Abstract
Contamination of toxic metals in P. viridis mussels has been prevalently reported; hence, health risk assessment for consuming this aquaculture product as well as the surrounding surface seawater at its harvesting sites appears relevant. Since Kampung Pasir Puteh, Pasir Gudang is the major harvesting site in Malaysia, and because the last heavy metal assessment was done in 2009, its current status remains unclear. Herein, flame atomic absorption spectrometry and flow injection mercury/hydride system were used to determine the concentrations of Pb, Cd, Cu and total Hg in P. viridis mussels and surface seawater (January–March 2015), respectively. Significantly higher concentrations of these metals were found in P. viridis mussels (p < 0.05) than that of surface seawater samples. The concentrations for Pb (4.27–6.55 μg/g) and Cd (1.55–2.21 μg/g) in P. viridis mussels exceeded the maximum permitted proportion prescribed by the Malaysian law. The concentrations of all metals in surface seawater also violated the Malaysia Marine Water Quality Criteria and Standards. Significant (p < 0.05) and high strength of association (r = 0.787) observed between Pb concentration in P. viridis mussel with the surface seawater indicates its possible application for inferring Pb concentrations in the mussel. Since both the calculated target hazard quotient and hazard index for Pb and Cd exceeded 1, the possible detrimental health impacts on human for consuming P. viridis mussels from this rearing site cannot be ignored. Hence, promoting continuous monitoring programmes and developing efficient toxic metal removal techniques prior to entering the market are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perna viridis (family: Mytilidae) is an economically important coastal bivalve mollusc, known as the Asian green mussel, Philippine green mussel or green-lipped mussel in certain parts of the world. The mussels are commonplace along the coastal marine waters of the Indo-Pacific region (Gosling 2003), as well as at several portions of coastal areas of Peninsular Malaysia (Ismail et al. 2000). Since P. viridis mussels contain about 60% of protein for every 100 g of its dry weight (Choo and Ng 1990) with substantial amounts of vitamins and trace elements (Gopalakrishnan and Vijayavel 2009), they become an important source of nutrients for human (Yap et al. 2004a). Alarmingly, toxic metals from natural and anthropogenic sources that continuously enter aquatic ecosystem would increase the possibility of posing serious threat towards aquatic organisms due to their toxicity, long-term persistence, bioaccumulation and biomagnification in the food chain (Papagiannis et al. 2004; Chua et al. 2014; Edbeib et al. 2016, 2017). Bivalves like P. viridis have the ability to accumulate toxic metals in their soft tissues to relatively higher levels than that of their environment without exhibiting any detrimental effects (Wagner and Boman 2004). Therefore, the use of P. viridis as a biomonitoring agent for heavy metal pollutions within the coastal environment has also been suggested (Nicholson and Szefer 2003; Arockia et al. 2012; Hadibarata et al. 2012), attributable to its cost effectiveness, as well as reliability (Yap et al. 2006).
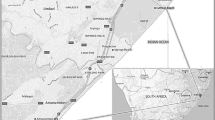
Interestingly, while Johor has been the largest producer for P. viridis mussels in Malaysia (Department of Fisheries 2013), its major harvesting area (i.e. Kampung Pasir Puteh) is located adjacent to the Pasir Gudang Seaport and industrial zones (Yap et al. 2004b). Kampung Pasir Puteh has been exposed to petrochemical and its related industries, as well as shipping, land reclamation and urbanisation (Yap et al. 2004b). Pertinently, the cumulative rearing area for this important aquaculture species at Kampung Pasir Puteh has soared tremendously from 133,501 m2 (in 2009) to 287,248 m2 (in 2015), a twofold increase over the span of 6 years (Department of Fisheries Malaysia 2015). In addition, the million freight weight tonnes (FWT) managed by the Pasir Gudang Seaport has increased from 23.9 million FWT in 2009 to 32.9 million FWT for 2015, a staggering increment of 38% of commodity load over 6 years (MMC Corporation Berhad 2015). Since the strait that borders Malaysia and Singapore is narrow and because the rearing site is limited within the Malaysian border alone, the ever-increasing rearing area has grown closer to the bustling Pasir Gudang Seaport and JSEDC Industrial Estate (Fig. 1a–c). Hence, the possibility of increasing contamination by toxic metals from the seaport and industrial activities into the surrounding seawater where the mussels are reared could not be ruled out.
Since ecotoxicological studies performed on P. viridis mussel at Kampung Pasir Puteh between 1998 to 2009 (Yap et al. 2002, 2003, 2004a, b, 2005, 2006; Ng et al. 2013) reported substantial amounts of lead (Pb) and cadmium (Cd), and because similar analysis on such mussels sampled beyond 2009 remains unavailable, this present study that evaluated such aspect acquires considerable pertinence. Considering the adjacent increasing industrial activities, especially those related to mercury (Hg), as well as public awareness on toxicities of Hg associated with seafood consumption (Hajeb et al. 2012; Dong et al. 2015), its presence in P. viridis mussel was also assessed here. Similarly, Cu contamination may originate from industrial effluents, municipal waste outfalls and sediment dredging as well as from leaching of antifouling paints of ships, entering marine and estuary waters. Although Cu is considered as an essential element for aquatic environment, its toxicity can occur when the concentration exceeds certain ranges (Baltas et al. 2016). To date, only the Indian jurisdiction (Food Safety and Standards Authority of India, FSSAI 2011) has provided the maximum permitted proportion for Cu in mussels. Interestingly, while investigation on the physiological response P. viridis when exposed to Cu (in view of its potential use as cytological marker of pollution) has been reported (Nicholson 2001), the health risk assessment of Cu in the mussel for inferring potential human toxicity following its ingestion remains unreported.
Acute exposure to high levels of toxic metals may result in brain damage, paralysis, anaemia and disruption of gastrointestinal system. On the other hand, chronic exposure of such contaminants has been associated with damages of the kidneys, as well as reproductive, immune, nervous, respiratory and cardiovascular systems (Cope et al. 2004; Flanagan et al. 2008). In addition, miscarriage, stillbirth, premature birth and low birth weight, as well as malformations of foetus among pregnant women exposed to toxic metals, have been reported (World Health Organisation, WHO 2016). In view of such toxicological risks towards public health, continuous assessment of toxic metals in seafood such as P. viridis mussel merits serious consideration. In this context, determining if the concentrations of such contaminants in P. viridis mussels and surrounding seawater samples are within the maximum permitted proportions of metal contaminants prescribed by the Malaysian and international laws proves relevance.
Hence, this cross-sectional research that assessed the concentrations of Pb, Cd, Cu and total Hg in the harvested P. viridis mussels, as well as its surrounding seawater samples, from different sampling sites within Kampung Pasir Puteh harvesting area during January–March 2015 acquires significance. The findings may be beneficial for the environmental and health authorities for formulating suitable intervention programmes for managing this significant issue of public interest for benefiting the community at large.
Materials and methods
Standards and reagents
The reagents used in this study were analytical-grade stannous chloride dihydrate and potassium permanganate purchased from Merck (Darmstadt, Germany) and used without further purification. Standards for Pb, Cd and Cu in 2% HNO3 and total Hg in 10% HNO3 were both purchased from Merck (Germany) (1000 mg/L for each standard). All standards and sample solutions were prepared using deionised water produced by Sartorius arium® pro ultrapure water system (Gottingen, Germany). Concentrated acids, i.e. HNO3 (65%), H2SO4 (95–97%) and HCl (37%), as well as H2O2 (35%), for sample digestion were acquired from QRëC (Malaysia).
Sampling and sample preparation
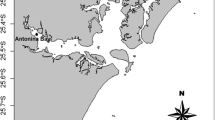
In three replicates, P. viridis mussels and surrounding surface seawater samples were collected from the five different harvesting sites (labelled as S1–S5) of Kampung Pasir Puteh, Pasir Gudang, Johor, Malaysia (1°25′–26′N, 103°55′–57′E) (Fig. 1a–c) during January, February and March 2015. Each P. viridis sample (a pooled of 15 mature mussels) was collected from each harvesting site, placed in a cold box that contained ice cubes, transported to the laboratory and kept at − 20 °C prior to analysis. Using the same cold box, the surface seawater samples (in the different acid-washed, 10% HNO3, polyethylene bottles) collected from all the harvesting sites were acidified using concentrated HNO3 (67%, 5 mL). The samples were then transported to the laboratory and kept at 4 °C before analysis.
To prevent contamination from sand and dirt from the external surface of mussel, the shell surface was cleaned using running tap water and a brush. Then, the mussel was thawed at room temperature (about 26 °C) on a clean tissue paper with the posterior part facing downwards for draining the water content in the shell. Upon completion, the flesh of each mussel was removed and dried at 60 °C for 72 h to a constant dry weight. All the 15 P. viridis mussels that formed a sample were then homogenised by grinding with a porcelain mortar and pestle to a fine powdery form and stored in an airtight plastic container at room temperature, prior to analysis. Considering better homogeneity of samples as well as the availability of suitable guidelines for inferring toxicity, dry weight of P. viridis mussel was used in this research. All P. viridis mussels and surface seawater samples collected during the three different sampling intervals (January, February and March 2015) were analysed quantitatively for Pb, Cd, Cu and total Hg.
For analysing Pb, Cd and Cu using flame atomic absorption spectrometry (FAAS), the P. viridis sample was acid-digested following the method described by previous researchers (Rahman et al. 2012) with several modifications. The modifications introduced here included the use of hotplate instead of water bath, initial heating temperature at 40 °C for 1 h, as well as longer duration of digestion (from 20 min to 2 h). A sample (0.5 g dry weight) of powdered mussel was placed in a conical flask through a filter funnel, followed by the addition of concentrated H2SO4 (2 mL) and HNO3 (4 mL). After the initial vigorous reaction subsided, the mixture was heated on a hot plate for 1 h at 40 °C. Upon completion, H2O2 was added drop wise into the mixture until a clear solution was obtained. For total Hg analysis using flow injection mercury/hydride system (FI-MHS), the P. viridis sample was acid digested following the method described by Chahid et al. (2014). A sample (0.5 g dry weight) was digested using a mixture that consisted of concentrated HNO3 (4 mL), H2SO4 (2 mL) and HCl (1 mL). The mixture was heated at 80 °C until it was fully digested. Upon cooling to room temperature, both mixtures were filtered using Whatman No. 1 filter papers into two different 50-mL volumetric flasks, subsequently diluted with deionised water and subjected to analysis with FAAS and FI-MHS, respectively.
Surface seawater sample was digested according to the standard method (U.S. EPA 3005A) prescribed by the US Environmental Protection Agency (US EPA 1992). Fifty millilitres of well-mixed sample was transferred into a 100-mL beaker, followed by the addition of concentrated HNO3 (1 mL) and HCl (2.5 mL). The sample was heated on a hot plate at about 90 °C until the volume was reduced to approximately 10 mL. The sample was allowed to cool to room temperature, filtered into a 50-mL volumetric flask and made up to the mark with deionised water. For total Hg determination, the surface seawater sample was filtered using a Whatman No. 1 filter paper and analysed directly without any pre-treatment.
Analysis of toxic metals with FAAS and FI-MHS
Air-acetylene FAAS (Perkin Elmer PinAAcle 900 T, USA) was used for quantifying the concentrations of Pb, Cd and Cu in all P. viridis and surface seawater samples, while total Hg was determined using FI-MHS (Perkin Elmer FIAS 100, U.S.A.). The conditions for FAAS were air flow 10 L/min, acetylene flow 4 L/min and wavelengths at 283.3, 228.8 and 324.8 nm for Pb, Cd and Cu, respectively, with a slid width of 0.7 nm. The conditions for FI-MHS were argon flow 90 mL/min, 20 s integration time, wavelength at 253.7 nm and a slid width of 0.7 nm.
The working standards for Pb (0–5 mg/L), Cd (0–1 mg/L), Cu (0–1 mg/L) and total Hg (0–10 μg/L) were prepared by diluting the concentrated stock solutions (1000 mg/L) with suitable amounts of deionised water. As for total Hg standard, 3% HCl solution was used. For total Hg analysis, a reducing agent (1.1% SnCl2) was prepared by placing 11 g of SnCl2.2H2O into a 50-mL beaker. Then, 10 mL of deionised water and 3 mL of concentrated HCl were added into the beaker, and the solution was left to stand for 10 min for complete dissolution. The solution was transferred into a 1000-mL volumetric flask, diluted up to the mark using deionised water and then inverted and shaken well. A carrier solution (3% HCl) for the FI-MHS analysis was prepared by transferring 30 mL of concentrated HCl into a 1000-mL volumetric flask. The solution was diluted by adding deionised water. A KMNO4 solution (5%) was prepared by placing 1.25 g of KMNO4 into a 25-mL volumetric flask. The compound was then diluted using deionised water. The calibration standard solutions of total Hg and blank were stabilised by adding four drops of a 5% KMNO4 solution into 100 mL of solution. The concentrations of toxic metals in P. viridis mussels were expressed in μg/g dry and wet weights, while mg/L was used for the surface seawater samples. For enabling appropriate comparison with the concentrations of toxic metals in molluscs as prescribed by different international standards, the dry weight was converted to wet weight using a conversion factor of 0.17 recommended by previous researchers (Yap et al. 2004a).
Analytical method validation
This research utilised the analytical methods for analysing Pb, Cd and Cu (Rahman et al. 2012) as well as total Hg (Chahid et al. 2014) in seafood described by previous researchers, and the methods were partially validated once again here. Calibration curves for Pb (0, 1, 2, 3, 4 and 5 mg/L), Cd (0, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/L), Cu (0, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/L) and total Hg (0, 2, 4, 6, 8 and 10 μg/L) were prepared by spiking the samples with known amount of analyte via serial dilution. Using the coefficient of determination (R2), the calibration curves were constructed using the absorbance values versus the concentrations of analyte added. The obtained calibration curves were accepted only when (1) the coefficient of determination (R2) was > 0.995 and (2) the relative standard deviation (RSD) was ≤ 20% (US EPA 2010). The limit of detection (LOD) and limit of quantitation (LOQ) for all the heavy metals were mathematically determined from the standard calibration curves using the equations (Sanagi et al. 2009) described below:
Using six replicates, the recovery of Pb, Cd, Cu and total Hg in P. viridis mussel and surface seawater was investigated. Standard solutions containing Pb, Cd, Cu and total Hg were individually used to spike samples of P. viridis mussel (0.5 g) and surface seawater (50 mL). After acid digestion, the spiked sample in a calibrated volumetric flask was further added with deionised water to the mark, prior to analysis. The percentage recovery of each of the four heavy metals analysed was calculated using Eq. 3 (Vipulanandan 2004) described below:
where AT refers to the total amount measured in the spike sample, Ai represents the amount measured in the unspiked sample and Aadded is the amount of known analyte (standard) added to the sample.
Estimation of daily intake of toxic metals and health risk assessment
The adult estimated daily intake (EDI) for Pb, Cd, Cu and total Hg through ingestion of P. viridis mussel was calculated following the equation suggested by Song et al. (2009) (Eq. 4), and compared with the provisional tolerable daily intakes (PTDI) suggested by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1999, 2003) and the US EPA (2011):
EDI refers to an adult daily intake of a heavy metal via consumption of P. viridis mussels (in μg/g/day), Cmetal is the concentration of a heavy metal in such mussels (in μg/g, wet weight), Wmussels being the daily consumption of mussels (in g/day), and Bw represents the body weight of an adult (in kg).
Because specific data on daily consumption of mussels remains unavailable for Malaysia, Wmussels was estimated from the data reported by the US EPA (2000), i.e. 17.5 g. In addition, the Malaysian Adult Nutrition Survey reported 63 kg as the average Bw for Malaysians (Azmi et al. 2009). For assessing the risk of Pb, Cd, Cu and total Hg through consumption of P. viridis mussels, the target hazard quotient (THQ) was also calculated based on the US EPA Region III risk-based concentration table (US EPA 2000) (Eq. 5):
While EFr represents the exposure frequency (350 days/year), ED, Wmussels and \( {C}_i^{metal} \) are the exposure duration (70 years), daily consumption of P. viridis mussels (in g/day) and the individual concentration of heavy metal in P. viridis mussels (in μg/g, wet weight), respectively. In addition, RfD refers to the oral reference dose (in μg/g/day) provided by the US EPA (2011), Bw is the average body weight of an adult (63 kg) and ATn being the average exposure time for non-carcinogens (ED × 365 days/year).
Since the RfD value for Pb remains unreported and there is no evidence of a threshold below which a non-harmful intake could be allowed for Pb (USEPA 2004), the THQ for Pb was calculated using the equation suggested by Jović and Stanković (2014) detailed below:
where C represents Pb concentration in mussels (mg/kg w/w) and MRL (maximum residue limit) set by the Regulation (EC) No 1881/2006 (European Communities 2006).
Considering the exposure of multiple toxic metals within the P. viridis mussels to human, the total hazard index (HI) was computed by summing all the calculated THQ values of toxic metals as suggested by Li et al. (2013) (Eq. 7):
while THQi refers to the target hazard quotient of an individual heavy metal, HI is the total hazard index for all the four toxic metals analysed here viz. Pb, Cd, Cu and total Hg with the n value of 4.
Statistical analysis
All data were analysed using the IBM SPSS version 20.0. Kolmogorov–Smirnov and Shapiro-Wilk tests were used to determine the normality of the distribution of data. Because the data were all normally distributed, the one-way ANOVA with Tukey-Kramer post hoc test was used to determine the significant differences among groups (January, February and March 2015). Independent samples t test was used for comparing the concentrations of Pb, Cd, Cu and total Hg between P. viridis mussel with that of surrounding surface seawater. For studying the association between concentrations of Pb, Cd, Cu and total Hg in P. viridis mussel with that of surface seawater samples, Pearson correlation coefficient analysis was utilised. For defining the strength of correlation coefficient, the categorical definition (very high 0.90–1.00; high 0.70–0.89; moderate 0.50–0.69; low 0.26–0.49; little 0.00–0.25) suggested by Munro (2005) was used. Level of significance of 0.05 (α < 0.05) was utilised for determining the significant differences among groups (Munro 2005).
Results and discussion
Analytical method validation
The calibration parameters, LOD and LOQ, as well as percentage recovery, are presented in Table 1. It has been indicated that to accept a calibration curve, the R2 must be greater than 0.995 with RSD of less than or equal to 20% (Man et al. 2006). Therefore, considering that all the calibration curves included in this present research had fulfilled such prescribed criteria, it can be construed that the responses obtained were linear and hence determination of those analytes in the P. viridis mussel and surface seawater samples proved to be appropriate. LOD is the lowest concentration of an analyte determined with signal-to-noise ratio of at least 3:1, while LOQ refers to the lowest concentration of an analyte in a calibration curve with signal to noise ratio of at least 10:1 (Sanagi et al. 2009). Using the data from calibration curves, the LODs for Pb, Cd, Cu and Hg were calculated as 0.140, 0.026, 0.022 and 0.360 μg/L, respectively (Table 1). In addition, the LOQs were 0.460, 0.087, 0.075 and 1.200 μg/L, correspondingly (Table 1). Results also revealed that the percentage recoveries for Pb, Cd, Cu and Hg in P. viridis mussel ranged between 94.0 and 95.9%, 101 and 113%, 97.5 and 102% and 80.0 and 83.0%, respectively (Table 1). While the percentage recovery for Hg in surface seawater was not determined because the sample was analysed directly without any digestion, the percentage recoveries for Pb, Cd and Cu in this matrix ranged between 97.3 and 98.0%, 94.2 and 102% and 99.3 and 102%, respectively (Table 1). Considering that the percentage recoveries for all the analytes analysed here fell well within the acceptable range (75.0–120%) prescribed by previous researchers (AOAC 2002; Ryu et al. 2016), the analytical method used in this present research appears reliable.
Toxic metals in P. viridis mussel and surface seawater at Kampung Pasir Puteh, Pasir Gudang, Johor
Concentrations of four toxic metals (Pb, Cd, Cu and total Hg) that are commonly associated with various adverse effects in human as well as environment in P. viridis mussels were investigated. While high concentrations of Pb, Cd and Cu in the soft tissues of P. viridis mussels collected from Kampung Pasir Puteh, Johor had been reported between 2002 and 2013 (Yap et al. 2002, 2003, 2004a, b, 2005, 2006; Ng et al. 2013), status of contamination beyond 2013 remains unclear. Yap et al. (2016) reanalysed Cd, Cu, Fe, Ni, Pb and Zn concentrations in P. viridis mussels reported by the previous studies conducted in Peninsular Malaysia (between year 2002 and 2009), including those from Kampung Pasir Puteh (10 June 2006, 10 May 2007, 13 May 2008 and 28 August 2009). The authors (Yap et al. 2016) concluded that because the Pb THQ values were high, ‘the Pb levels in some mussel populations could create a health risk problem’ and therefore, ‘the consumption amounts of mussels should be limited for minimizing potential health risks of heavy metals to the HLM consumers’. Because a recent sampling conducted at a nearby P. viridis harvesting site (Kong Kong Laut, about 60 km up north) revealed a moderate level of contamination of Pb and Cu in catfish and P. viridis mussel (Mohamat-Yusuff et al. 2015), investigating the current status at the Kampung Pasir Puteh harvesting site becomes pertinent. This aspect is further exacerbated by the fact that the harvesting sites at Kampung Pasir Puteh are located close to a bustling seaport and industrial areas, possible sources of contaminations (Fig. 1a, c).
In Malaysia, the maximum permitted proportions for Pb, Cd and total Hg for mussels (wet weight) as prescribed by law (Food Act 1983 (Act 281) and Regulations 2013) are 1.5, 2.0 and 0.5 μg/g, respectively. As for the general marine water, the maximum levels for Pb, Cu and total Hg are 8.5, 2.9 and 0.16 μg/L, respectively (Department of Environment Malaysia, DOE 2015). Interestingly, while the maximum permitted proportion for Pb, Cd and Hg in bivalve molluscs have been gazetted by the Malaysian law, the same remains lacking for Cu. The lack of maximum permitted proportion for Cu also prevails in the European Community (EC 2006), Food Standards Australia New Zealand Act (FSANZ 2015) and the US Food and Drug Administration (US FDA 2007). The only available standard, i.e. the Indian jurisdiction, prescribes the maximum limit for Cu in mollusc as 30 μg/g (FSSAI 2011). Taking into account that excessive amount of Cu can still lead to undesired human toxicity (Baltas et al. 2016), and because mussels like P. viridis are consumed routinely, establishing its specific maximum permitted proportion in the Malaysian Food Act appears necessary.
The concentrations of Pb, Cd, Cu and total Hg in P. viridis mussel as well as the surrounding surface seawater samples during January–March 2015 are presented in Table 2. The means concentrations of Pb, Cd, Cu and total Hg (Table 2) in P. viridis mussel (dry weight) during the 3 months of sampling period ranged between 25.10 and 38.60 μg/g, 9.10 and 13.00 μg/g, 11.20 and 13.80 μg/g and 30.10 and 38.90 ng/g, respectively. These concentrations were equivalent to 4.27–6.55 μg/g, 1.55–2.21 μg/g, 1.90–2.35 μg/g and 5.12–6.60 ng/g of Pb, Cd, Cu and total Hg in wet weight of P. viridis mussels, correspondingly. It was observed that the concentration of Pb in P. viridis mussel differed significantly (p < 0.05) among the 3 months of sampling period. While the highest concentrations of Pb in the P. viridis mussel (dry weight) (38.60 ± 2.22 μg/g) was recorded during January, its lowest concentration (25.10 ± 2.11 μg/g) was observed during March (Table 2, p < 0.05). Like Pb, the highest mean concentrations for Cd (13.00 ± 0.20 μg/g), Cu (13.80 ± 2.10 μg/g) and total Hg (38.90 ± 4.20 ng/g) in P. viridis mussel were observed during January (Table 2, p < 0.05). Interestingly, while the lowest mean concentration of Pb in P. viridis mussel was observed during March, the lowest mean concentrations of Cd, Cu and total Hg were all found during February. Notably, the means concentrations of Pb and Cd in P. viridis mussel reported here were greater than the previous studies conducted at the same harvesting site (Yap et al. 2004a, 2005). Such observations indicate the possibility of temporal changes that might have taken place at this important mussel harvesting site, possibly due to more extensive industrial and seaport activities that resulted in severe contamination. Alarmingly, because the ranges of mean concentration of Pb and Cd in P. viridis mussel reported here exceeded the maximum permitted proportions (μg/g wet weight) prescribed by the Malaysian Food Act 1983 (Act 281) and Regulations (2013), its negative impacts on human health could not be ruled out.
In addition, significantly lower mean concentrations of Pb (2.62–3.62 mg/L), Cd (0.72–0.78 mg/L), Cu (0.27–0.38 mg/L) and total Hg (0.21–1.49 μg/L) were observed in the surrounding surface seawater samples when compared with that of P. viridis mussels (Table 2, p < 0.05). Such finding appears consistent with those reported by previous researchers (Hadibarata et al. 2012; Putri et al. 2012; Tewari et al. 2001), attributable to higher bioaccumulation ability of the bivalves (Hadibarata et al. 2012; Thorp 2010). Furthermore, the high bioaccumulation ability of P. viridis mussel towards marine contaminants has resulted in its utilisation as a biomonitoring agent for pollution (Yap et al. 2002; Nicholson and Szefer 2003; Arockia et al. 2012).
As for the surrounding surface seawater sample, the mean concentrations of Pb, Cd and Cu as well as total Hg (Table 2) ranged between 2.62 and 3.62 mg/L, 0.72 and 0.78 mg/L, 0.27 and 0.38 mg/L and 0.21 and 1.49 μg/L, correspondingly. In view of regulating the level of contaminations in marine water areas where seafood for human consumption is applicable, the DOE (2015) via its Malaysia Marine Water Quality Criteria and Standards (MMWQCS) has prescribed that the levels of Pb, Cd, Cu and total Hg shall not exceed 8.5, 3.0, 2.9 and 0.04 μg/L, respectively. Since the levels of Pb, Cd, Cu and total Hg in the surface seawater samples exceeded the maximum levels allowable by the MMWQCS (DOE 2015), the possibility of such water being a source of contamination for seafood (especially P. viridis mussels) for human consumption proves alarming. This observation appears to be consistent with the moderate Marine Water Quality Index (MWQI) for the coastal water around Pasir Gudang seaport categorised in the Malaysia Environmental Quality Report (DOE 2015).
While the highest concentration of Pb in surface seawater samples was recorded during January (3.62 ± 0.21 mg/L), the lowest concentration of such analyte was observed during March (2.62 ± 0.26 mg/L) (Table 2, p < 0.05). Moreover, the highest mean concentrations of Cd and Cu and total Hg were all observed in the surrounding surface seawater during February (Table 2, p < 0.05). Although the lowest mean concentrations of Cd and Cu in the surrounding surface seawater was observed during January (Table 2, p < 0.05), the lowest mean concentration of total Hg in the same matrix was evident during March (Table 2, p < 0.05). In this context, it is pertinent to indicate that higher temperature (Lee and Lee 2005; Mubiana and Blust 2007) as well as lower salinity (Belabed et al. 2013) and pH (Pascal et al. 2010) in the surrounding seawater would increase the proportion of ionic metals, leading to better bioaccumulation of toxic metals in molluscs such as P. viridis. The significant temporal variations observed may further be explained by differences in amount of pollutants entering the surrounding marine ecosystem, in addition to the possible run-off effect of rainfall and water current. Hence, further studies incorporating the temporal physicochemical characterisation (temperature, salinity and pH) of surrounding surface seawater and its possible influence on bioaccumulation of toxic metals by P. viridis mussel at Kampung Pasir Puteh, Pasir Gudang, Johor merit consideration.
Estimation of daily intake of toxic metals and health risk assessment
Presence of toxic metals in coastal areas, especially from anthropogenic discharges, would lead to their unwanted bioaccumulations in aquaculture products (e.g. P. viridis mussels), causing detrimental health implications towards human, particularly over long-term consumption (Li et al. 2013; Chua et al. 2014; Edbeib et al. 2017). Therefore, continuous assessments of health risk indices such as THQ and HI, following the consumption of contaminated P. viridis mussels harvested at Kampung Pasir Puteh, Pasir Gudang, Johor may prove necessary. Being a ratio of the consumed dose of toxic metals through oral ingestion, THQ has been commonly used for evaluating such toxic metal intake through contaminated foods (Chary et al. 2008). While it is likely that the exposed population with THQ > 1 may ‘experience obvious deleterious effects’, higher THQ has been associated with the probability of having greater hazard risk (Li et al. 2013). In addition, because multiple toxic metals can occur in aquaculture products such as P. viridis mussels, estimating the HI values for explaining the risk of having non-cancer human health impacts deserves public health consideration. In this context, occurrence of non-cancer human health impact is the least expected whenever the HI < 1, and vice versa (Griffin 2009).
To enable calculations of THQ and HI values, the EDI values for Pb, Cd and Cu and total Hg for an adult via consumption of mussels were calculated. The calculated EDI values, as well as the relevant PTDI data suggested by the JECFA (1999, 2003), are provided in Table 3. Results revealed high EDI values for Pb (7.12–10.94 μg/kg/day) and Cd (2.58–3.69 μg/kg/ day) in P. viridis mussels examined here when compared with that reported by previous researchers (Franco et al. 2002; Yap et al. 2004a; Li et al. 2013) and the PTDI values (JECFA 1999, 2003). While the EDI values for Pb and Cd observed here were (1219–1873% and 2504–3582%, respectively) substantially higher than that reported by a previous study in Malaysia (Yap et al. 2004b), the values were also 199–306% and 258–369% higher than the PTDI reported by JECFA (1999, 2003). Although the EDI value for total mercury in P. viridis mussels (0.01 μg/kg/day) was observably higher than the EDI values (0.005–0.054 μg/kg/day) reported by previous researchers (Franco et al. 2002; Li et al. 2013), the value remains lower than the reported PTDI for Hg (0.228 μg/kg/day) (JECFA 1999, 2003). The fact that the EDI and PTDI values for Cu in mussels remain unavailable, suitable comparison with the findings reported in this present research could not be made.
Considering that specific study focusing on the risk assessment of ingesting toxic metals viz. Pb, Cd, Cu and total Hg in P. viridis mussels in Malaysia and its implication on public health remains lacking, this present study that utilised THQ and HI indices for assessing the health risk of consuming such mussels deserves consideration. The THQ and HI values of Pb, Cd, Cu and total Hg by consuming P. viridis mussels for a healthy adult are presented in Table 4. Like the finding reported from countries surrounding the Adriatic Sea (Jović and Stanković 2014), the THQ values for Cu (0.089–0.194) and total Hg (0.030–0.081) observed here were also largely lower than 1, indicating that the amount of Cu and total Hg in P. viridis mussels may not likely cause negative health effect on human. However, because results also highlighted on the alarmingly high THQ values for Pb (16.719–25.712) and Cd (2.600–7.426) by consuming P. viridis mussels harvested at Kampung Pasir Puteh, Johor, Malaysia, their roles in causing detrimental health effects on humans cannot be completely ruled out. Such a proposition is further supported by the extremely high values of HI reported in this present research (23.47–33.41) when compared to those reported by Jović and Stanković (2014). In fact, chronic exposures to Pb and Cd have been associated with numerous health problems that include toxicity of haematopoietic, nervous, reproductive, respiratory and urinary systems, as well as impairment of cognitive ability and tumours (Cope et al. 2004; Flanagan et al. 2008). Therefore, having the ability to screen the concentrations of these toxic metals in P. viridis mussels, probably by associating their concentrations in the surface seawater surrounding its harvesting sites, attempted in this present research too, may prove useful.
Association between the concentrations of Pb, Cd, Cu and total Hg in P. viridis mussel with that of surface seawater
While moderate to high strengths of associations between concentrations of Pb, Cd and Cu in P. viridis mussels and surface sediments in the west coast of Peninsular Malaysia have been reported (Yap et al. 2002), specific studies that explore the association between those elements in P. viridis mussel and surface seawater in Malaysia remain lacking. The fact that ‘suspended filter feeder for P. viridis is not in actual contact with the sediments’ (Yap et al. 2002), and because the sampling of surface seawater is relatively easier than that of sediments, exploring such association for inferential purposes merits consideration.
Table 5 represents the correlation coefficients and regression parameters observed between the concentrations of Pb, Cd, Cu and total Hg in P. viridis mussels and the surrounding surface seawater at Kampung Pasir Puteh harvesting site. It was found that the concentration of Pb in the P. viridis mussel was highly and positively correlated (r = 0.787; p < 0.05) with that of the surrounding surface seawater. Such significant and high strength of association observed between the concentrations of Pb, in P. viridis mussel, with that of surface seawater indicates the possible application of surface seawater for inferring the concentrations of Pb, in P. viridis mussel. In contrast, associations between the concentrations of Cd (r = − 0.620; p < 0.05) and Cu (r = − 0.794; p < 0.05) in P. viridis mussels versus that of the surrounding surface seawater were negatively moderate and negatively high, respectively. Such negative associations observed for Cd and Cu although statistically significant (p < 0.05) may not provide meaningful insights for inferring their concentrations in the P. viridis mussels from those found in the surface seawater samples. Similar conclusion can also be made for total Hg, considering the insignificant (p > 0.05) as well as negative and little (r = − 0.110) strength of association was observed in the P. viridis mussels with that of surface seawater samples.
Review of literature also reveals no specific study, focusing on the association between Hg concentration in P. viridis mussel with that of sediments and/or surface seawater in Malaysia. Therefore, further studies for exploring the associations between concentrations of various toxic metals that are prevalently found in the coastal waters with that of economically important marine commodities such as P. viridis mussel over a longer duration appear useful. This is due to relatively easier analysis of toxic metals in water than that of animal tissues that would enable more frequent periodic assessments to be carried out for ensuring safer human consumption of this important aquaculture product.
Conclusions
This present research assessed the concentrations of Pb, Cd, Cu and total Hg in P. viridis mussel and surface seawater samples collected at Kampung Pasir Puteh, Pasir Gudang, Johor, Malaysia during January to March 2015. It was found that the concentrations (dry weight) of Pb, Cd, Cu and total Hg in P. viridis mussel during the 3 months of sampling period ranged between 25.10 and 38.60 μg/g, 9.10 and 13.00 μg/g, 11.20 and 13.80 μg/g and 30.10 and 38.90 ng/g, respectively. Since enormous values of THQ for Pb and Cd as well as HI were found, the possibility that prolonged consumption of such mussels would lead to unwanted detrimental impact on public health appears disturbing. Therefore, continuous monitoring on the level of toxic metals in such mussels and developing an efficient toxic metal removal technique prove pertinent. The fact that significant and moderate correlation was observed between the concentration of Pb, in P. viridis mussels and the surrounding surface seawater, its application for monitoring activities deserves to be further explored.
Limitations
Because P. viridis is a suspension feeder, and since the biogeochemical cycle of trace metals depends on pH and salinity, analyses of trace metals in suspended particulate matter (SPM) along with the data on seawater pH and salinity would provide more accurate interpretation relating to the temporal changes. Considering that in this present research the sampling was done and completed in year 2015, analysing new samples from the site now may no longer be representative to the actual conditions that prevailed during that time. Therefore, further studies focusing on these aspects at the same P. viridis harvesting site merit consideration.
References
AOAC (2002) AOAC guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals. U.S.A: Official methods of analysis of the association of official analytical chemists international
Arockia VL, Revathi P, Arulvasu C, Munuswamy N (2012) Biomarkers of metal toxicity and histology of Perna viridis from Ennore estuary, Chennai, south east coast of India. Ecotoxicol Environ Saf 84:92–98
Azmi MY, Junidah R, Siti Mariam A, Safiah MY, Fatimah S, Norimah AK, Poh BK, Kandiah M, Zalilah MS, Wan Abdul Manan WM, Siti Haslinda MD, Tahir A (2009) Body mass index (BMI) of adults: findings of the Malaysian adult nutrition survey (MANS). Mal J Nutr 15(2):97–119
Baltas H, Dalgic G, Bayrak EY, Sirin M, Cevik U, Apaydin G (2016) Environmental study on copper uptake capacity in the Mediterranean mussel (Mytilus galloprovincialis). Environ Sci Pollut Res 23:10983–10989
Belabed BE, Laffray X, Dhib A, Fertouna-Belakhal M, Turki S, Aleya L (2013) Factors contributing to heavy metal accumulation in sediments and in the intertidal mussel Perna perna in the Gulf of Annaba (Algeria). Mar Pollut Bull 74:477–489
Chahid A, Hilali M, Benlhachimi A, Bouzid T (2014) Contents of cadmium, mercury and lead in fish from the Atlantic Sea (Morocco) determined by atomic absorption spectrometry. Food Chem 147:357–360
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox Environ Safe 69:513–524
Choo SE, Ng CS (1990) Enzyme hydrolysis of green mussel (Perna viridis) to produce an enhanced taste extract. Singap J Primary Ind 18:48–53
Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ Sci Technol 48:8127–8134. https://doi.org/10.1021/es405717z
Cope WG, Leidy RB, Hodgson E (2004) Classes of toxicants: use classes. In: Hodgson E (ed) A textbook of modern toxicology, 3rd edn. John Wiley & Sons, Inc., New Jersey
Department of Environment Malaysia, DOE (2015) Malaysia environmental quality report 2014, Department of Environment Malaysia, Putrajaya
Department of Fisheries (2013) Annual fisheries statistics 2013. Department of Fisheries Malaysia, Putrajaya
Department of Fisheries Malaysia (2015). Annual fisheries statistics 2005–2015. Retrieved from https://www.dof.gov.my/index.php/pages/view/82
Dong Z, Jim RC, Hatley EL, Backus ASN, Shine JP, Spengler JD, Schaider LA (2015) A longitudinal study of mercury exposure associated with consumption of freshwater fish from a reservoir in rural south central USA. Environ Res 136:155–162
Edbeib MF, Wahab RA, Huyop F (2016) Halophiles: biology, adaptation and their role in decontamination of hypersaline environments. World J Microbiol Biotechnol 32(8):135. https://doi.org/10.1007/s11274-016-2081-9.
Edbeib MF, Wahab RA, Kaya Y, Huyop F (2017) In silico characterization of a novel dehalogenase (DehHX) from the halophile Pseudomonas halophila HX isolated from Tuz Gölü Lake, Turkey: insights into a hypersaline-adapted dehalogenase. Annal Microbiol 67(5):371–382
European Community, EC (2006) Commission regulation (EC) no 1881/2006: setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364:5–24
Flanagan RJ, Kala M, Braithwaite R, de Wolff FA (2008) Other substances encountered in clinical and forensic toxicology. In: Jickells SNA (ed) Clarke’s analytical forensic toxicology. Pharmaceutical Press, London, pp 105–108
Food act 1983 (Act 281) & regulations (2013) Laws of Malaysia. Internation Law Book Services, Petaling Jaya
Food Safety and Standards Authority of India, FSSAI (2011) Food Safety and Standards (Contaminants, Toxins and Residues) Regulations, 2011, F.No. 2–15015/30/2010. Food Safety and Standards Authority of India
Franco J, Borja A, Solaun O, Perez V (2002) Heavy metals in molluscs from the Basque coast (northern Spain): results from an 11-year monitoring programme. Mar Pollut Bull 44:973–976
Gopalakrishnan S, Vijayavel K (2009) Nutritional composition of three estuarine bivalve mussels, Perna viridis, Donax cuneatus and Meretrix meretrix. Int J Food Sci Nutr 60:458–463
Gosling EM (2003) Bivalve molluscs: biology, ecology and culture. In: Fishing News Books. Blackwell Science, Oxford
Griffin RG (2009) Principles and Harzadous materials management. Taylor and Francis Group, Boca Raton. Florida
Hadibarata T, Abdullah F, Yusoff ARM, Ismail R, Azman S, Adnan N (2012) Correlation study between land use, water quality, and heavy metals (Cd, Pb, and Zn) content in water and green lipped mussels Perna viridis (Linnaeus.) at the Johor Strait. Water Air Soil Pollut 223:3125–3136
Hajeb P, Jinap S, Ismail A, Mahyudin NA (2012) Mercury pollution in Malaysia. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer Science+Business Media, New York, p 61
Ismail A, Yap, CK, Zakaria MP, Tanabe S, Takada H, Ismail AR (2000) Green-lipped mussel Perna viridis (L.) as a biomonitoring agent for heavy metals in the west coast of peninsular Malaysia. In: Shariff M. Y, F. M., Gopinath, N., Ibrahim, H. M., & Nik Mustapha, A (editor), Owards sustainable management of the straits of Malacca, technical and financial options Malacca Straits Research and Development Centre (MASDEC). Malacca Straits Research and Development Centre (MASDEC), Malacca
Joint FAO/WHO Expert Committee on Food Additives, JECFA (1999) Reports of the 53rd meeting of the joint FAO/WHO expert committee on food additives (JECFA). In: JECFA/53/TRS. Rome, Italy
Joint FAO/WHO Expert Committee on Food Additives, JECFA (2003): Summary and conclusions of the 61st meeting of the joint FAO/WHO expert committee on food additives (JECFA). JECFA/61/SC; Rome, Italy.
Jović M, Stanković S (2014) Human exposure to trace metals and possible public health risks via consumption of mussels Mytilus galloprovincialis from the Adriatic coastal area. Food Chem Toxicol 70:241–251
Lee J-S, Lee B-G (2005) Effects of salinity, temperature and food type on the uptake and elimination rates of Cd, Cr, and Zn in the asiatic Clamcorbicula fluminea. Ocean Sci J 40:79–89
Li J, Huang ZY, Yue H, Yang H (2013) Potential risk assessment of heavy metals by consuming shellfish collected from Xiamen. China Environ Sci Pollut Res 20:2937–2947
Man CN, Gam LH, Ismail S, Lajis R, Awang R (2006) Simple, rapid and sensitive assay method for simultaneous quantification of urinary nicotine and cotinine using gas chromatography-mass spectrometry. J Chromatogra B 844:322–327
MMC Corporation Berhad (2015). Annual report 2007–2015. Retrieved from https://www.mmc.com.my/page13.html
Mohamat-Yusuff F, Yun LS, Wan ECK, Zulkifli SZ (2015) Profile of heavy metals level in catfish (Hexanematichthys sagor) and green mussel (Perna viridis) from Kong Kong Laut, Johor Straits. Acta Biol Malaysia 4:46–50
Mubiana VK, Blust R (2007) Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar Environ Res 63:219–235
Munro BZ (2005) Statistical methods for health care research, 5th edn. Lippincott Williams & Wilkins, Philadelphia
Ng YJE, Yap CK, Zakaria MP, Tan SG (2013) Assessment of heavy metal pollution in the straits of Johore by using transplanted caged mussel, Perna viridis. Pertanika. J Sci Technol 21:75–96
Nicholson S (2001) Ecocytological and toxicological responses to copper in Perna viridis (L.) (Bivalvia: Mytilidae) haemocyte lysosomal membrane. Chemosphere 45:399–407
Nicholson S, Szefer P (2003) Accumulation of metals in the soft tissues, byssus and shell of the mytilid mussel Perna viridis (Bivalvia: Mytilidae) from polluted and uncontaminated locations in Hong Kong coastal waters. Mar Poll Bull 46:1040–1043
Food Standards Australia New Zealand Act, FSANZ (2015) Food Standards Australia New Zealand Act 1991 Standard 1.4.1, Contaminants and natural toxicants, Federal Register of Legislative Instruments F2015C00052
Papagiannis I, Kagalou I, Leonardos J, Petridis D, Kalfakakou V (2004) Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environ Int 30:357–336
Pascal P-Y, Fleeger JW, Galvez F, Carman KR (2010) The toxicological interaction between ocean acidity and metals in coastal meiobenthic copepods. Mar Pollut Bull 60:2201–2208
Putri LSE, Prasetyo AD, Arifin Z (2012) Green mussel (Perna viridis L.) as bioindicator of heavy metals pollution at Kamal estuary, Jakarta Bay. Indonesia J Environ Res Develop 6:389–396
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka. Bangladesh Food Chem 134:1847–1854
Ryu D, Choi B, Kim N, Koh E (2016) Validation of analytical methods for ethyl carbamate in nine food matrices. Food Chem 211:770–775
Sanagi MM, Ling SL, Nasir Z, Hermawan D, Ibrahim WAW, Abu Naim A (2009) Comparison of signal-to-noise, blank determination, and linear regression methods for the estimation of detection and quantification limits for volatile organic compounds by gas chromatography. J AOAC Int 92:1833–1838
Song B, Lei M, Chen T, Zheng Y, Xie Y, Li X, Gao D (2009) Assessing the health risk of heavy metals in vegetables to the general population in Beijing. China J Environ Sci 21(12):1702–1709
Tewari A, Joshi HV, Raghunathan C, Sravan-Kumar VG, Khambhaty Y (2001) Effect of heavy metal pollution on growth, carotenoid content and bacterial flora in the gut of Perna viridis (L.) in in situ condition. Curr Sci 81:819–828
Thorp JH (2010) Ecology and classification of north American freshwater invertebrates, 3rd edn. Academic Press, London
US Environmental Protection Agency, US EPA (1992) Method 3005A: Acid digestion of waters for total recoverable or dissolved metals for analysis by FLAA or ICP spectroscopy. Environmental Protection Agency, Washington
US Environmental Protection Agency, US EPA (2000) Risk-based concentration table. Environmental Protection Agency, Washington
US Environmental Protection Agency, US EPA (2004) Integrated risk information system (IRIS) on lead and compounds (inorganic). National Center for Environmental Assessment, Washington
US Environmental Protection Agency, US EPA (2010) Calibration curves: program use/needs. Environmental Protection Agency, Washington
US Environmental Protection Agency, US EPA (2011) Risk-based concentration table. Environmental Protection Agency, Washington
US Food and Drug Administration, FDA (2007) Guidance documents chapter II. Growing areas: action levels, tolerances and guidance levels for poisonous or deleterious substances in seafood. U.S.: national shellfish sanitation program guide for the control of molluscan shellfish. US FDA, US
Vipulanandan C (2004) Environmental technology verification protocol water quality protection center: verification protocol for the verification of grouting materials for infrastructure rehabilitation. Diane Publishing Co, USA
Wagner A, Boman J (2004) Biomonitoring of trace elements in Vietnamese freshwater mussels. Spectrochim. Acta B 59:1125–1132
World Health Organization, WHO (2016) Lead poisoning and health. Retrieved from http://www.who.int/mediacentre/factsheets/fs379/en/
Yap CK, Ismail A, Tan SG, Omar H (2002) Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of peninsular Malaysia. Environ Int 28:117–126
Yap CK, Ismail A, Tan SG (2003) Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from peninsular Malaysia. Mar Pollut Bull 46:1044–1048
Yap CK, Ismail A, Tan SG (2004a) Heavy metal (Cd, Cu, Pb and Zn) concentrations in the green-lipped mussel Perna viridis (Linnaeus) collected from some wild and aquacultural sites in the west coast of peninsular Malaysia. Food Chem 84:569–575
Yap CK, Ismail A, Tan SG, Ismail AR (2004b) The impact of anthropogenic activities on heavy metal (Cd, Cu, Pb and Zn) pollution: comparison of the metal levels in green-lipped mussel Perna viridis (Linnaeus) and in the sediment from a high activity site at kg. Pasir Puteh and a relatively low activity site at Pasir Panjang. Pertanika J. Trop. Agric Sci 27:73–78
Yap CK, Ismail A, Tan SG (2005) Cadmium, copper, lead and zinc levels in the green-lipped mussel P. viridis (L.) from the west coast of peninsular Malaysia: safe as food? Pertanika J. Trop Agric Sci 28:41–47
Yap CK, Ismail A, Edward FB, Tan SG, Siraj SS (2006) Use of different soft tissues of Perna viridis as biomonitors of bioavailability and contamination by heavy metals (Cd, Cu, Fe, Pb, Ni, and Zn) in a semi-enclosed intertidal water, the Johore Straits. Toxicol Environ Chem 88:683–695
Yap CK, Cheng WH, Karami A, Ismail A (2016) Health risk assessments of heavy metal exposure via consumption of marine mussels collected from anthropogenic sites. Sci Total Environ 553:285–296
Acknowledgements
Clerical assistance provided by Miss Aida Rasyidah Azman is appreciated.
Funding
Universiti Teknologi Malaysia for provided research grants (Q.J130000.2526.12H51 and Q.J130000.2526.16H14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mahat, N.A., Muktar, N.K., Ismail, R. et al. Toxic metals in Perna viridis mussel and surface seawater in Pasir Gudang coastal area, Malaysia, and its health implications. Environ Sci Pollut Res 25, 30224–30235 (2018). https://doi.org/10.1007/s11356-018-3033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3033-8