Abstract
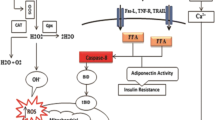
Arsenic (As) is a toxic and hazardous metalloid. Unfortunately, its presence in drinking water together with wrong nutritional patterns is associated with an increase in the occurrence of metabolic disorders in young people. Degradation of mitochondria is presented by a specific form of autophagy called mitophagy which is an important landmark leading to apoptosis during lipotoxicity. Lipotoxicity and cellular toxicity due to arsenic intake can lead to changes in mitophagy and apoptosis. The protein derived from SQSTM1 gene, also called p62, plays an important role in energy homeostasis in the liver, and it can contribute to the regulation of autophagic responses given its effect on signaling of mTOR, MAPK, and NF-KB. Consequently, changes in Sqstm1, mitophagy (BNIP3), and apoptotic (caspase 3) genes in the livers of NMRI mice were examined with the use of real-time RT-PCR Array followed by exposure to an environmentally relevant and negligible cytotoxic concentration of arsenite (50 ppm) in drinking water while being fed with a high-fat diet (HFD) or low-fat diet (LFD) for 20 weeks (LFD-As and HFD-As groups). While LFD-As and HFD groups showed a decrease in BNIP3 expression, a significant increase was observed in the HFD-As group. P62 gene showed downregulation in LFD-As and HFD groups, and upregeneration was observed in the HFD-As group. Caspase 3 showed increased expression as the key factor associated with apoptotic liver cell death in the three groups, with the highest value in HFD-As group. Overall, the changes observed in the expression of Sqstm1, BNIP3, and caspase 3 in this study can be related to the level of liver damage caused by exposure to arsenic and HFD and probably, BNIP3 pro-apoptotic protein is associated with an increased cell death due to HFD and As.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “arsenic” is known among most people in the world given its long history of use as a poison. However, most people are unaware of the fact that due to the presence of this substance in the environment and its use in commercial products and medical applications, they are constantly exposed to its toxic effects. Although, the normal levels of this substance in nature are safe, there are many areas in the world with high levels of arsenic which have toxic effects (Hughes et al. 2011). Although, the highest levels of arsenic are reported in the US drinking water in quantities less than 5 ppb, most people in different countries of the world, such as Mexico, Argentina, China, Bangladesh, India, and Iran, use potable water with high amount of arsenic. Lots of studies have been conducted on the effects arsenic in drinking water have on the health of people in different parts of the world. The results obtained from these studies show that arsenic in potable water is clearly associated with an increased risk of cancer (in bladder, lungs, kidney, liver, and prostate), cardiovascular diseases, skin effects, neurological complications, and diabetes in particular (Del Razo et al. 2011; Islam et al. 2012; Meliker et al. 2007; Navas-Acien et al. 2006; Tseng et al. 2000).In previous studies, it was found that oxidative stress and liver mitochondrial damage were involved in hepatotoxicity, effects of chronic exposure to inorganic arsenic and the use of HFD simultaneously (Ahangarpour et al. 2018).
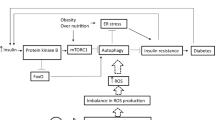
Non-adipose cells and tissues suffer a kind of cytotoxicity when they receive high levels of cholesterol or triglyceride fatty acids known as lipotoxicity (Schaffer 2003; Unger 2002; Unger 2003; Van Herpen and Schrauwen-Hinderling 2008). When the plasma levels of these compounds rise to an amount that adipose tissue cannot absorb, non-adipose tissue is overloaded with lipids, and lack of metabolic balance leads to metabolic diseases. If these tissues suffer weak oxidative capacity, lipotoxicity will result in poor functioning of the cell and the complications consequently resulting in cell death. So far, various consequences of lipotoxicity have been discussed repeatedly. The results of these studies confirm the role of endoplasmic reticulum stress and mitochondria in this regard. Recent studies specifically stressed mitochondrial involvement (Schrauwen 2004; Schrauwen and Hesselink 2004; Schrauwen et al. 2002; Schrauwen et al. 2003; Turner and Heilbronn 2008). The depletion of mitochondria because of a specific form of autophagy called mitophagy is considered as an important landmark which ends in apoptosis during lipotoxicity. Changes in cell responses following lipotoxicity occurs in different forms. For instance, an increase in substrates induced by excessive lipids can lead to a change in oxidative mitochondrial function. As a result of the oxidation of fatty acids, ROS production and lipotoxic products, mitochondrial maladaptive impairment can be observed. The lipotoxicity-activated kinases can disrupt transporters functioning through interaction with the internal mitochondria membrane that results in mal-functioning of mitochondria due to ionic imbalance. All these occurrences can result in not only apoptosis, but can also activate the mitophagy process as a manifestation of lipotoxic cell damage (Bülow et al. 2018; Duchen 2004; Pang et al. 2018; Pickles et al. 2018). So far, limited studies have been conducted on arsenic-induced changes in autophagy path and cytoprotective or cell-induced effects in liver cells (Bai et al. 2016; Liu et al. 2016; Pan et al. 2016; Zeinvand-Lorestani et al. 2018).
Although, recent studies have proven the relationship between arsenic and metabolic disorders, the results of studies on animal models vary and the main mechanism of arsenic effects is unknown. Diagnosis of arsenic functioning in healthy people and prevention of its effect on the progression and spread of diseases confirms the need for further studies and comparison of the effect of this metal element in the expression and presentation of a new perspective (Ahangarpour et al. 2017; Huang et al. 2015; Paul et al. 2011; Walton et al. 2004).
Anyway, the types of mitochondrial collisions with the surrounding cell’s environment have a significant effect on cellular fate. Thus, studies that specifically emphasize the mechanism of mitochondrial participation in a variety of stress disorders not only improve knowledge in this regard but also provide appropriate information for therapeutic purposes for the betterment of the health status of humans. Thus, this study examined the interactions caused by chronic exposure to arsenic and the use of the HFD regime as two important factors in the development of hepatotoxicity and the role of mitophagy in this regard.
Materials and methods
Animal treatment
Mature male NMRI mice with a weight of 30–35 g were obtained from the animal clinic of Ahvaz Jundishapur University of Medical Sciences (AJUMS). Mice were kept in each cage at room temperature (24 ± 2 °C), 10% moisture, and 12 h light/12 h dark cycle in polycarbonate cages with corncob bedding. The mice used in this trial were tested in accordance with the detailed guidelines of AJUMS animal care guidelines and ethics committee.
Chemicals
Sodium arsenite (99% pure) was purchased from Sigma-Aldrich (St. Louis, MO). Low-fat diet (LFD; 11% of all calorie supply from fat) and high-fat diet (HFD; 57% of all calorie supply from fat) were obtained from Javaneh Khorasan lab. Iran. RNeasy Mini kit (Qiagen, Valencia, CA), RT2 First Strand Kit (Qiagen, Frederick, MD, USA), and 96-well RT2 Profiler PCR Arrays-Mouse Autophagy (Qiagen, Frederick, MD, USA) were purchased from Qiagen.
Methods
The mice were divided into four groups and in the form of chronic studies as follows: low-fat diet (LFD) intake as control group (11, 16, and 72% for fat, protein, and carbohydrate kcal/g, respectively) and the other as high-fat diet (HFD) intake group (58, 16.4, and 25.5% for fat, protein, and carbohydrate kcal/g, respectively).To evaluate the effects of chronic arsenic exposure, the other two groups received chronic arsenic with dietary intake of HFD and LFD (LFD-As and HFD-As groups). Mice food was prepared from Javaneh Khorasan Food Co., Mashhad, and arsenic was determined by atomic absorption by the Jahad Daneshgahi University of Tehran (Perkin-Elmer4100 Perkin Elmer Norwalk, Connecticut). The arsenic in the HFD was about 5 ppb and in the LFD, it was about 7 ppb, which was negligible given the 50 ppm applied concentration of arsenic. Ten times higher concentration of drinking water arsenic (50 ppm) is needed to achieve liver arsenic concentrations similar to those seen in humans exposed to arsenic in West Bengal. Therefore, in the current study, the liver of mice that drank diH2O containing 50 ppm arsenic was used (Paul et al. 2011). Decreased ATG7 expression (key indicator of autophagy) occurs following HFD at week 16 and ends at week 22, so the duration of the experimental period was regarded as 20 weeks in this study (Yang et al. 2010).
Study samples
Twenty weeks later, at the end of the test, the overnight fasting animals were killed and their livers were removed from the abdominal cavities through a U-shaped incision. After washing with normal saline, liver tissue samples were placed in − 80 °C freezer for use in PCR array.
Liver sampling and extraction of total cellular RNA
Thirty (≥30) milligrams of each sample was taken and prepared according to the protocol of the RNeasy Plus Mini Kit. Thus, appropriate volume of the RLT buffer (600 μL) was added to the amount separated from the tissue and centrifuged for 3 min at maximum speed and supernatant was collected. Then, the homogenized solution was transferred to a gDNA Eliminator spin column placed in a 2-ml collection tube and centrifuged for 30 s at high speed 8000×g (≥ 10,000 rpm). A volume (600 μL) of 70% ethanol was added to the product of the previous step, and after pipetting and mixing 700 μL of the samples, they were transferred to a RNeasy spin column and centrifuged at ≥ 8000×g for 15 s. Then, 700 μL of RW1 buffer was added and centrifuged at ≥ 8000×g for 15 s. At this step, the RPE buffer (500 μL) was added to the RNeasy spin column and centrifuged at ≥ 8000×g for 15 s and repeated for 2 min at the column. Finally, RNeasy spin column was transferred to the collection tube and centrifuged after the addition of about 30–50 μL of RNase-free water for 1 min at ≥ 8000×g speed. The precipitated liquid which contained RNA was used in the experiments.
The cDNA synthesis
To make RNA concentrations of the same size using spectrophotometer (Nano Drop 1000, Thermo Scientific, Pittsburgh, PA) at 260 and 280 nm, RNAs concentration was determined in all samples and the required volume of RNA sample was calculated. RT2 First Strand Kit was used to make the cDNA. Firstly, the genomic DNA elimination mix was prepared by the addition of the required amounts of RNA to GE buffer and RNase-free water to reach 10 μL. It was then incubated for 5 min at 42 °C then immediately placed in ice for 1 min.
The reverse-transcription mix was also prepared according to the kit protocol using the specified values of 5× Buffer BC3, control P2, RE3 Reverse Transcriptase Mix and RNase-free water, and was taken to the required volume. To each tube, 10 μL of reverse-transcription mix and genomic DNA elimination mix were added, pipetted, and incubated at 42 °C for 15 min, then incubated at 95 °C for 5 min after which the reaction was immediately stopped by setting it on ice. After adding 91 μL of RNase-free water and pipetting for real-time PCR, it was kept in the freezer from − 15 to − 30 °C.
The real-time PCR for RT2 profiler PCR arrays
After centrifuging RT2 SYBR Green Mastermix (for 10–15 s), PCR components mix was prepared using the values required for RT2 SYBR Green Mastermix, cDNA synthesis reaction and RNase-free water according to the kit protocol. Twenty-five microliters of PCR components mix was added to each well from the plate and kept at room temperature (15–25 °C) for 1 min at 1000 g then centrifuged to eliminate bubbles. Then, the real-time cycler program was defined based on the protocol specified in the kit for Roche LightCycler 480. Upon determination of threshold cycle (CT) using real-time cycler software, data analysis was performed based on the ΔΔCT method. In accordance with the kit protocol, fold change above 1 was reported as fold upregulation and less than 1 as fold downregulation.
In this study, Actin, beta (Actb), Beta-2 microglobulin (B2m), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), and Heat shock protein 90 alpha (cytosolic), class B member 1(Hsp90ab1), genes were considered as housekeeping gene (HKG).
To normalize the expression level of a gene of interest (GOI) to a HKG, the expression levels of the two genes are divided:
To determine fold change in gene expression, the normalized expression of the GOI in the experimental sample was divided by the normalized expression of the same GOI in the control sample:
The complete calculation is as follows:
Histopathological studies
After blood collection, the mouse livers were removed immediately and fixed in 10% formalin solution. Then, dehydrated in graded alcohol concentrations and, embedded in paraffin. Sections of 4–6 μm were prepared and, stained with hematoxylin and eosin (H&E). Six microscopy slides per animal were examined for assessment of histological changes such congestion of RBCs, infiltration of inflammatory cells, nuclear pyknosis, and fat deposits. For assessment of nuclear pyknosis, the average percentage of nuclear pyknosis was determined by dividing the number nucleus with the pyknosis in a randomly microscopic field by the total number of nucleuses in the same field and the result multiplied by 100. Infiltration of inflammatory cells, fat deposits, and congestion of RBCs were graded into four categories: normal (0), weak (1), moderate (2), or intense (3) and the averages were considered. For each slide, the mean of six field was calculated. Slides were read in a “blind” fashion.
Statistical analyses
Data were presented as means ± SE. All the results were analyzed using Graph Pad Prism (version 7.03). Statistical significance was determined with the use of the one-way analysis of variance with the Tukey’s post-hoc test and non-parametric Kruskal-Wallis test. Statistical significance was set at p < 0.05.
Results
Liver distribution of arsenic
After determination of the presence of arsenic in the liver by an atomic absorption spectrophotometer, it was shown that exposure to arsenic 50 ppm resulted in accumulation of this substance in the liver of the mice that received LFD and HFD (Table 1) (p < 0.001) (Chilakapati et al. 2015).
Effect of arsenic exposure and diet on liver weight to body weight ration
The average ratio of liver weight to body weight in the HFD group was higher than that of LFD (p < 0.001). Although, receiving arsenic in the LFD group did not have a significant effect on the mean liver weight to body weight ratio in the LFD group, this significantly decreased in the HFD group exposed to arsenic simultaneously (p < 0.001) (Table 1).
Changes in expression of Sqstm1, BNIP 3, and Caspase 3 genes in LFD-As-treated groups
The expression of the caspase 3 increased (2.83-fold changes), but both Sqstm1 and BNIP3 showed reduced expression to 0.83- and 0.84-fold changes, respectively (Fig. 1 and Table 2).
Changes in expression of Sqstm1, BNIP 3, and Caspase 3 genes in HFD-treated groups
The expression of the caspase 3 increased in 1.06-fold change, but both Sqstm1 and BNIP3 showed reduced expression to 0.6- and 0.41-fold changes, respectively (Fig. 2 and Table 2).
Changes in expression of Sqstm1, BNIP 3, and Caspase 3 genes in HFD-As-treated groups
In this group, the expression of Sqstm1, BNIP 3, and caspase 3 genes increased by 1.95-, 1.81-, and 4.84-fold, respectively (Fig. 3 and Table 2).
Histopathological analysis of liver tissue
Administration of arsenic led to substantial pathological liver injuries including cellular damages, inflammatory cells aggregation, bleeding, and sinusoidal dilation. In the control LFD, some hepatocytes contained pyknotic nuclei. In LFD-As groups, the liver cells tended to have an increased number of pyknotic nuclei. Infiltration of inflammatory cells was also observed. In the control HFD, fatty changes were higher than in the control LFD fed mice. In HFD-As animals, infiltration of inflammatory cells, number of pyknotic nuclei, and congestion of red blood cells were higher as compared to the control HFD group. Arsenic significantly increased the HFD-induced liver damage in the liver of HFD-As-treated groups (Fig. 4 and Table 3).
Histopathological analysis of liver tissue. a In the control LFD, some hepatocytes contained pyknotic nuclei. b In LFD-As groups, the liver cells tended to have an increased number of pyknotic nuclei. Infiltration of inflammatory cells was also observed. c In the control HFD, fat deposits were higher than in the control LFD fed mice. d In HFD-As animals, infiltration of inflammatory cells, number of pyknotic nuclei, and congestion of red blood cells were higher as compared to the control HFD group. Arsenic significantly increased the HFD-induced liver damage in liver of HFD-As-treated groups. F fat deposit, i infiltration of inflammatory cells, C congestion of red blood cells, NP nuclear pyknosis; a LFD; b LFD + As; c HFD; d HFD + As
Discussion
Based on epidemiological studies, arsenic is one of the global pollutants. Chronic exposure to it is associated with many diseases and liver injuries, e.g., fibrosis, cirrhosis, and cancer (Biswas et al. 2018; Grau-Perez et al. 2018; Huang et al. 2015; Nesha et al. 2018; Rana et al. 2016; Santra 2015).Unfortunately, the complications associated with exposure to arsenic and malnutrition are not well known due to the greater sensitivity of humans than laboratory animals and the differences in arsenic metabolism in these animals (Abernathy et al. 2003; Brown and Ross 2002; Vahter and Concha 2001).
Limited studies have examined the hypothalamus and showed that “arsenic and malnutrition can simultaneously increase the damage caused by this substance in the liver and other organs” (Yang and Blackwell 1961). A study by Mitra et al. (2004) showed that low levels of vegetable fiber, calcium, folate, and low animal protein intake may be effective in increasing arsenic damage in people exposed to arsenic intake via water (Mitra et al. 2004). Similar studies also show the role of carbohydrates, low intake of micronutrients, and poor nutritional status in increasing the risk of disease and cancers by arsenic (Bhowmick et al. 2018; Engel and Receveur 1993; Hsueh et al. 1995; Tsuji et al. 2014; Yang and Blackwell 1961).
However, the harmful effects of nutritional pattern on the damage caused by arsenic in various organs are limited and requires further investigation (Bhowmick et al. 2018; Dutta et al. 2014; Hou et al. 2017; Tsuji et al. 2014).
The most common cell death in hepatic and pancreatic diseases is apoptosis and necrosis. Recently, autophagy was introduced for induction of cell death in B cell (Zhu et al. 2014). Changes in autophagy have been confirmed in many systemic diseases, such as cancer, neurodegeneration, cardiomyopathies, and metabolic dysfunction (Perluigi et al. 2015; Schneider and Cuervo 2014; Varga et al. 2015; Wang et al. 2016a). So far, many studies have been carried out on the changes in autophagy and cytoprotective effects or destructive effects in exposure to arsenic. The results of these studies indicate that the role of arsenic in the regulation of autophagy is highly dependent on the type of cellular stress as well as the type of cell, and can be varied (Guo et al. 2017; Sun et al. 2018; Wang et al. 2017; Wu et al. 2018; Zhu et al. 2014). The liver has the highest number of organelles, mitochondria which show the highest damage to oxidative and nutritional stress. An increase in energy intake as well as exposure to nutritional stress lead to induction of inhibitory signals on autophagy, and these result in inefficient organ function (Rautou et al. 2010; Yang et al. 2010).
In this study, for the first time, the activity of BNIP3 in the mice liver was shown following the simultaneous toxicity of HFD and As. An increase of BNIP3 expression was associated with increased expression of caspase3 and SQSTM1. In previous studies, it seemed that this cell death was due to apoptosis, which led to toxic effects of arsenic on the liver (Choudhury et al. 2016; Li et al. 2018; Xia et al. 2018; Yousefsani et al. 2018). Nevertheless, from the present study, the data obtained showed that events occurring in the liver due to metabolic and oxidative stresses are much more complicated than we can imagine and various types of cell death can be involved in this process in different conditions.
Some studies have confirmed that autophagy and, especially, mitophagy are involved in a variety of metabolic stresses and arsenic toxicity (Cavaliere et al. 2014; Law et al. 2015; Niu et al. 2016; Zeinvand-Lorestani et al. 2018). In this study, it was indicated that BNIP3 gene clearly showed downregulation in LFD-As and HFD but in the HFD-As group, it showed upregulation. BNIP3 is stated to interact with LC3 to target the damaged mitochondria by autophagosomes and start the mitophagic process (Graier et al. 2009; Shi et al. 2014). Moreover, the researchers have previously found that BNIP3 induced cell death through mitochondrial dysfunction. Thus, homodimeric BNIP3 tends to attach to the mitochondrial outer membrane and leads to increased permeability and release of cytotoxic proteins from mitochondria (Li et al. 2016; Nakamura et al. 2012). In our previous study, increased oxidative stress and mitochondrial involvement were observed at the time of simultaneous use of HFD and As (Ahangarpour et al. 2018). In this study, the hypothesis proposed was that mitochondrial BNIP3 could be activated as an upstream signal to increase oxidative stress and maybe, BNIP3 upregulation caused increased liver cell death. In confirmation of the hypothesis set forth in this study, caspase 3 showed increase in expression in all three groups and the highest was in the HFD-As group. As caspase 3 was identified as a key trigger for apoptotic cell death; the increased expression of BNIP3 and caspase 3 simultaneously in the HFD-As group showed the association of BNIP3 as a co-regulator of autophagy and apoptosis when both HFD and As were received (Wang et al. 2016b).
The protein derived from SQSTM1 gene, known as p62, is eliminated through autophagy, and is often discussed in studies together with the determination of the activity of a variety of autophagy (Manley et al. 2013). Moreover, SQSTM1 is an important regulatory protein for setting antioxidant responses by Nrf-2 (Jain et al. 2010; Katsuragi et al. 2016). In this study, it was observed that the SQSTM1 gene clearly showed downregulation in the LFD-As and HFD groups, but in the HFD-As group, it showed upregulation. SQSTM has a tissue-specific role in adipogenesis and energy regulation. Findings from studies recently conducted confirmed that the expression of SQSTM in cells is determined by three factors which are autophagy degeneration type, transcriptional upregulation, and availability of lysosomal aminoacids, which are not always inversely associated with autophagic activity (Sahani et al. 2014).
In general, all these results suggest that probably, BNIP3 contribute to increased cell death, which resulted, from HFD and As. Thus, this study provides a new insight into the role of BNIP3 gene in the regulation of liver cell death.
Conclusion
According to the results, it can be stated that there may be a relationship between the increase in the activity of the BNIP3 gene and the increase in cell death. The results of this study can be effective in improving our knowledge on the damaging effects of environmental pollutants such as arsenic in potable water and metabolic stress due to the unusual nutritional pattern, on liver in most people worldwide.
References
Abernathy CO, Thomas DJ, Calderon RL (2003) Health effects and risk assessment of arsenic. J Nutr 133:1536S–1538S
Ahangarpour A, Oroojan AA, Rezae M, Khodayar MJ, Alboghobeish S, Zeinvand M (2017) Effects of butyric acid and arsenic on isolated pancreatic islets and liver mitochondria of male mouse. Gastroenterology and Hepatology From Bed to Bench 10:44–53
Ahangarpour A, Alboghobeish S, Rezaei M, Khodayar MJ, Oroojan AA, Zainvand M (2018) Evaluation of diabetogenic mechanism of high fat diet in combination with arsenic exposure in male mice. Iranian Journal of Pharmaceutical Research 17
Bai J, Yao X, Jiang L, Zhang Q, Guan H, Liu S, Wu W, Qiu T, Gao N, Yang L, Yang G, Sun X (2016) Taurine protects against As2O3-induced autophagy in livers of rat offsprings through PPARγ pathway. Sci Rep 6:27733. https://doi.org/10.1038/srep27733
Bhowmick S, Pramanik S, Singh P, Mondal P, Chatterjee D, Nriagu J (2018) Arsenic in groundwater of West Bengal, India: a review of human health risks and assessment of possible intervention options. Sci Total Environ 612:148–169
Biswas A, Das A, Deb D, Ghose A, Mazumder DNG (2018) Cancer risk estimation from dietary arsenic, a new approach from longitudinal cohort study. Stoch Env Res Risk A 32:1035–1050
Brown KG, Ross GL (2002) Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul Toxicol Pharmacol 36:162–174
Bülow MH et al (2018) Unbalanced lipolysis results in lipotoxicity and mitochondrial damage in peroxisome-deficient Pex19 mutants. Mol Biol Cell 29:396–407
Cavaliere V, Lombardo T, Costantino SN, Kornblihtt L, Alvarez EM, Blanco GA (2014) Synergism of arsenic trioxide and MG132 in Raji cells attained by targeting BNIP3, autophagy, and mitochondria with low doses of valproic acid and vincristine. Eur J Cancer 50:3243–3261
Chilakapati J, Wallace K, Hernandez-Zavala A, Moore T, Ren H, Kitchin KT (2015) Pharmacokinetic and genomic effects of arsenite in drinking water on mouse lung in a 30-day exposure. Dose-Response 13:1559325815592392. https://doi.org/10.1177/1559325815592392
Choudhury S, Ghosh S, Mukherjee S, Gupta P, Bhattacharya S, Adhikary A, Chattopadhyay S (2016) Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J Nutr Biochem 38:25–40
Del Razo LM et al (2011) Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health 10:73
Duchen MR (2004) Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Asp Med 25:365–451
Dutta M et al (2014) High fat diet aggravates arsenic induced oxidative stress in rat heart and liver. Food Chem Toxicol 66:262–277
Engel RR, Receveur O (1993) Re:“Arsenic ingestion and internal cancers: a review”. Am J Epidemiol 138:896–897
Graier WF, Malli R, Kostner GM (2009) Mitochondrial protein phosphorylation: instigator or target of lipotoxicity? Trends Endocrinol Metab 20:186–193. https://doi.org/10.1016/j.tem.2009.01.004
Grau-Perez M et al (2018) Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ Pollut 235:948–955
Guo Z et al (2017) The optimal dose of arsenic trioxide induced opposite efficacy in autophagy between K562 cells and their initiating cells to eradicate human myelogenous leukemia. J Ethnopharmacol 196:29–38
Hou H, Yu Y, Shen Z, Liu S, Wu B (2017) Hepatic transcriptomic responses in mice exposed to arsenic and different fat diet. Environ Sci Pollut Res 24:10621–10629
Hsueh Y-M, Cheng G, Wu M, Yu H, Kuo T, Chen CJ (1995) Multiple risk factors associated with arsenic-induced skin cancer: effects of chronic liver disease and malnutritional status. Br J Cancer 71:109
Huang C-F et al (2015) Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 123:1138
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332
Islam MR et al (2012) Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 11:38
Jain A et al (2010) p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285:22576–22591. https://doi.org/10.1074/jbc.M110.118976
Katsuragi Y, Ichimura Y, Komatsu M (2016) Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr Opin Toxicol 1:54–61. https://doi.org/10.1016/j.cotox.2016.09.005
Law BA, Roddy P, Liao X, Schulze P, Cowart L (2015) Lipid oversupply to cardiomyocytes induces sphingolipid-dependent oxidative stress and induction of mitophagy through ceramide synthase 2. Am Heart Assoc,
Li S et al (2016) Epigallocatechin-3-gallate attenuates apoptosis and autophagy in concanavalin A-induced hepatitis by inhibiting BNIP3 Drug design. Dev Ther 10:631
Li Y, Zhang Y, Gao Y, Zhang W, Cui X, Liu J, Wei Y (2018) Arsenic induces Thioredoxin 1 and apoptosis in human liver HHL-5 cells. Biol Trace Elem Res 181:234–241
Liu X et al (2016) MicroRNA-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cells. Toxicol Lett 252:1–10. https://doi.org/10.1016/j.toxlet.2016.04.015
Manley S, Williams JA, Ding W-X (2013) The role of p62/SQSTM1 in liver physiology and pathogenesis. Experimental biology and medicine (Maywood, NJ) 238:525–538 https://doi.org/10.1177/1535370213489446
Meliker JR, Wahl RL, Cameron LL, Nriagu JO (2007) Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health 6:4
Mitra SR et al (2004) Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect 112:1104
Nakamura Y et al (2012) BNIP3 and NIX mediate Mieap-induced accumulation of lysosomal proteins within mitochondria. PLoS One 7:e30767. https://doi.org/10.1371/journal.pone.0030767
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Perspect 114:641
Nesha M, Islam M, Ferdous N, Nazrul FB, Rasker JJ (2018) Chronic arsenic exposure through drinking water and risk of type 2 diabetes mellitus: a study from Bangladesh. J Family Med Prim Care Open Acc: JFOA-113 DOI 10
Niu Z, Zhang W, Gu X, Zhang X, Qi Y, Zhang Y (2016) Mitophagy inhibits proliferation by decreasing cyclooxygenase-2 (COX-2) in arsenic trioxide-treated HepG2 cells. Environ Toxicol Pharmacol 45:212–221
Pan J-A et al (2016) TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol Cell 61:720–733. https://doi.org/10.1016/j.molcel.2016.02.007
Pang L et al (2018) Differential effects of reticulophagy and mitophagy on nonalcoholic fatty liver disease. Cell Death Dis 9:90
Paul DS, Walton FS, Saunders RJ, Stýblo M (2011) Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119:1104–1109. https://doi.org/10.1289/ehp.1003324
Perluigi M, Di Domenico F, Butterfield DA (2015) mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 84:39–49
Pickles S, Vigié P, Youle RJ (2018) Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol 28:R170–R185
Rana T, Bera AK, Das S, Bhattacharya D, Pan D, Das SK (2016) Inhibition of oxidative stress and enhancement of cellular activity by mushroom lectins in arsenic induced carcinogenesis. Asian Pac J Cancer Prev 17:4185–4197
Rautou P-E, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R (2010) Autophagy in liver diseases. J Hepatol 53:1123–1134
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10:431–441. https://doi.org/10.4161/auto.27344
Santra A (2015) Arsenic-induced liver injury. In: Handbook of arsenic toxicology. Elsevier, pp 315–334
Schaffer JE (2003) Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14:281–287
Schneider JL, Cuervo AM (2014) Autophagy and human disease: emerging themes. Curr Opin Genet Dev 26:16–23
Schrauwen P (2004) The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc Nutr Soc 63:287–292
Schrauwen P, Hesselink MK (2004) Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53:1412–1417
Schrauwen P, Hesselink MK, Vaartjes I, Kornips E, Saris WH, Giacobino J-P, Russell A (2002) Effect of acute exercise on uncoupling protein 3 is a fat metabolism-mediated effect. Am J Physiol Endocrinol Metab 282:E11–E17
Schrauwen P et al (2003) Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J 17:2272–2274
Shi RY, Zhu SH, Li V, Gibson SB, Xu XS, Kong JM (2014) BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS Neurosci Ther 20:1045–1055
Sun X et al (2018) Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus. J Inorg Biochem 178:54–62
Tseng C-H et al (2000) Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108:847
Tsuji JS, Alexander DD, Perez V, Mink PJ (2014) Arsenic exposure and bladder cancer: quantitative assessment of studies in human populations to detect risks at low doses. Toxicology 317:17–30
Turner N, Heilbronn LK (2008) Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab 19:324–330
Unger RH (2002) Lipotoxic diseases. Annu Rev Med 53:319–336
Unger RH (2003) Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144:5159–5165
Vahter M, Concha G (2001) Role of metabolism in arsenic toxicity Pharmacology & Toxicology. MiniReview 89:1–5
Van Herpen N, Schrauwen-Hinderling V (2008) Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav 94:231–241
Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P (2015) Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta (BBA)-Mol Basis Dis 1852:232–242
Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M (2004) Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Tox Appl Pharmacol 198:424–433. https://doi.org/10.1016/j.taap.2003.10.026
Wang S-F, Wu M-Y, Cai C-Z, Li M, Lu J-H (2016a) Autophagy modulators from traditional Chinese medicine: mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J Ethnopharmacol 194:861–876
Wang S, Pacher P, De Lisle RC, Huang H, Ding WX (2016b) A mechanistic review of cell death in alcohol-induced liver injury. Alcohol Clin Exp Res 40:1215–1223
Wang G et al (2017) Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free Radic Biol Med 106:24–37
Wu W et al (2018) Pancreatic islet-autonomous effect of arsenic on insulin secretion through endoplasmic reticulum stress-autophagy pathway. Food Chem Toxicol 111:19–26
Xia Y, Liu X, Liu B, Zhang X, Tian G (2018) Enhanced antitumor activity of combined megestrol acetate and arsenic trioxide treatment in liver cancer cells. Exp Ther Med 15:4047–4055
Yang T, Blackwell R (1961) Nutritional and environmental conditions in the endemic blackfoot area. Formos Sci 15:101–129 Find this article online
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010) Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11:467–478
Yousefsani BS, Pourahmad J, Hosseinzadeh H (2018) The mechanism of protective effect of crocin against liver mitochondrial toxicity caused by arsenic III. Toxicol Mech Methods 28:105–114
Zeinvand-Lorestani M et al (2018) Autophagy upregulation as a possible mechanism of arsenic induced diabetes. Sci Rep 8:11960
Zhu X-X et al (2014) Sodium arsenite induces ROS-dependent autophagic cell death in pancreatic β-cells. Food Chem Toxicol 70:144–150. https://doi.org/10.1016/j.fct.2014.05.006
Acknowledgements
This paper is issued from Ph.D. thesis of Marzieh Zeinvand-Lorestani.
Funding
This paper was financially supported by Toxicology Research Center (Grant number TRC-9505) of Ahvaz Jundishapur University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zeinvand-Lorestani, M., Kalantari, H., Khodayar, M.J. et al. Dysregulation of Sqstm1, mitophagy, and apoptotic genes in chronic exposure to arsenic and high-fat diet (HFD). Environ Sci Pollut Res 25, 34351–34359 (2018). https://doi.org/10.1007/s11356-018-3349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3349-4