Abstract
Honey has multifaceted beneficial properties, but polluted environment and unapproved apicultural practices have led to its contamination. In this study, QuEChERS method followed by chromatographic analysis by GC-μECD/FTD and GC-MS was validated and used for determination of 24 pesticides in 100 raw honey samples from various floral origins of Northern India. Matrix-matched calibrations showed that the method was selective and linear (r2 > 0.99) with detection limit < 9.1 ng g−1 for all the studied pesticides except for monocrotophos (21.3 ng g−1). The average recoveries at different fortification levels ranged from 86.0 to 107.7% with relative standard deviation < 20%. Pesticide residues were detected in 19.0% samples, and most prevalent compounds detected were dichlorvos in 6.0% samples followed by monocrotophos (5.0%), profenofos (5.0%), permethrin (4.0%), ethion (3.0%), and lindane (3.0%) with concentrations ranging from 58.8 to 225.5, from 96.0 to 430.1, from 14.6 to 43.2, from 27.8 to 39.6, from 25.6 to 28.0, and from 19.6 to 99.2 ng g−1, respectively. Honey samples originating from cotton, sunflower, and mustard crops (33.3%) that tested positive for pesticide residues were found to be significantly higher (p < 0.05) than the honey originating from natural and fruity vegetation (13.5%). Therefore, considering the contamination of environmental compartments due to extensive application of pesticides in the study area and their potential for subsequent transfer to honey by the expeditious bees, the results of present study proclaim that honey may be used as an indicator of environmental pollution. Further, estimated daily intakes of all contaminants were found to be at levels well below their acceptable daily intakes suggesting that consumption of honeys at current levels does not pose deleterious effects on human health. However, precautionary measures should always be taken considering the customary honey feeding in infants and cumulative effect of these chemicals in the foreseeable future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food is essential for well-being of human, but it may also act as a major source of worry, pleasures, and stress. One of the reasons behind the stress and worry is the adverse effects caused as a result of contaminated food (Rather et al. 2017). Therefore, concerns about food safety are incessantly increasing in both developed and developing regions of the world.
India, bestowed with rich biodiversity, has a long history of apiculture and, alone with over 1200 million people, is a huge market for honey. Indian subcontinent is now emerging as a major producer and exporter of high-quality, mild honey with versatility for various countries. The annual production of honey for the year 2016–2017 has been estimated to be 94, 500 metric tons; out of which, approx. 40,000 metric tons was exported from India (NBB 2017).
Honey, due to its multiple beneficial properties, is being consumed since time immemorial by children, elderly and ill people across the globe (Tsipi et al. 1999). It has myriad uses and finds place as an ingredient in many pharmaceuticals, cosmetics and food products. Thus, like any other food, it must be free from contaminants and safe for human consumption. However, nowadays, honey is being produced in an environment contaminated by various pollutants (Christodoulou et al. 2015; Mitchell et al. 2017).
India, owing to agricultural green revolution, has established its position as a progressive agrarian country. But, at the same time pesticides has been produced and applied massively for the purpose of enhancing crop production and improving health by destroying pests of food crops and vectors of various arthropod borne diseases like malaria, dengue, and encephalitis. India occupies fourth position in the world after the USA, Japan, and China in terms of total pesticides production, and the average per hectare usage of pesticides in India has gradually increased from 1.2 g/ha in 1953–1954 to 600 g/ha during 2014–2015 (Chahal et al. 2016). Pesticides have been instrumental in country’s green revolution as they have contributed to almost double the food production during the last century (Mittal et al. 2014). They are also imperative in modern agricultural practices because current need to increase food production to feed a rapid growing human population maintains pressure on modern agricultural practices with judicious use of pesticides and fertilizers. However, their widespread use and indiscriminate application on crops can contaminate blossoms from which bees collect nectar for honey production, resulting in transfer of pesticide residues to honey and finally to consumers. Furthermore, at environmental levels, honey bees (e.g., Apis mellifera) on their foraging expeditions of up to 3 km in all directions can also pick and transfer pollutants from contaminated soil, water, and air to their respective hives leading to contamination of beehive products (Abou-Shaara 2014; Kujawski et al. 2014; Mullin et al. 2010; Mitchell et al. 2017; Naccari et al. 2014; Porrini et al. 2003). Therefore, honey can provide sufficient information on the extent of pesticide usage in the field crops surrounding the beehives, and this way, it can also act as an excellent sentinel for monitoring contaminations in the environment (Balayiannis and Balayiannis 2008; Przybyowski and Wilczyńska 2001).
The presence of agrochemical residues such as organochlorines (OCs), organophosphates (OPs), and synthetic pyrethroids (SPs), which have been extensively spayed on nearby crops, not only influences the honey bee colony performance but can also devalue honey’s use for human consumption. Such contaminated honey may pose potential risks to human health by causing deleterious effects like genetic mutations and cellular degradations (Aliferis et al. 2010; Panseri et al. 2014). Therefore, determination of pesticide residues in honey has become essential and growing concern in recent years for maintaining its healthful characteristics and protecting public health.
For detection of pesticides in honey, both gas chromatography (GC) and liquid chromatography (LC) methods have been used. But owing to the volatile nature and thermal stability of most of the pesticides, GC, due to its high separation power and availability of selective detectors such as electron capture (ECD), flame thermionic (FTD), and mass spectrometry (MSD) detectors, seems to be the most promising methodology for detection and quantification of organochlorine, synthetic pyrethroid, and organophosphorus pesticides (Fernandez et al. 2002). Most of these analytical methods based on the traditional pretreatment procedures such as liquid-liquid extraction (LLE) and solid phase extraction (SPE) have been applied by various researchers for determination of pesticide residues in honey (Blasco et al. 2003; Debayle et al. 2008; De Pinho et al. 2010; Rissato et al. 2007). Every method has its own limitations, with some lacking selectivity, others requiring large volumes of solvents, are time consuming, and quite expensive to execute in routine analysis. Hence, to trounce these issues, quick, easy, cheap, effective, rugged, safe (QuEChERS) method was originally developed for fruits and vegetables (Anastassiades et al. 2003), employing acetonitrile extraction/partitioning and dispersive solid-phase extraction (d-SPE), tended to be one of the most promising and frequently employed multiresidue method for determination of pesticide residues in honey (Barakat et al. 2007; Barganska et al. 2014; Irungu et al. 2016; Malhat et al. 2015; Nadaf et al. 2015; Vilca et al. 2012; Wiest et al. 2011).
Residues of pesticides in food commodities are becoming a major concern for food regulators all over the world, and it is of paramount importance to safeguard human health. Therefore, food safety organizations have established and enforced acceptable daily Intake (ADI) and maximum residual limits (MRLs) for various food items. But, despite the fact that there is substantial production and trade in honey worldwide, there are very limited numbers of standards for pesticide residues in honey (CAC 2001; EC 2005). In India, all the MRLs with regard to pesticides are only for the honey which is to be exported to European Union (EIC 2013).
Concerning the above facts, in the present study, QuEChERS method for simultaneous extraction and cleanup of pesticide residues from honey matrix as well as analytical methods for multiresidue detection of 13 organochlorines, 3 synthetic pyrethroids by GC-μECD, and 8 organophosphates by GC-FTD was optimized and validated. The method was applied for determination of pesticide residues in raw honey samples belonging to various floral origins. Additionally, human health risk assessments were also performed by comparing estimated daily intakes (EDIs) with acceptable daily intakes (ADIs) for all the detected pesticides. To the best of our knowledge, this is the first in-depth multiclass, multiresidue analysis of pesticides in raw honeys from India which has highlighted the importance of honey as an indicator of environmental pollution.
Materials and methods
Chemicals and reagents
Certified reference materials of the targeted pesticides belonging to organophosphates (dichlorvos, monocrotophos, phorate, chlorpyrifos, profenofos, ethion, malathion, quinalphos), organochlorines (α-BHC, heptachlor, lindane, aldrin, heptachlor epoxide, fipronil, endosulfan I, α-chlordane, p,p′-DDE, o,p′-DDD, endrin, p,p′-DDD, methoxychlor), and synthetic pyrethroids (λ-cyhalothrin, permethrin, cypermethrin) with purity > 98% were procured from Sigma-Aldrich (Germany). The standard operating procedures were followed for obtaining, labelling, storing, and handling of pesticide standards. Reference standards were initially stored in deep freezer (− 20 °C) under dry storage conditions and were brought to room temperature prior to preparing stock solutions, while the liquid standards were sonicated for proper mixing.
Individual stock standard solutions (100 mg/L) were prepared in volumetric flasks by dissolving 10 mg of each compound in 100 mL of HPLC grade n-hexane:acetone (1:1) and were stored at − 20 °C. Intermediate standards solutions of 10 mg/L were prepared by dissolving 1 mL of stock solutions in 9 mL n-hexane:acetone (9:1) and kept in refrigerator at 4 °C. For multiresidue analysis of pesticides, multicomponent working standard calibration solutions in the concentrations range of 5–500 ng/mL were then prepared by mixing and properly diluting the calculated volumes of each intermediate standard solution with n-hexane and acetone (9:1). For the method validation and recovery studies, the blank honey samples were spiked before the sample preparation. Matrix matching was used in analysis to compensate for matrix effects and for that purpose, control blank honey matrix fortified at the final reconstitution step was also run on GC.
The QuEChERS kits with salt packets containing 6 g of anhydrous magnesium sulfate and 1.5 g sodium acetate and 15 mL d-SPE polypropylene tubes with 0.4 g primary secondary amine (PSA) sorbent and 1.2 g anhydrous magnesium sulfate were purchased from Agilent Technologies, USA. The deionized water (< 10 μS/cm electrical conductivity) used was purified by Milli-Q water system (Millipore Corporations, USA). All other organic solvents and reagents were of analytical grade and purchased from standard commercial suppliers.
Sample collection
A total of 100 raw honey samples comprising of 83 unifloral and 17 multifloral honeys were collected randomly from apiaries of North Indian states (J&K, Himachal Pradesh, Punjab, Haryana, and Rajasthan) with similar managemental practices, during the honey harvesting season (Supplementary Data, Table S 1). Floral origins of honey samples were provided by the beekeepers’ in relation to the locations where the beehives were situated. The source of nectars in the surrounding areas of apiaries was further verified by the researchers during sample collection. To classify and differentiate unifloral honeys originating from different botanical origins, the samples were also subjected to sensory, physicochemical, and principal component analysis (Kumar et al. 2018). All honey samples weighing between 100 and 250 g were subsequently stored at − 20 °C until extraction and analysis to prevent any possible matrix alteration (fermentation phenomena). One blank (reference) honey sample was also collected from an apiary placed in a low anthropized area of Northwestern Himalayan Region of India and was checked for no contamination.
Sample preparation
For multiresidue analysis of pesticides in honey, QuEChERS-AOAC 2007.01 method was used (AOAC 2007) with slight modifications for honey matrix. Five grams of honey weighed in a 50-mL polypropylene centrifuge tube was dissolved in 10 mL of ice-cold deionized water by shaking for 1 min to make a homogenous mixture. Ten milliliters of acetonitrile acidified with glacial acetic acid (1%) and contents of “QuEChERS” salt kit (6.0 g MgSO4 and 1.5 g CH3COONa) were then added to the homogenized sample. The mixture was immediately hand shaken vigorously for 1 min and subsequently centrifuged at 4000 rcf for 5 min in a refrigerated centrifuge at 4 °C. Six millilieters of the upper clear solution (acetonitrile fraction) was transferred into 15 mL dSPE polypropylene centrifuge tubes containing 0.4 g PSA sorbent and 1.2 g MgSO4. The tube was immediately capped, and then the extract with the sorbent and desiccant was shaken vigorously by hand for 1 min, vortexed for 30 s, and finally centrifuged at 4000 rcf for 3 min in a refrigerated centrifuge at 4 °C. Four milliliters of the upper clear solution was then transferred into a clean glass beaker and evaporated in a vacuum concentrator at 40 °C to dryness. The pesticide residues were re-dissolved in 2 mL of n-hexane:acetone (9:1), and 1 μL of the sample was injected into a gas chromatography-flame thermionic detector (GC-FTD) and 2 μL into the gas chromatography-electron capture detector (GC-μECD).
Chromatographic analysis
The residues in cleaned up extracts were detected and quantified using gas chromatography coupled with μECD and FTD. The compounds were identified and quantified by comparing retention time and peak area of the sample chromatogram with those of matrix-matched calibration standards run under the same operating conditions. Each chromatographic sequence included a reagent blank (procedural blank), matrix-matched calibration standards, honey samples, and quality control samples (spike sample and blank sample) to check for contamination, peak identification, and quantification.
GC-μECD analysis
For the detection and quantification of the organochlorines and synthetic pyrethroids, chromatographic analysis was performed by GC 7890B equipped with an electron capture detector (μECD) and capillary column DB-5 (30 m × 0.32 mm i.d. × 0.25 μm film thickness) of 5% diphenyl and 95% dimethyl polysiloxane (Agilent Technologies, USA). The temperature program consisted of initial oven temperature, 170 °C (13 min hold) increased to 270 °C (10 min hold) at a rate of 3 °C min−1 for a total run time of 56.33 min. The temperature of the injection port and detectors was set at 280 °C and 300 °C, respectively. The carrier gas (N2) flow was maintained at 32.904 mL/min and 2.7 mL/min through column with split ratio 1:10. OpenLAB EZChrom software was used for instrument control and data analysis.
GC-FTD analysis
For the detection and quantification of the organophosphorus pesticides, analysis was performed by FTD analytical system of Shimadzu equipped with a flame thermionic detector (FTD) and a RTX-5 (30 m × 0.25 mm i.d. × 0.25 μm film thickness) capillary column. The temperature program applied was as follows: initial temperature 180 °C, held for 2 min; a 10 °C min−1 ramp to 270 °C, held for 3 min; and a 5 °C min−1 ramp to 280 °C, held for 5 min, leading to a total run time of 21 min. The temperature of the injection port and detectors was set at 280 °C. The carrier gas (N2) flow was maintained at 9 mL/min and 1.0 mL/min through column with split ratio 1:5. Shimadzu GCsolution software was used for instrument control and data analysis.
GC-MS analysis
Suspected samples were analyzed by GC-MS to confirm the GC-μECD and GC-FTD results. For the qualitative confirmation of pesticide residues in honey, GC-MS analysis was carried out on a Shimadzu GCMS-QP2010 Plus model (Shimadzu, Japan) with auto sampler AOC 20i operated through GCMSsolution-software-based data acquisition. RTX-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) and helium as carrier gas were used. The oven temperature programmed was as follows: initial temperature of 80 °C, held for 3 min; increased to 180 °C at 20 °C min−1, held for 2 min; increased to 190 °C at 2 °C min−1 and held for 2 min; and then raised to 280 °C at 5 °C min−1 and held at this temperature for 10 min. The temperatures of ion source and interface were 200 °C and 290 °C, respectively. The injector temperature was 285 °C, and 1 μL of sample was injected in the splitless mode with 60 s purge off. Analysis was performed in selected ion monitoring (SIM) mode, monitoring specific ions of each analyte. The suspected compounds were confirmed on the basis of their retention time and fragment ions (m/z) (Supplementary Data Table S 2 and Figs. S 1a, S 1b, S 1c).
Method validation and quality control
The method for extraction, clean up, detection, and quantification of 24 pesticide residues from honey matrix was optimized and validated in compliance with the European Commission guidance document SANTE 11945/2015 (EC 2016) by evaluating the following performance parameters: linearity, limit of detection, limit of quantification, accuracy, precision, and selectivity.
Linearity was evaluated by the calculation of a 5-point linear plot with three replicates based on linear regression and coefficient of determination (r2). Five point matrix-matched calibrations were performed in the concentration range of 5–100 ng g−1 for organochlorines and synthetic pyrethroid pesticide and in the concentration range between 10 ng g−1 and 200 ng g−1 for each organophosphorus pesticide.
The limit of detection (LOD) and limit of quantification (LOQ) were estimated from the calibration curve using the equation; \( \mathrm{LOD}=\frac{3.3\ X\ \upsigma\ }{m} \), where, m is the slope of the calibration curve and σ is the residual standard deviation. The limit of quantification was calculated as LOQ = 3 × LOD (ICH 2005; Panseri et al. 2014).
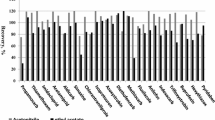
The trueness of the method was evaluated by estimating the accuracy (expressed as recovery percentage) and precision (% relative standard deviation). Recovery experiments, concerning 24 pesticides, were carried out by spiking 5 g of the homogenized, known negative, blank honey sample with working standard solutions of multicomponent calibration mixtures at 3 fortification levels: 25, 50, and 100 ng g−1 for organochlorines and synthetic pyrethroids, and at 4 fortification levels: 25, 50, 100, and 200 ng g−1 for organophosphates. Spiking was done in such a way that the first level corresponded approximately to the LOQ of the method for each pesticide, with three replicates for each level. Since, LOQ for monocrotophos was found to be relatively high (63.9 ng g−1) and first fortification level, i.e., 25 ng g−1, was well below quantification limits, therefore, for organophosphates, 200 ng g−1 as a fourth level of fortification was used. After spiking, the samples were let stand at room temperature for 2 h to achieve sample equilibration. Peak areas of the compounds in the blank matrix sample spiked before sample preparation and in the blank matrix sample added just before analysis (as matrix matched standards) were used to calculate recovery percentage.
The precision (repeatability) of the method is a measure of variability of the results and was determined in terms of the relative standard deviation (RSD) of three identical extractions of honey samples spiked with pesticides at same as well as at different fortification levels. The method’s selectivity was evaluated by analyzing blank honey matrix and procedural (reagent) blank to determine any interference from endogenous compounds around the retention time window of target analytes.
Results and discussion
Method validation and quality control
The optimized analytical conditions of GC-μECD and GC-FTD resulted in effective identification and separation of all the targeted pesticides with good resolution of the peaks (Supplementary Data Figs. S 2a and S 2b). Method validation and quality control parameters for determination of pesticides in honey and their corresponding acceptable criteria’s are included in Table 1.
Five point matrix-matched calibrations in triplicates showed that the method was linear with a coefficient of determination (r2) value > 0.994 for all the compounds in the investigated range. The limits of detection (LOD) obtained were lower than 9.1 ng g−1 for most of the analytes except for monocrotophos, with LOD value of 21.3 ng g−1. In India, MRLs for pesticide residues have not yet been established for honey. Therefore, LOD and LOQ values obtained in the present study were found to be comparable with the MRLs established by Export Inspection Council of India.
The pesticide residues were recovered in the range between 86.0 and 107.7% with pooled relative standard deviation values lower than 20%, which is in compliance to European Commission guidance document SANTE 11945/2015 (EC 2016). Analysis of blank honey samples (n = 10) showed that there were no interference peaks from the endogenous compounds present in the honey matrix around the retention time window of target analytes (Figs. 1 and 2). The results showed that the method was efficacious, sensitive, and reliable, enabling the multiresidue determination of all the targeted pesticides in honey that have toxicological relevance at low concentrations.
Application of method to real samples
Pesticide residues in honey have recently become a major consumer concern, and there are several reports of their detection in commercial honey and other apicultural matrices from various countries (Mullin et al. 2010; Wiest et al. 2011; Panseri et al. 2014; Malhat et al. 2015). But, the information on presence of pesticide residues in raw honey belonging to different floral origins and their potential association with agricultural farming practices is probably lacking from India.
In the present study, the optimized and validated QuEChERS method was applied for determination of pesticide residues in 100 raw unifloral and multifloral honey samples. Residues of one or more investigated pesticides were detected in 19% of honey samples. Six out of 19 samples were found to contain more than 1 pesticide residues. Further, out of 24 targeted pesticides, 9 were detected in positive honey samples which included organophosphates (6/8), synthetic pyrethroids (2/3), and organochlorine (1/13). Among the different groups, predominantly detected pesticides were organophosphates in 15% of the samples followed by synthetic pyrethroids (5%) and organochlorine (3%). As evident from the results summarized in Table 2, the compounds detected were dichlorvos in 6 samples followed by monocrotophos (5), profenofos (5), permethrin (4), ethion (3), and lindane (3) with concentrations ranging from 58.8 to 225.5 ng g−1, from 96.0 to 430.1 ng g−1, from 14.6 to 43.2 ng g−1, from 27.8 to 39.6 ng g−1, from 25.6 to 28.0 ng g−1, and from 19.6 to 99.2 ng g−1, respectively. Phorate, chlorpyrifos, and cypermethrin were also detected individually in 3 different honey samples with residual concentration of 24.5 ng g−1, 60.5 ng g−1, and 26.0 ng g−1, respectively. The presence of pesticides was qualitatively confirmed by GC-MS-SIM.
Except for the presence of lindane in 3 samples, no other targeted organochlorine pesticide (i.e., α-BHC, heptachlor, aldrin, heptachlor epoxide, fipronil, endosulfan I, α-chlordane, p,p′-DDE, o,p′-DDD, p,p′-DDD, endrin, and methoxychlor) was detected in any of the raw honey samples analyzed. Although the usage of organochlorine pesticides has been curtailed in India from last two decades, but the results obtained in the present study could be expected, because lindane has been extensively used in agricultural and associated practices before its recent ban in the year 2013.
The results obtained in the present investigation are contrary to the earlier studies conducted on honey and other apicultural matrices, where organochlorines dominated the contaminant lists followed by apicultural pesticides and organophosphates (Erdoğrul 2007; Yavuz et al. 2010; Singh and Venkataramegowda 2015). In the present study, the occurrence of organophosphorus pesticides in honey samples points out towards the change in tendency of farmers towards pesticide application during the last few years. The obtained analytical results suggest the shift in the pattern of pesticide usage from organochlorines to organophosphates and/or synthetic pyrethroids. Various studies conducted on agricultural soils (Kumari et al. 2008), surface and ground water (Lari et al. 2014), vegetables (Ananda and Somashekar 2012; Chandra et al. 2014), fruits (Harinathareddy et al. 2014), milk and butter (Bedi et al. 2016), fish (Ramesh and Selvanayagam 2015) etc. have also showed contamination of different types of organophosphates throughout India. Further, to substantiate these findings, the critical review of data available from India also revealed that during the last 6 years, i.e., 2010–2011 to 2015–2016, the overall indigenous chemical pesticide’s demand was remarkably higher for insecticides with organophosphates accounting for the major share of the insecticides (65%) trailed by carbamates (13%), pyrethroids (9.5%), organochlorines (7.5%), and other newer formulations (5%) (Anonymous 2016).
Since, honey samples were collected directly from the beehives colonized by the bees foraging in nearby known floral sources; the outcome of this study suggested the pesticide usage on those floral sources and subsequently their detection in honey samples. The outcome of present study revealed that 66.7% of cotton honey (4/6), 33.3% of sunflower honey (2/6), and 25% of mustard honey (6/24) samples contained pesticide residues. The mean concentration values as high as 303.2 ng g−1 for monocrotophos and 225.5 ng g−1 for dichlorvos were observed among positive cotton honey samples. Similarly, 2 samples of sunflower honey individually contained monocrotophos and dichlorvos with concentrations of 237.7 ng g−1 and 82.9 ng g−1, respectively. All the 6 positive mustard honey samples were also found to be contaminated with dichlorvos, permethrin, ethion, and profenofos residues with the mean concentrations of 155.8, 34.7, 26.8, and 18.6 ng g−1, respectively (Table 3). These findings could probably be explained by the fact that, currently, among the various groups of pesticides that are being used in India, organophosphorus pesticides form the major and most widely used group (Kumar et al. 2016). These pesticides are used extensively to control broad spectrum of pests affecting variety of crops such as cotton, mustards, sunflower, paddy, wheat, sugarcane, and vegetables (Bhat et al. 2012). Hence, as a consequence of their application in agriculture, the presence of pesticide residues on such floras as well as in different compartments of the environment, such as soil, water, and air, is obvious, which may result in transfer of these harmful residues through multifarious routes to honey and finally to consumers.
Altogether, 33.3% (12/36) of the samples belonging to 3 floral sources, i.e., cotton, sunflower, and mustard, were found positive to at least one pesticide, whereas, only 13.5% (7/52) honey samples originating from other floral sources like eucalyptus (3/16), rosewood (1/7), Egyptian clover (1/8), multifloral (1/17), and coriander (1/4) were found to contain pesticide residues. Twelve samples comprising of jujube (7) and litchi (5) honey were found to be free from targeted contaminants. These variations in contamination frequency of honey from different floral origins may be due to the fact that pesticides are generally not applied on floral sources like eucalyptus, rosewood, or their use on Egyptian clover, and coriander-like floral species is almost negligible in comparison to their application on cash crops like cotton, mustard, and sunflower. Therefore, it might be safe to assume that in most of the cases, honey gets contaminated either due to extensive application of pesticides in the agricultural areas surrounding beehives or due to environmental contamination, and not by the beekeepers’ practices resulting in unavoidable presence of these toxic substances in honey originating especially from such floral sources. This is in concordance with the findings of other investigators who observed that honey from natural vegetation is less frequently contaminated as compared to the honey originating from blossoms of agricultural cash crops like mustard, cotton, and sunflower (Choudhary and Sharma 2008; Deka et al. 2004; Tiwari et al. 2009).
There is no homogeneity on MRLs as different national regulations have established their own maximum residual limits for presence of pesticides in honey. In the absence of MRLs set for honey in India, the MRLs established by Export Inspection Council of India for the honey meant for export to EU and European Union set MRLs were employed and where no MRL existed, it was presumed at 10 ng g−1 which is the default MRL for pesticides with no specific value set as recommended in European Commission regulation No. 396/2005 (EC 2005). The results of the present study indicated that 18% of the honey samples had pesticide residues above MRLs but the number could probably be more, if higher sensitive instrumentation has been used. Moreover, due to stringent food safety and quality standards followed by the European food agencies, the quantification limits comparable with the MRLs established by EU could not be attained for all pesticides. Therefore, further studies employing sensitive analytical methods such as tandem mass spectrometry may prove to be more advantageous for pesticide determination in honey. Further, all the positive samples represented; raw, unprocessed, and unblended honey taken directly from the beehives. These types of honeys from various floral origins have different sensorial and physicochemical characteristics. So, in order to bring uniformity, different raw honeys are usually blended and processed. Hence, risk assessment studies are required to determine potential human health risks associated with consumption of raw honeys containing pesticide residues. But, undoubtedly, such raw honeys act as excellent indicators to mirror the environmental contaminations.
Human health risk assessments
The assessment of health risks associated with consumption of honey containing pesticide residues was evaluated by considering the residue analysis results obtained in the present study and annual honey intake per person in India (70 g/person/year). Hazard index (HI) model was used to assess toxicological significance of human exposure to the pesticide residues detected in honey wherein the EDIs calculated for each pesticide was compared with their corresponding ADIs established by WHO/FAO organizations (WHO and FAO 2016), as shown in Table 4.
The EDIs of the detected pesticides for both adults (body weight 60 kg) and children (body weight 10 kg) were found to be lower than the ADIs, i.e., hazard index less than unity, which indicates that consumers are adequately protected from consumption of honey at current levels of residues and per capita availability of honey. Although, the per cent contribution of total dietary intake of honey to ADI (% ADI) was less than 1%, but keeping in view, the levels of pesticides and customary feeding of honey to the infants in India, precautionary measures should always be taken considering the floral origins and cumulative exposures of these chemicals in the foreseeable future. Further, it is emphasized that the estimated daily intakes of detected residues were calculated only on the basis of dietary consumption of honey which is merely 70 g/person/year in India (Lalhmangaihi and Laha 2017) and did not include the other major commodities of daily food basket such as grains, pulses, fruits, vegetables, oils, milk, meat, and eggs along with drinking water. Assessing the real-life chronic exposure to mixture of pesticides with possible additive or synergistic effects has been an enduring challenge for ecotoxicological studies and environmental health research, which requires an in-depth research (Eggen et al. 2004; Monosson 2005). Some of the reports have indicated that pesticides in combination may lead to more severe impact on human health when compared to their individual effects (Kortenkamp 2007; Laetz et al. 2009; Singh et al. 2017). Therefore, further monitoring studies involving as many as possible food commodities together with occupational exposures should be performed to ensure overall food safety and protect public health.
Conclusion
The optimized and validated multiresidue method was applied to determine pesticide residues in raw honey sample of various floral origins. The method proved to be simple, cost-effective, and expeditious. In absence of apicultural applications of pesticides, the detection of their residues in honeys originating from different flora sources suggested that the source of contamination could factually be the ambient environment (nectar sources, water, soil, air, etc.). This earnestly affirms that beehive products like honey can be used as sentinel to monitor contamination level of the environment surrounding beehives. Toxicological risk associated with consumption of honey was found to be minimal, but residue levels reported here may prove to be detrimental for honey bee colonies which need further investigations. Such results are not underestimations and rather they emphasize the potential risk posed by pesticides for the environment as well as for the humans. Therefore, precautionary measures should always be taken considering the customary feeding of honey to the infants and possible overall exposures to these chemicals from other major food commodities in the foreseeable future. Therefore, in an endeavor to assess the conceivable human health risks, continuous monitoring studies involving as many as possible food commodities together with occupational exposures should be performed to ensure overall food safety and protect public health. Moreover, this could also present an opportunity for the food safety organizations and other public health agencies to be proactive in preventing incidents caused by consumption of contaminated honey.
References
Abou-Shaara HF (2014) The foraging behaviour of honey bees, Apis mellifera: a review. Vet Med 59:1): 1–1):10
Aliferis KA, Tarantilis PA, Harizanis PC, Alissandrakis E (2010) Botanical discrimination and classification of honey samples applying gas chromatography/mass spectrometry fingerprinting of headspace volatile compounds. Food Chem 121(3):856–862. https://doi.org/10.1016/j.foodchem.2009.12.098
Ananda GSR, Somashekar RK (2012) Evaluation of pesticide residues in farmgate samples of vegetables in Karnataka, India. Bull Environ Contam Toxicol 89(3):626–632
Anastassiades M, Lehotay SJ, Tajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Anonymous (2016) State-wise estimated demand of chemical pesticides during 2011-12 to 2015-16 & projected demand for 2016-17. Directorate of plant protection, quarantine & storage, Government of India. Available at: http://ppqs.gov.in/PMD%20Old.htm. Accessed 3 Feb 2017
AOAC (Association of Official Analytical Chemist) (2007) AOAC official method 2007.01: pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate. AOAC Int, Gaithersburg Available at: http://www.weber.hu/PDFs/QuEChERS/AOAC_2007_01.pdf. Accessed 8 Oct 2016
Balayiannis G, Balayiannis P (2008) Bee honey as an environmental bioindicator of pesticides’ occurrence in six agricultural areas of Greece. Arch Environ Contam Toxicol 55(3):462–470. https://doi.org/10.1007/s00244-007-9126-x
Barakat AMA, Badawy H, Salama E, Attallah E, Maatook G (2007) Simple and rapid method of analysis for determination of pesticide residues in honey using dispersive solid phase extraction and GC determination. J Food Agric Environ 5(2):97–100
Barganska Ż, Ślebioda M, Namieśnik J (2014) Determination of pesticide residues in honeybees using modified QUEChERS sample work-up and liquid chromatography-tandem mass spectrometry. Molecules 19(3):2911–2924. https://doi.org/10.3390/molecules19032911
Bedi JS, Gill JPS, Aulakh RS, Kaur P (2016) Occurrence and spatial distribution of pesticide residues in butter and ghee (clarified butter fat) in Punjab (India). Environ Monit Assess 188(2):100. https://doi.org/10.1007/s10661-016-5115-8
Bhat BA, Bhat IA, Vishwakarma S, Verma A, Saxena GA (2012) Comparative study on the toxicity of a synthetic pesticide, dichlorvos and a neem based pesticide, neem-on to Labeo rohita (Hamilton). Curr World Environ 7(1):157–161
Blasco C, Fernández M, Pena A, Lino C, Silveira MI, Font G, Picó Y (2003) Assessment of pesticide residues in honey samples from Portugal and Spain. J Agric Food Chem 51(27):8132–8138. https://doi.org/10.1021/jf034870m
CAC (Codex Alimentarius Commission) (2001) Revised codex standard for honey. Codex Alimentarius 12-1981:1–7
Chahal KK, Kumar A, Kataria D, Singh T, Chadha BK (2016) Dynamics of demand and consumption of pesticides in agriculture-an overview. Indian J Econ Dev 12(1):171–177
Chandra S, Kumar M, Mahindrakar AN, Shinde LP (2014) Effect of washing on residues of chlorpyrifos and monocrotophos in vegetables. Int J Adv Res 2:744–750
Choudhary A, Sharma DC (2008) Pesticide residues in honey samples from Himachal Pradesh (India). Bull Environ Contam Toxicol 80(5):417–422. https://doi.org/10.1007/s00128-008-9426-5
Christodoulou DL, Kanari P, Kourouzidou O, Constantinou M, Hadjiloizou P, Kika K, Constantinou P (2015) Pesticide residues analysis in honey using ethyl acetate extraction method: validation and pilot survey in real samples. Int J Environ Anal Chem 95(10):894–910. https://doi.org/10.1080/03067319.2015.1070408
De Pinho GP, Neves AA, de Queiroz MELR, Silvério FO (2010) Optimization of the liquid–liquid extraction method and low temperature purification (LLE–LTP) for pesticide residue analysis in honey samples by gas chromatography. Food Control 21(10):1307–1311. https://doi.org/10.1016/j.foodcont.2010.03.006
Debayle D, Dessalces G, Grenier-Loustalot MF (2008) Multi-residue analysis of traces of pesticides and antibiotics in honey by HPLC-MS-MS. Anal Bioanal Chem 391(3):1011–1020. https://doi.org/10.1007/s00216-008-2003-2
Deka SC, Barman N, Baruah AALH (2004) Monitoring of pesticide residues in honey samples of Jorhat district, Assam, (India). Pestic Res J 16(1):83–85
EC (European Commission) (2005) Regulation (EC) No 396/2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending council directive 91/414/EEC. Official Journal of European Communities L 70/1-L 70/16. Available at: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0396. Accesses 13 Aug 2017
EC (European Commission) (2016) Document number SANTE/11945/2015. Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. Available at: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_11945.pdf. Accessed 13 Aug 2017
Eggen RIL, Behra R, Burkhardt-Holm P, Escher BI, Schweigert N (2004) Challenges in ecotoxicology. Environ Sci Technol 38(3):59A–64A
EIC (Export Inspection Council) (2013) EIC’s residue monitoring plan (RMP) 2013–14.EIC/RMP/HN/2013–14,Rev 0. Available at: http://www.eicindia.gov.in/Services/Pre-Compliance/RMP-2013-14-HONEY.pdf Accessed 27 July 2018
Erdoğrul Ö (2007) Levels of selected pesticides in honey samples from Kahramanmaraş, Turkey. Food Control 18(7):866–871. https://doi.org/10.1016/j.foodcont.2006.05.001
Fernandez M, Picó Y, Mañes J (2002) Analytical methods for pesticide residue determination in bee products. J Food Prot 65(9):1502–1511. https://doi.org/10.4315/0362-028X-65.9.1502
Harinathareddy A, Prasad NBL, Devi LK (2014) Pesticide residues in vegetable and fruit samples from Andhra Pradesh, India. J Biol Chem Research 31:1005–1015
ICH (International Conference on Harmonisation) (2005) Harmonised tripartite guideline validation of analytical procedures: text and methodology Q2 (R1)
Irungu J, Raina S, Torto B (2016) Determination of pesticide residues in honey: a preliminary study from two of Africa’s largest honey producers. Int J Food Contam 3(1):14. https://doi.org/10.1186/s40550-016-0036-4
Kortenkamp A (2007) Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 115:98–105. https://doi.org/10.1289/ehp.9357
Kujawski MW, Barganska Z, Marciniak K, Miedzianowska E, Kujawski JK, Slebioda M, Namiesnik J (2014) Determining pesticide contamination in honey by LC-ESI-MS/MS- comparison of pesticide recoveries of two liquid-liquid extraction based approaches. LWT Food Sci Technol 56:517–523. https://doi.org/10.1016/j.lwt.2013.11.024
Kumar A, Gill JPS, Bedi JS, Manav M, Ansari MJ, Walia GS (2018) Sensorial and physicochemical analysis of Indian honeys for assessment of quality and floral origins. Food Res Int 108:571–583. https://doi.org/10.1016/j.foodres.2018.04.005
Kumar S, Kaushik G, Villarreal-Chiu JF (2016) Scenario of organophosphate pollution and toxicity in India: a review. Environ Sci Pollut Res 23(10):9480–9491. https://doi.org/10.1007/s11356-016-6294-0
Kumari B, Madan VK, Kathpal TS (2008) Status of insecticide contamination of soil and water in Haryana. India Environ Monit Assess 136(1):239–244. https://doi.org/10.1007/s10661-007-9679-1
Laetz CA, Baldwin DH, Collier TK, Hebert V, Stark JD, Scholz NL (2009) The synergistic toxicity of pesticide mixtures: implications for risk assessment and the conservation of endangered pacific salmon. Environ Health Persp 117(3):348–353
Lalhmangaihi R, Laha RC (2017) Beekeeping: a nature eco-friendly business. In: Lalchhandama K (ed) Science and technology for shaping future of Mizorum-Proceedings of the Mizorum Science Congress. Allied Publishers, New Delhi, pp 35–39 ISBN: 976-93-85926-49.5
Lari SZ, Khan NA, Gandhi KN, Meshram TS, Thacker NP (2014) Comparison of pesticide residues in surface water and ground water of agriculture intensive areas. J Environ Health Sci Eng 12(1):11
Malhat FM, Haggag MN, Loutfy NM, Osman MAM, Ahmed MT (2015) Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 120:457–461. https://doi.org/10.1016/j.chemosphere.2014.08.032
Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A (2017) A worldwide survey of neonicotinoids in honey. Science 358:109–111
Mittal S, Kaur G, Vishwakarma GS (2014) Effects of environmental pesticides on the health of rural communities in the Malwa region of Punjab, India: a review. Hum Ecol Risk Assess 20(2):366–387. https://doi.org/10.1080/10807039.2013.788972
Monosson E (2005) Chemical mixtures: considering the evolution of toxicology and chemical assessment. Environ Health Perspect 113:383–390
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, vanEngelsdorp D, Pettis JS (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5(3):e9754. https://doi.org/10.1371/journal.pone.0009754
Naccari C, Macaluso A, Giangrosso G, Naccari F, Ferrantelli V (2014) Risk assessment of heavy metals and pesticides in honey from Sicily (Italy). J Food Res 3(2):107. https://doi.org/10.5539/jfr.v3n2p107
Nadaf HA, Yadav GS, Kumari B (2015) Validation and monitoring of pesticide residues in honey using QuEChERS and gas chromatographic analysis. J Apic Res 54(3):260–266. https://doi.org/10.1080/00218839.2016.1143693
NBB (National Bee Board) (2017) Minutes of meeting to discuss details for developing data base for beekeeping and honey production in the country. Department of Agriculture, Cooperation and Farmers Welfare (DAC & FW), Ministry of Agriculture and Farmers Welfare, Govt of India vide F. No. 20-5/2008-NBB. Available at: http://nbb.gov.in/pdf/15022017.pdf. Accessed 2 Sept 2017
Panseri S, Catalano A, Giorgi A, Arioli F, Procopio A, Britti D, Chiesa LM (2014) Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control 38:150–156. https://doi.org/10.1016/j.foodcont.2013.10.024
Porrini C, Gloria Sabatini A, Girotti S, Ghini S, Medrzycki P, Grillenzoni F, Bortolotti L, Gattavecchia E, Celli G (2003) Honey bees and bee products as monitors of the environmental contamination. Apiacta 38:63–70
Przybyowski P, Wilczyńska A (2001) Honey as an environmental marker. Food Chem 74(3):289–291. https://doi.org/10.1016/S0308-8146(01)00153-4
Ramesh BK, Selvanayagam M (2015) Pesticides pollution in water, sediment and fishes of Kolavai lake in Chengalpet, Tamil Nadu, India. Int J Chemical Concepts 1:9–14
Rather IA, Koh WY, Paek WK, Lim J (2017) The sources of chemical contaminants in food and their health implications. Front Pharmacol 8:830. https://doi.org/10.3389/fphar.2017.00830
Rissato SR, Galhiane MS, de Almeida MV, Gerenutti M, Apon BM (2007) Multiresidue determination of pesticides in honey samples by gas chromatography–mass spectrometry and application in environmental contamination. Food Chem 101(4):1719–1726. https://doi.org/10.1016/j.foodchem.2005.10.034
Singh N, Gupta VK, Kumar A, Sharma B (2017) Synergistic effects of heavy metals and pesticides in living systems. Front Chem 5:70. https://doi.org/10.3389/fchem.2017.00070
Singh C, Venkataramegowda S (2015) Organochlorine residues in bees and hive products in Karnataka. Int J Sci Res 4(5):1954–1960
Tiwari R, Karnatak AK, Kale SP (2009) Pesticide residue analysis of fresh and stored honey of Tarai region of Uttarakhand (India) using multi-residue analysis method. Pant J Res 7(1):29–32
Tsipi D, Triantafyllou M, Hiskia A (1999) Determination of organochlorine pesticide residues in honey, applying solid phase extraction with RP-C18 material. Analyst 124(4):473–475
Vilca F, Correia-Oliveira M, Marques R, Leal P, Monteiro S, Zanardi O, Carlos Marchini L, Luiz Tornisielo V (2012) Quechers approach for the determination of seven pesticide residues in Brazilian honey samples using GC-μECD. J Food Sci Eng 2:163–169
WHO and FAO (2016) Pesticide residues in food 2016. Report of the joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 229. Available at: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report2016/JMPR_2016_Report_full.pdf. Accsessed 13 Sept 2017
Wiest L, Buleté A, Giroud B, Fratta C, Amic S, Lambert O, Pouliquen H, Arnaudguilhem C (2011) Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J Chromatogr A 1218(34):5743–5756. https://doi.org/10.1016/j.chroma.2011.06.079
Yavuz H, Guler GO, Aktumsek A, Cakmak YS, Ozparlak H (2010) Determination of some organochlorine pesticide residues in honeys from Konya, Turkey. Environ Monit Assess 168:277–283. https://doi.org/10.1007/s10661-009-1111-6
Acknowledgments
Authors are thankful to Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab (India) for providing the funds to carry out the present research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 308 kb)
Rights and permissions
About this article
Cite this article
Kumar, A., Gill, J.P.S., Bedi, J.S. et al. Pesticide residues in Indian raw honeys, an indicator of environmental pollution. Environ Sci Pollut Res 25, 34005–34016 (2018). https://doi.org/10.1007/s11356-018-3312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3312-4