap water at 22 ± 2 °C, with a 12 h light/dark cycle and fed a semi-synthetic diet (Mucedola S.r.L., Settimo Milanese, Italy). Embryos were obtained from natural breeding of adult pairs and the experiment run according to the Frog Embryo Teratogenesis Assay-Xenopus, FETAX, protocol (ASTM 1998), lightly modified. In particular, we planned a late exposure, being interested in the possible effects of ingested PSμPs and not to their developmental toxicity. Embryos were thus exposed prior to mouth opening, which happens at stage 40 (Nieuwkoop and Faber 1994), and not at the classic midblastula stage (stage 8). At the end of the test (stage 46), FETAX endpoints, i.e., mortality and growth inhibition, were considered. Exposure tests were performed in FETAX solution (0.01 M NaCl, 1 mM NaHCO3, 0.4 mM KCl, 0.1 mM CaCl2, 0.35 mM CaSO4 and 2H2O, and 0.6 mM MgSO4, at pH 7.6–8.0).
After breeding, adults were removed and embryos collected in plastic Petri dishes. Fertilized eggs were dejelled with 2% l-cysteine solution (pH 8.0) and rinsed several times with FETAX solution. Normally, cleaved embryos were selected, transferred to plastic Petri dishes filled with 10 mL of FETAX solution, and allowed to develop until stages 36–37, according to Nieuwkoop and Faber (1994). Thirty tadpoles at stages 36–37 were seeded in Petri dishes and exposed to a nominal concentration of 0.125, 1.25, and 12.5 μg mL−1 PSμPs in FETAX. The test was performed in semi-static conditions every single day. All groups were incubated in a thermostatic chamber at 22 ± 0.5 °C, and both control and PSμPs exposure groups duplicated. Tadpoles were not fed during the experiment and allowed to develop until stage 46, end of the exposure test. At this point, 20 tadpoles from each group were transferred to a small Petri dish filled with 5 mL of culture medium to be video-tracked. Then, all tadpoles were anesthetized with MS222 at a final concentration of 100 mg L−1 and evaluated for single malformations under a dissecting microscope. At the end of the analysis, all samples were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer solution (pH 7.4) for growth retardation measurements and for the subsequent microscopical analyses.
Microscopy analyses
For light microscopy analyses, 26 tadpoles per replicate were dehydrated in ethanol (EtOH) up to 70% and examined under a Leica DMRA2 microscope. Images were collected with a Leica DC300F digital camera and tadpole body lengths measured using Fiji freeware software (Schindelin et al. 2012). For electron microscopy analyses, 10 tadpoles from each treatment group were randomly selected, post-fixed in 1% OsO4 for 2 h at 4 °C, and critical-point dried in a Balzers Unions CPD 020 apparatus (Balzers Unions, Lichtenstein). Under a stereomicroscope, the digestive tract and gills of each tadpole were dissected, mounted onto standard SEM stubs, gold sputtered, and observed under a Zeiss LEO 1430 SEM at 20 kV.
Swimming activity analysis
The effects on swimming activity of tadpoles were evaluated by a video tracking analysis. Twenty tadpoles per treatment, including control, were randomly selected from exposure Petri dishes and individually transferred to another Petri dish (Ø = 60 mm) filled with 5 mL of culture medium. Because of their high motility, tadpoles were enclosed in a small arena (Ø = 10 mm) placed in the center of the Petri dish where they stopped movements and acclimatized for 3 min to new conditions. After acclimatization, the small arena was removed, the tadpoles restarted to swim, and its movements were filmed by an iPhone 6 for 10 s. The obtained 1080p Full HD videos were analyzed by using the ImageJ plugin Animal Track (Gulyás et al. 2016). The distance moved (mm) and mean swimming speed (cm s−1) were considered as swimming activity endpoints.
Statistical analysis
The effect of PSμPs exposure on the body length and the swimming activity of tadpoles was investigated by using linear mixed models (LMMs) including the treatment as fixed factor, while the identity of the Petri dish as a random factor. As no mortality occurred in all the experimental groups, no statistical analysis on this endpoint was performed. The analyses were run using SPSS 21.0 statistical package.
Results and discussion
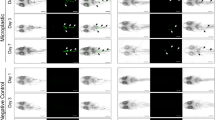
The present work showed that X. laevis tadpoles can ingest polystyrene microplastic beads during early-life stages, even though these particles did not significantly affect survival, body growth, and swimming activity. Stereomicroscopy analyses showed the presence of PSμPs in the whole digestive tract of tadpoles, mostly after the exposure to 1.25 μg mL−1 and 12.5 μg mL−1, while the lower concentration seems not to show the presence of PSμPs (Fig. 2). However, the SEM analyses showed the presence of PSμPs in the digestive tract of tadpoles from all the treatments, including 0.125 μg mL−1 (Fig. 3). As expected, the digestive tract from tadpoles exposed to 12.5 μg mL−1 of PSμPs was completely full of particles, while the amount of microbeads was notably lower in the individuals from the other treatments. SEM analyses suggest the absence of mechanical damage to the walls of the epithelium. Our findings are in agreement with previous studies demonstrating that polystyrene μPs of different size can be easily ingested and accumulated in the digestive tract of different aquatic organisms. For instance, PSμPs (1.7–30.6 μm in size) were observed in the digestive tract of 13 marine zooplanktonic organisms (Cole et al. 2013), while 100 nm–10 μm PSμPs filled up the digestive tract of the cladoceran Daphnia magna (Ma et al. 2016; Rist et al. 2017). Moreover, similar results were also obtained in Xenopus tropicalis, whereby fluorescent polystyrene μPs (1 and 10 μm in size) were clearly observed in alimentary canal, stomach, and intestine of tadpoles already after 1 h of exposure (Hu et al. 2016). However, in our study, no PSμPs were found on tadpole gills at each tested concentration (Fig. S2), contrasting previous results on X. tropicalis that showed the presence of 1 and 10 μm on the gills of tadpoles (Hu et al. 2016). Similarly, 8–10 μm PSμPs were found on the gills of the crab Carcinus maenas (Watts et al. 2014). Such findings suggested that the ingestion, the transfer, and the accumulation of different μPs in specific body districts greatly depend on the concentration and the size of the particles, as well as on the size of the focal model species (Wright et al. 2013). Thus, we suppose that the discrepancy in the presence of μPs on the gills of two Xenopus species might be due to their different body size. In fact, X. tropicalis is smaller than X. laevis, and consequently, it owns smaller gills and thick filaments, which allowed a more efficient trapping of PSμPs. This anatomic feature could also explain the higher accumulation of PSμPs in X. tropicalis compared to X. laevis although the exposure concentration selected by Hu et al. (2016) was notably lower (concentration range of 1 μm PSμPs 10–105 particles mL−1 and concentration range of 10 μm PSμPs 0.1–103 particles mL−1) than those tested in our study (concentration range of 3 μm PSμPs 1 × 105–8.6 × 105 particles mL−1.
Although PSμPs filled up the digestive tract of tadpoles, no tadpole died over the exposure period neither in the control nor in all the treatment groups. Our results are consistent with previous studies showing no mortality on diverse aquatic organisms after the exposure to diverse concentrations of dissimilar μP polymers, including invertebrates (e.g., Imhof et al. 2017; Rist et al. 2017; Weber et al. 2018) and vertebrates (e.g., Hu et al. 2016; Chen et al. 2017). However, the ingestion of PSμPs might cause sub-lethal effects, including the reduction of food assimilation and body growth (Cole et al. 2015; Xu et al. 2017). No significant differences in body length of tadpoles at stage 46 were noted between the treatment groups and the control (F3,203 = 1.137; P = 0.335; Fig. 4), suggesting that PSμPs ingestion did not affect body growth of tadpoles during early-life stages. Our results are in contrast with previous studies demonstrating that the ingestion of PSμPs negatively affected body growth of diverse organisms (Besseling et al. 2014; Lo and Chan 2018). These discrepancies might be due to the duration of the exposure and/or the size of the tested μPs. In fact, 14-day exposure to PSμPs (Ø = 2–2.4 μm) reduced the growth of the onyx slipper snail Crepidula onyx (Lo and Chan 2018). Moreover, the 21-day exposure to polystyrene nanoplastics (Ø = 70 nm) reduced the growth of Daphnia magna (Besseling et al. 2014), while the exposure up to 7 days post-fertilization to polystyrene nanoplastics (Ø = 50 nm) altered the early development of X. laevis (Tussellino et al. 2015). We may suppose that ingested PSμPs did not affect body growth of X. laevis tadpoles because they do not interfere with the assimilation of yolk reserves used during early-life stages. Alternatively, polystyrene microbeads were ingested and egested quickly by tadpoles (Hu et al. 2016) and did not affect the development.
Despite no developmental effects, the ingestion of μPs could affect tadpole swimming activity because particles can represent an additional weight for tadpoles and consequently a high energy demanding effort to be supported. According to results on body growth, PSμPs ingestion did not affect the swimming activity of tadpoles (Fig. 5a, b); no significant differences in terms of distance moved (F3,73 = 0.677; P = 0.569) and mean swimming speed (F3,73 = 0.196; P = 0.899) occurred between the treatment groups and the control. On the contrary, a previous study of the amphipod Platorchestia smithi showed that the ingestion of polyethylene μPs (Ø = 35–45 μm) caused a decrease of the jump height (Tosetto et al. 2016). This discrepancy can be due to species-specific differences, different ontogenetic stage, and/or to the type of analyzed swimming activity of the model organisms. In fact, in the present study, we monitored the horizontal swimming of tadpoles, while Tosetto et al. (2016) monitored the vertical hopping of amphipods. In addition, the rate of μP ingestion/egestion, the size and the composition of plastic used for exposures (3 μm polystyrene used in our study versus 35–45 μm polyethylene particles used by Tosetto et al. 2016), and their exposure concentration can affect the swimming activity and explain the differences of the responses after μP exposure. Lastly, Tosetto et al. (2016) “doped” the polyethylene μPs administered to amphipods with contaminated marine water and doped μPs adsorbed on their surface 0.007 μg g−1 of PAHs, which could cause the observed behavioral changes. This hypothesis is supported by a previous study of zebrafish larvae showing that negative effects on swimming activity occurred only when organisms where co-exposed to μPs and α-ethynylestradiol, while no swimming alteration was noted when larvae were exposed to μPs alone (Chen et al. 2017).
Estimated marginal means (± standard error) of distance moved (a) and swimming speed (b) measured in X. laevis tadpoles (stage 46). Letters above histograms indicate differences between groups, whereby similar letters indicate no significant differences. No significant differences were found (p > 0.05)
Conclusion
Our findings showed that 3 μm PSμPs are quickly ingested by X. laevis tadpoles at all the tested concentrations, but the exposure period does not induce negative effects on the body growth and swimming activity, also at high unrealistic concentrations. Further studies should be planned in order to evaluate if long-term exposure can impact the development and post-metamorphic stages of X. laevis. Lastly, investigations on the potential effects due to smaller polystyrene spherical particles or to fragments, foams, and pellets, which are predominant in freshwater ecosystems, should be necessary to understand the real impact of PSμPs on aquatic organisms.
References
American Society for Testing and Materials (ASTM) (1998) Standard guide for conducting the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). p E1439–E1498
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B 364:1985–1998
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48:12336–12343
Chen Q, Gundlach M, Yang S, Jiang J, Velki M, Yin D, Hollert H (2017) Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci Total Environ 584:1022–1031
Cohen JT, Carlson G, Charnley G, Coggon D, Delzell E, Graham JD, Greim H, Krewski D, Medinsky M, Monson R (2002) Comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene. J Toxicol Environ Health B 5:1–263
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47:6646–6655
Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS (2015) The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol 49:1130–1137
Collard F, Gilbert B, Compère P, Eppe G, Das K, Jauniax T, Parmentier E (2017) Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Envrion Pollut 229:1000–1005
Desforges JW, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 15:94–99
Dris R, Imhof H, Sanchez W, Gasperi J, Galgani F, Tassin B, Laforsch C (2015) Beyond the ocean: contamination of freshwater ecosystems with (micro-)plastic particles. EnvironChem 12:539–550
Fendall LS, Sewell MA (2009) Contributing to marine pollution by washing your face: microplastics in facial cleanser. Mar Pollut Bull 58:1225–1228
Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, Minutoli R (2012) Are baleen whales exposed to the threat of microplastic? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar Pollut Bull 64:2374–2379
Frydkjaer C, Iversen N, Roslev P (2017) Ingestion and egestion of microplastics by the Cladoceran Daphnia magna: effects of regular and irregular shaped plastic and Sorbed Phenanthrene. Bull Environ Contam Toxicol 99:655–661
Graham ER, Thompson JT (2009) Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Biol Ecol 368:22–29
Gregory MR (1996) Plastic “scrubbers” in hand cleansers: a further (and minor) source for marine pollution identified. Mar Pollut Bull 32:867–871
Gulyás M, Bencsik N, Pusztai S, Liliom H, Schlett K (2016) Animal tracker: an ImageJ-based tracking API to create a customized behaviour analyser program. Neuroinformatics 14:479–481. https://doi.org/10.1007/s12021-016-9303-z
Hämer J, Gutow L, Köhler A, Saborowski R (2014) Fate of microplastics in the marine isopod Idotea emarginata. Environ Sci Technol 48:13451–13458
Hu L, Su L, Xue Y, Mu J, Xu J, Shi H (2016) Uptake, accumulation and elimination of polystyrene microspheres in tadpoles of Xenopus tropicalis. Chemosphere 164:611–617
Hu L, Chernick M, Hinton DE, Shi H (2018) Microplastics in small waterbodies and tadpoles from Yangtze River Delta, China. Environ Sci Technol 52:8885–8893
Imhof HK, Rusek J, Thiel M, Wolinska J, Laforsche C (2017) Do microplastic particles affect Daphnia magna at the morphological, life history and molecular level? PlosOne DOI 12:e0187590. https://doi.org/10.5061/dryad.9d84
Lavers JL, Bond AL, Hutton I (2014) Plastic ingestion by flesh-footed shearwaters (Puffinus carneipes): implications for fledgling body condition and the accumulation of plastic-derived chemicals. Environ Pollut 187:124–129
Lee K, Shim WJ, Kwon O, Kang J (2013) Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ Sci Technol 47:11278–11283
Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D (2018) Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 619:1–8
Li WC, Tse HF, Fok L (2016) Plastic waste in the marine environment: a review of sources, occurrence and effects. Sci Total Environ 566:333–349
Lo HKA, Chan KYK (2018) Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ Pollut 233:588–595
Lusher A (2015) Microplastics in the marine environment: distribution, interactions and effects. Marine Anthropogenic Litter 245:307
Ma Y, Huang A, Cao S, Sun F, Wang L, Guo H, Ji R (2016) Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ Pollut 219:166–173
Messinetti S, Mercurio S, Parolini M, Sugni M, Pennati R (2018) Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ Pollut 237:1080–1087
Murphy F, Quinn B (2018) The effects of microplastic on freshwater Hydra attenuate feeding, morphology & reproduction. Environ Pollut 234:487–494
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis
Rist S, Baun A, Hartmann N (2017) Ingestion of micro- and nanoplastics in Daphnia magna—quantification of body burdens and assessment of feeding rates and reproduction. Environ Pollut 228:398–407
Scherer C, Brennholt N, Reifferscheid G, Wagner M (2017) Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci Rep 7:17006
Schindelin J, Arganda-Carreras I, Frise E (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Stefatos A, Charalampakis M, Papatheodorou G, Fereninos G (1999) Marine debris on the seafloor of the Mediterranean Sea: examples from two enclosed gulfs in Western Greece. Mar Pollut Bull 38:389–393
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A (2015) Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci U S A 113:2430–2435
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838
Tosetto L, Broen C, Williamson J (2016) Microplastics on beaches: ingestion and behavioural consequences for beachhoppers. Mar Biol 163:199
Tussellino M, Ronca R, Formiggini F, De Marco N, Fusco S, Netti PA, Carotenuto R (2015) Polystyrene nanoparticles affect Xenopus laevis development. J Nanops 17:70
Watts AJR, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Gallowat TS (2014) Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol 48:8823–8830
Weber A, Scherer C, Brennholt N, Reifferscheid G, Wagner M (2018) PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ Pollut 234:181–189
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492
Xu XY, Lee WT, Chan AKY, Lo HS, Shin PKS, Cheung SG (2017) Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar Pollut Bull 124:798–802
Zbyszewski M, Corcoran PL, Hockin A (2014) Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J Great Lakes Res 40:288–299
Acknowledgments
We thank Dr. Marco Ortenzi and Dr. Stefano Antenucci for additional analyses of polystyrene μPs characterization.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Rights and permissions
About this article
Cite this article
De Felice, B., Bacchetta, R., Santo, N. et al. Polystyrene microplastics did not affect body growth and swimming activity in Xenopus laevis tadpoles. Environ Sci Pollut Res 25, 34644–34651 (2018). https://doi.org/10.1007/s11356-018-3408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3408-x