Abstract
The rotating packed bed (RPB) as a continuous flow reactor performs very well in degradation of nitrobenzene wastewater. In this study, acidic nitrobenzene wastewater was degraded using ozone (O3) combined with hydrogen peroxide and titanium ions (Ti(IV)/H2O2/O3) or using only H2O2/O3 in a RPB. The degradation efficiency of nitrobenzene by Ti(IV)/H2O2/O3 is roughly 16.84% higher than that by H2O2/O3, and it reaches as high as 94.64% in 30 min at a H2O2/O3 molar ratio of 0.48. It is also found that the degradation efficiency of nitrobenzene is significantly affected by the high gravity factor, H2O2/O3 molar ratio, and Ti(IV) concentration, and it reaches a maximum at a high gravity factor of 40, a Ti(IV) concentration of 0.50 mmol/L, a pH of 4.0, a H2O2/O3 molar ratio of 0.48, a liquid flow rate of 120 L/h, and an initial nitrobenzene concentration of 1.22 mmol/L. Both direct ozonation and indirect ozonation are involved in the reaction of O3 with organic pollutants. The indirect ozonation due to the addition of different amounts of tert-butanol (·OH scavenger) in the system accounts for 84.31% of the degradation efficiency of nitrobenzene, indicating that the nitrobenzene is dominantly oxidized by ·OH generated in the RPB-Ti(IV)/H2O2/O3 process. Furthermore, the possible oxidative degradation mechanisms are also proposed to better understand the role of RPB in the removal of pollutants.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
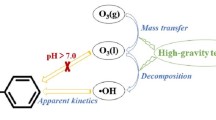
Many toxic aromatic compounds such as nitrobenzene are widely used in explosives, pesticides, paper pulp production, and colorant industries (Zhang et al. 2014a). Nitrobenzene is frequently found in wastewater and listed as a priority pollutant by the US Environmental Protection Agency due to its strong mutagenesis and carcinogenesis in humans (Elshafei et al. 2014). The presence of nitro-groups makes nitrobenzene more stable, and thus treatment of wastewater containing toxic nitrobenzene by conventional biological methods is often inadequate to remove the pollutant completely. Although a number of technologies have been proposed to remove nitrobenzene (Yang et al. 2016), there is still a need to find a more efficient and cost-effective method for degrading nitrobenzene-containing wastewater. H2O2/O3 is considered an effective treatment for different organic pollutants due to their strong oxidative capacity in an alkaline environment (Chidambara Raj and Quen 2005; Martins and Quinta-Ferreira 2011; Kordkandi and Forouzesh 2014; Kordkandi and Motlagh 2017). The decomposition of O3 in the alkaline environment can be expressed as
However, H2O2/O3 has a low nitrobenzene degradation efficiency in the acidic environment because of the low amount of ·OH (Zeng et al. 2012). In order to solve this problem, the discharge standard can be met by adding alkali to neutralize the acid and then using the advanced oxidation processes (AOPs) to degrade pollutants. This, however, is achieved at the expense of increased economic costs. Homogeneous ozonation catalyzed by transition metals, such as manganic ions (Mn(II)) (Arslan et al. 2000), ferrous ions (Fe(II)) (Yao et al. 2013), and Ti(IV) (Tong et al. 2011), has emerged as a cost-effective alternative to degrade acidic wastewater. Ti(IV) is considered one of the most effective transition metals for the degradation of organic pollutants under acidic conditions (Yang et al. 2007; Lin et al. 2010). Lin et al. showed that the Ti(IV) in the acidic environment with H2O2 could produce multiple complexes that could catalyze O3 to produce ·OH. In 2007, Yang et al. also reported that the high oxidative efficiency of Ti(IV)/H2O2/O3 could be attributed to a yellow complex compound (Ti2O52+) that could initiate the chain reaction of ozone decomposition to generate ·OH. These results suggest the possible application of Ti(IV) as a promising homogeneous catalyst in the removal of organic pollutants in an acidic environment. Homogeneous catalysis can also produce ·OH to degrade organic pollutants by catalyzing O3 in water (Ma and Graham 2000). However, most previous studies have focused on the conventional batch reactors, which have been shown to have low O3 mass transfer efficiency and treatment capacity (Lin and Wang 2003). Thus, it is necessary to find a more efficient contactor to improve the gas-liquid mass transfer and the degradation efficiency.
Rotating packed bed (RPB) is a new efficient gas-liquid contactor that can improve the gas-liquid mass transfer and micro-mixing. For these reasons, RPB has been widely used not only in the gas-liquid contacting process but also in processes such as absorption (Mandal et al. 2004; Yi et al. 2009), distillation (Mondal et al. 2012; Luo et al. 2012; Li et al. 2014), stripping (Lin et al. 2008; Yuan et al. 2016), and wastewater treatment (Chen et al. 2005; Zeng et al. 2013; Keen et al. 2015). In recent years, RPB has also been widely used in ozonation, because it could increase the interfacial area between gas and liquid phases and decrease their transfer resistance. This allows O3 molecules in the gas phase to easily enter into the liquid phase. Wang et al. examined the absorption of O3 by deionized water in a RPB and found that the mass transfer coefficient of the liquid phase kLa was 1.912 × 10−2s−1, which was twice as much as that of the conventional batch reactor (Wang et al. 2008).
To the best of our knowledge, there has been no study investigating the degradation of acidic nitrobenzene wastewater using Ti(IV)/H2O2/O3 in a RPB. In this study, we investigated the degradation of nitrobenzene through H2O2/O3 catalyzed by Ti(IV), and its performance was compared with that through the classic H2O2/O3 under the same conditions. In addition, the effects of high gravity factor, H2O2/O3 molar ratio, liquid flow rate, and pH on the degradation efficiency were also evaluated. Finally, the intermediates formed during the degradation of nitrobenzene were determined by gas chromatography/mass spectroscopy (GC-MS), and the possible degradation pathways of nitrobenzene were proposed.
Experimental
Materials
All chemicals were of analytical grade and commercially available from Tianjin Tianli Chemical Reagent Co., Ltd. (China), without further purification. All working solutions were prepared in deionized water.
Experimental setup
Nitrobenzene was dissolved in deionized water to prepare the nitrobenzene solution with a concentration of 1.22 mmol/L. The initial pH of the solution was adjusted to 4.0 with H2SO4. All experiments were performed at a temperature of 25 ± 0.1 °C.
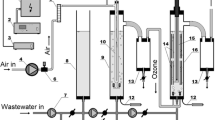
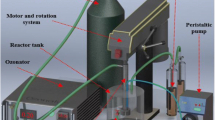
Figure 1 shows the experimental setup for the degradation of nitrobenzene. O3 was generated from oxygen by an ozone generator (NPO10P-2, Shandong Lvbang Photoelectric Equipment Co., Ltd., China). The gas mixture of O2 and O3 was measured by the gas flow meter and then passed through the RPB vertically through its bottom. The nitrobenzene solution added with Ti(IV) was pumped into the RPB via a storage tank and sprayed onto the inner edge of the RPB. The gas and liquid streams were contacted in a cross-flow mode in the RPB, and then O3 was absorbed by the liquid to react with nitrobenzene. Then, the reacted liquid flowed into the storage tank for cycling and unreacted O3 was absorbed by KI solution. The experiments were performed at a temperature of 25 °C, a nitrobenzene concentration (\( {C}_{{\mathrm{NB}}_0} \)) of 1.22 mmol/L, a high gravity factor (β) of 40, a pH of 4.0, a Ti(IV) molar concentration (CTi(IV)) of 0.50 mmol/L, a H2O2/O3 molar ratio (\( {r}_{{\mathrm{H}}_2{\mathrm{O}}_2/{\mathrm{O}}_3} \)) of 0.48, a gas flow rate (QG) of 75 L/h, a liquid flow rate (VL) of 120 L/h, and a reaction time of 30 min. The device was a cross-flow RPB packed with stainless wire mesh, as shown in Table 1.
Analytical methods
A high-performance liquid chromatograph (HPLC, Dionex UltiMate 3000, USA) was used to determine the concentration of nitrobenzene. Chromatographic column was C18 reversed phase column for the 5-mL sample from the liquid outlet, where the detection wavelength was 262 nm, the methanol-water (70:30) acted as mobile phases with a flow rate of 0.9 mL/min, the column temperature was 20 °C, and the sample quantity was 20 μL. The concentration of O3 in gas was measured by the iodometric titration method. The pH value was measured by a pH analyzer (PHS-3C, Shanghai Instrument Electric Scientific Instrument Co., Ltd., China), and the intermediate products were analyzed by GC-MS (Aglient, 5977A-7890B MSD, USA) equipped with a HP-5 ms column (30 m × 0.32 mm × 0.25 μm). The column temperature was held at 60 °C for 2 min and then increased to 300 °C at a heating rate of 10 °C/min. The samples of 1.0 L were extracted twice by dichloromethane, and then the extracted liquid was concentrated by a nitrogen gas to 1.0 mL for GC-MS. The degradation efficiency of nitrobenzene (ηB,%) was expressed as
where \( {C}_{{\mathrm{B}}_0} \) is the initial concentration of nitrobenzene, mmol/L, and \( {C}_{\mathrm{B}} \) is the concentration of nitrobenzene in the effluent solution, mmol/L.
Results and discussion
Effect of initial pH
The initial pH plays a key role in the Ti(IV)/H2O2/O3 treatment (Lin et al. 2010). Figure 2 shows the effect of initial pH on the degradation of nitrobenzene over the pH range of 2.5–5.0. Clearly, the degradation efficiency of nitrobenzene at first increases with the increase of pH until a peak of 95.10% is reached at pH 4.0 in 30 min, after which it decreases with further increase of pH. This phenomenon can be attributed to the following two reasons:
The first reason is that H2O2 can act as an initiator of O3 decomposition in an acidic environment, and a fraction of H2O2 is dissociated to HO2−, which can also act as an initiator of O3 decomposition. The mechanism of how H2O2 induces O3 to generate ·OH in the acidic environment is described in Eqs. (4)–(9) (where k is the reaction rate constant) (Hoigné et al. 1985; Stefan et al. 2015).
Both direct ozonation (O3 reacts directly with organic pollutants) and indirect ozonation (O3 is decomposed to produce ·OH, which in turn reacts with organic pollutants) are involved in the reaction of O3 with organic pollutants. Pera-Titus et al. reported that the direct ozonation was the dominating pathway at a pH below 4, and both direct ozonation and indirect ozonation were important in the pH range of 4.0–9.0 (Pera-Titus et al. 2004). Besides, as the direct ozonation between O3 and nitrobenzene was slow (Latifoglu and Gurol 2003), the degradation efficiency of nitrobenzene was limited at a pH below 4.0.
A second possible reason is that H2O2 could be easily protonated and converted into more stable chemicals such as H3O2+, which can no longer be decomposed to produce ·OH (Zhang et al. 2014b). This could explain the decrease of degradation efficiency of nitrobenzene with the increase of pH. However, it is noteworthy that the Ti(IV) could produce yellow Ti2O52+ with H2O2 (Eqs. 10–12), which is also an initiator of ·OH (Lin et al. 2010). The production of ·OH reaches a maximum at a pH of 4.0 (Fig. 2). Thus, the optimal pH is determined to be 4.0.
Effect of high gravity factor
The high gravity factor β is a dimensionless indicator of the strength of the high gravity field, which can be defined as the ratio of the centrifugal acceleration to the gravitational acceleration (Jiao et al. 2010):
where w is the angular velocity of the rotating rotor, /s; r is the rotor radius, m; g is the gravitational acceleration, m2/s; and N is the rotation speed of the rotor, r/min. These parameters could be controlled by adjusting the frequency of the converter.
The high gravity factor is proportional to the square of the rotation speed, and the high gravity field could be achieved by adjusting the rotor speed. The diffusion and interphase mass transfer of molecules are more pronounced in the high gravity field than in the conventional gravity field. The high shearing force in the high gravity field makes the mass transfer rate between gas and liquid phases one to three orders of magnitude higher than that in the conventional mass transfer equipment (Jiao et al. 2016).
Figure 3 shows the effect of high gravity factor β on the degradation of nitrobenzene (β = 20–80). It is observed that the degradation efficiency of nitrobenzene increases as the high gravity factor β increases from 20 to 60, and then decreases with further increase of the high gravity factor β to 80. The increase of high gravity factor β could enhance the mass transfer between O3 and nitrobenzene solution due to the decrease of the size of liquid droplets and the thickness of liquid films (Jiao et al. 2016). In addition, it can also increase the O3 mass transfer by increasing the renewal efficiency of the liquid surface. Nevertheless, the gas-liquid contact time decreases as the high gravity factor β increases, which is unfavorable for the mass transfer of O3 and thus can explain the non-significant effect of high gravity factor β on the degradation efficiency of nitrobenzene at a high gravity factor β higher than 60. This also demonstrates that the gas-liquid contact time plays an important role in the high gravity factor β (Zeng et al. 2012). As a high rotation speed is often associated with high energy consumption, the optimal high gravity factor β is taken to be 40 based on economic considerations.
Effect of Ti(IV) concentration
The effect of initial Ti(IV) concentration (0.25–1.00 mmol/L) on the degradation efficiency of nitrobenzene is shown in Fig. 4. The degradation efficiency of nitrobenzene increases with the increase of Ti(IV) concentration until a maximum is reached at a Ti(IV) concentration of 0.5 mmol/L, after which it decreases with further increase of Ti(IV) concentration. This is because Ti(IV) can react with H2O2 in the system to form more Ti2O52+, which could catalyze O3 to produce more ·OH as expressed in Eqs. (10)–(12). These results clearly reveal that Ti(IV) could improve the generation of ·OH and therefore improve the degradation efficiency of nitrobenzene. However, when the Ti(IV) concentration exceeds 0.50 mmol/L, self-extinguishing reaction occurs among ·OH through Eq. (14), and an excessive amount of H2O2 in the system could react with the generated ·OH through Eq. (15), leading to the decrease of the degradation efficiency of nitrobenzene (Zhang et al. 2014b).
In addition, the solution becomes more turbid as the Ti(IV) concentration increases, because the hydrolysis of Ti(IV) in water could generate TiO(OH)2 (Yang et al. 2007):
TiO(OH)2 could inhibit the participation of Ti(IV) in catalytic ozonation, which can also lead to a reduction in the degradation efficiency of nitrobenzene. Thus, the optimal Ti(IV) concentration is determined to be 0.50 mmol/L.
Effect of H2O2/O3 molar ratio
In this study, the H2O2/O3 molar ratios are 0.24, 0.48, 0.72, 1.0, and 1.2. Figure 5 shows that with the increase of the H2O2/O3 molar ratio, the degradation efficiency of nitrobenzene first increases and then decreases, because H2O2 can catalyze the formation of ·OH from O3 based on Eqs. (4)–(9). In addition, Ti(IV) can react with H2O2 to form Ti2O52+, which can also catalyze O3 decomposition to produce ·OH (Lin et al. 2010). In case of insufficient H2O2, less ·OH would be produced and thus the degradation efficiency of nitrobenzene would be limited, while in case of an excessive amount of H2O2, it could react with the generated ·OH to produce ·OOH based on Eq. (15), which has a lower oxidation ability than ·OH (Zeng et al. 2012). Thus, the optimal H2O2/O3 molar ratio is determined to be 0.48.
Effect of liquid flow rate
Figure 6 shows the effect of liquid flow rate (VL = 90–130 L/h) on the degradation efficiency of nitrobenzene. Also, the degradation efficiency of nitrobenzene increases from 36.90 to 95.10% in 30 min with the increase of liquid flow rate from 90 to 120 L/h. This is because the contact surface area between gas and liquid phases increases per unit time with the increase of liquid flow rate. The O3 mass transfer efficiency from the gas phase to the liquid phase and the amount of the generated ·OH increase, resulting in an increase in the degradation efficiency of nitrobenzene. However, the degradation efficiency decreases from 95.10 to 84.93% as the liquid flow rate increases from 120 to 130 L/h in 30 min, because increasing the liquid flow rate results in a decrease in the residence time and consequently a decrease in the degradation efficiency of nitrobenzene (Zeng et al. 2012). Thus, the optimal liquid flow rate is determined to be 120 L/h.
Comparison of different processes
In order to investigate the catalytic performance of different processes on O3 under acidic conditions, several ozonation and catalytic ozonation experiments (RPB-Ti(IV)/H2O2/O3, RPB-Ti(IV)/O3, RPB/H2O2/O3) were carried out.
As shown in Fig. 7, the degradation efficiency of nitrobenzene increases with the reaction time under the three conditions, but it differs significantly among different processes. At the same reaction time (30 min), the degradation efficiency of nitrobenzene by RPB-Ti(IV)/H2O2/O3, RPB/H2O2/O3, and RPB-Ti(IV)/O3 is 94.64, 77.80, and only 39.74%, respectively. Clearly, RPB-Ti(IV)/H2O2/O3 results in higher degradation of nitrobenzene than RPB/H2O2/O3 under acidic conditions, which can be attributed to the ability of Ti2O52+ to catalyze O3 to produce ·OH under acidic conditions. We also compared the degradation efficiency of nitrobenzene by the bubbling reactor (BR) (Guo et al. 2015) and RPB under the same conditions. The results show that the high-gravity technology can greatly improve the degradation efficiency of nitrobenzene, as shown in Table 2.
Effect of tert-butanol
In order to determine the dominant reaction for the degradation of nitrobenzene, several experiments were carried out with 0–20 ml of tert-butanol as ·OH scavenger (Liu et al. 2016). Tert-butanol may be oxidized by ·OH to some molecules or mineralized into CO2 and H2O. In 2013, Zeng et al. reported that the rate constant of the reaction between O3 and tert-butanol was 0.03 mol/(L·s) and that between ·OH and tert-butanol was 5 × 108 mol/(L·s), indicating that tert-butanol was a strong ·OH scavenger.
Figure 8 shows that with the addition of more tert-butanol into the system, the degradation efficiency of nitrobenzene is more strongly limited. The degradation efficiency of nitrobenzene with no tert-butanol (0 mL) is 94.64% in 30 min, while it decreases sharply to 15.14% with the addition of 10 mL of tert-butanol. This can be attributed to the high reaction rate of tert-butanol and ·OH, which results in the consumption of more ·OH in a short time and consequently a substantial reduction of ·OH in the solution that can inhibit the degradation of nitrobenzene.
In order to better understand the contribution of indirect ozonation and direct ozonation to the degradation efficiency of nitrobenzene, experiments were performed with higher amounts of tert-butanol. It is observed that the addition of 20 mL of tert-butanol results in a degradation efficiency of 14.85% in 30 min, which is close to that (15.14%) at a concentration of 10 mL. Thus, most ·OH in the system reacts with tert-butanol at an initial concentration of 10 mL, but the addition of more tert-butanol results in no significant decrease in the degradation efficiency of nitrobenzene, probably due to the limited amount of ·OH to be captured in the system. In this case, the degradation of nitrobenzene is mainly caused by O3 (direct ozonation) in the system.
The degradation efficiency of nitrobenzene with no tert-butanol (0 mL) is 94.64% in 30 min under both direct ozonation and indirect ozonation in the system, and the contribution of direct ozonation to nitrobenzene degradation is 14.85%. It can be inferred that the indirect ozonation accounts for 84.31% of the degradation efficiency of nitrobenzene, indicating that ·OH is still the main active substance responsible for degrading nitrobenzene in the acidic environment.
Electrical energy calculation
For the water treatment process, the energy consumption for degrading the wastewater is an important index. Since most advance ozonation processes are electric-energy-intensive, electric energy consumption can be very useful and informative (Bircher and Bolton 2001). Recently, IUPAC put forward the “efficiency index” as an index to evaluate ozonation process wastewater treatment (Bircher and Bolton 2001; Kordkandi and Ashiri 2015). Efficiency index is the electric energy in kilowatt-hours (kWh) required to bring about the degradation of a unit mass (e.g., 1 kg) of a contaminant in polluted water or air (Bircher and Bolton 2001). In order to compare the electric energy consumption of different processes, the electric energy consumption of 1 kg of nitrobenzene is used to characterize this process. The whole electric energy consumption (EEM) consists of two parts, one is ozone generation (EEM/O3), and the other is hydrogen peroxide production (EEM/H2O2). To calculate the theoretical energy required for different ozonation processes, we assumed an average energy requirement for 15 kWh/kg for O3 and of 10 kWh/kg for H2O2 production (Rosenfeldt et al. 2006).
The electric energy consumption of ozonation process can be calculated from the simple formulas, EEO (kWh/kg):
The electric energy consumption of the whole nitrobenzene wastewater treatment process can be expressed as follows:
For other water treatment technologies (such as UV/H2O2, Ultrasonic/H2O2), the whole process of consumption can be expressed as follows:
where P is the power of an ultrasonic or ultraviolet lamp (kw), t is the reaction time (h), C0 and Ct are the concentrations of nitrobenzene (mol/L) at the beginning of a test and at time t, respectively, V is the volume of the treated water, and M is the molar mass of the nitrobenzene (g/mol). The results of electric energy calculations are shown in Table 3 (García Einschlag et al. 2002; Xie et al. 2005).
The calculation results show that the RPB-Ti(IV)/H2O2/O3 system (106.58 kWh/kg) requires the least amount of electric energy to treat the acidic nitrobenzene wastewater, compared to the RPB-H2O2/O3 system (129.52 kWh/kg), the ultrasonic/H2O2/Fe2+ system (338.75 kWh/kg), the BR-H2O2/O3 system (1244.57 kWh/kg), and the UV/H2O2 system (1358.69 kWh/kg). The experimental results show that the RPB-Ti(IV)/H2O2/O3 system can improve the efficiency of acidic nitrobenzene wastewater treatment and reduce the cost of wastewater treatment.
Mechanism of nitrobenzene degradation
The experimental results suggest that the degradation of nitrobenzene can be attributed to the combined action of O3 and ·OH. In order to better understand the mechanism of nitrobenzene degradation by O3 and ·OH, the intermediates formed during the degradation of nitrobenzene by RPB-Ti(IV)/H2O2/O3 were determined by GC-MS. Figure 9 shows total ion chromatograms for nitrobenzene degradation by GC-MS, and the intermediate products of nitrobenzene oxidation detected by GC-MS are shown in Table 4. By GC-MS analysis, 2-nitrophenol, para-benzoquinone, catechol, hydroquinone, and oxalic acid were identified to be transformation products from nitrobenzene oxidation by the RPB-Ti(IV)/H2O2/O3 system. As was generally known, the concentration of intermediate products was low and unstable. Therefore, we proposed that nitrobenzene could be oxidized through two pathways from the GC-MS analysis.
Figure 10 shows that the proposed pathways of direct oxidation by O3 consist mainly of the oxidative ring-opening reaction, molecular rearrangement, free-radical substitution, isomerization, and mineralization. The nitro group has a strong electron withdrawing effect that allows for the attachment of carbon atoms, due to the dipole effect of O3. O3 is connected with the nitro group, and the carbon atoms undergo nucleophilic substitution reaction. First, oxygen atoms are bonded with carbon atoms to form unstable transition-state compounds, and the nitro group falls off from the nitrobenzene and is oxidized to nitrate. The energy required for this reaction is provided by the C-O bond release energy, and the unstable intermediate compounds are oxidized to phenols. Because the hydroxyl group is an electron-donating group, the density of the electron cloud on the benzene ring is greatly increased. According to the base effect, the positively charged oxygen atoms in the ozone molecule attack ortho-position and para-position of hydroxyl to form catechol and hydroquinone, which in turn can be oxidized to produce ortho-benzoquinone and para-benzoquinone. These quinones are easily oxidized to the open-loop structure to form maleic acid and oxalic acid, which are finally mineralized to CO2 and H2O (Zeng et al. 2013).
·OH has a higher oxidation capacity than O3 (Jiao et al. 2016), and thus the possible pathways of indirect oxidation by ·OH are shown in Fig. 11. In comparison with other oxidants, ·OH has a lone pair of electrons, resulting in more efficient and selective oxidation of organic compounds. The electrophilic energy is much higher than the activation energy required for the reaction, and the reaction between ·OH and other organic matters no longer meets the inter-site positioning effect of nitro group and produces some intermediates that do not accord with the rules of location. The ·OH and nitrobenzene can undergo the addition reaction and hydrogen extraction reaction, while nitrobenzene itself can undergo de-nitric acid and dehydration to form phenyl and nitrophenyl radicals, which are unstable and can produce phenol and nitrophenol (Zhang et al. 2014a). Due to the electron donating effect of ·OH, the density of the electron cloud of benzene ring is greatly improved, and it will be oxidized by O3 and ·OH to form bisphenol, which can be further oxidized to highly oxidizing quinones. The benzene ring opens to form small molecules, which are then mineralized to CO2 and H2O.
Conclusions
Ti(IV)/H2O2/O3 was used in this study for the degradation of nitrobenzene under acidic conditions in a RPB. It is found that Ti(IV)/H2O2/O3 is more effective than H2O2/O3, as Ti(IV) can promote the generation of ·OH. The degradation efficiency of nitrobenzene by Ti(IV)/H2O2/O3 is roughly 16.84% higher than that by H2O2/O3 in RPB. The optimal degradation efficiency of nitrobenzene by Ti(IV)/H2O2/O3 is obtained at an initial pH of 4.0, a high gravity factor of 40, a Ti(IV) concentration of 0.50 mmol/L, a H2O2/O3 molar ratio of 0.48, a liquid flow rate of 120 L/h, and a reaction time of 30 min. It is found that the indirect oxidation accounts for 84.31% of the total oxidation process. The GC-MS results show that the intermediates of nitrobenzene oxidation are primarily phenols (phenol, catechol, resorcinol, hydroquinone, and nitrophenol) that can be further oxidized to quinones (p-benzoquinone, o-benzoquinone, and m-benzoquinone). The Ti(IV)/H2O2/O3 method has promising applications in treating acidic nitrobenzene wastewater.
References
Arslan I, Balcioglu IA, Tuhkanen T (2000) Advanced treatment of dyehouse effluents by Fe(II) and Mn(II)-catalyzed ozonation and the H2O2/O3 process. Water Sci Technol 42:13–18
Bircher KG, Bolton JR (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC technical report). Pure Appl Chem 73:627–637
Chen YH, Chang CY, Su WL, Chiu CY, Yu YH, Chiang PC, Chang CF, Shie JL, Chiou CS, Chiang SI (2005) Ozonation of CI reactive black 5 using rotating packed bed and stirred tank reactor. J Chem Technol Biotechnol 80:68–75
Chidambara Raj CB, Quen HL (2005) Advanced oxidation processes for wastewater treatment: optimization of UV/H2O2 process through a statistical technique. Chem Eng Sci 60:5305–5311
Elshafei GMS, Yehia FZ, Dimitry OIH, Badawi AM, Eshaq G (2014) Ultrasonic assisted-Fenton-like degradation of nitrobenzene at neutral pH using nanosized oxides of Fe and Cu. Ultrason Sonochem 21:1358–1365
García Einschlag FS, Lopez J, Carlos L, Capparelli AL (2002) Evaluation of the efficiency of photodegradation of nitroaromatics applying the UV/H2O2 technique. Environ Sci Technol 36:3936–3944
Guo L, Jiao WZ, Liu YZ, Xu CC, Liu WL, Li J (2015) Treatment of nitrobenzene-containing wastewater using different combined processes with ozone. Chin J Energ Mater 5:702–708
Hoigné J, Bader H, Haag WR, Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water-III. Non-dissociating compounds and radicals. Water Res 19:993–1004
Jiao WZ, Liu YZ, Qi GS (2010) Gas pressure drop and mass transfer characteristics in a cross-flow rotating packed bed with porous plate packing. Ind Eng Chem Res 49:3732–3740
Jiao WZ, Luo S, He Z, Liu YZ (2016) Applications of high gravity technologies for wastewater treatment: a review. Chem Eng J 313:912–927
Keen OS, Love NG, Aga DS, Linden KG (2015) Biodegradability of iopromide products after UV/H2O2 advanced oxidation. Chemosphere 144:989–994
Kordkandi SA, Ashiri R (2015) Modeling and kinetics study of acid anthraquinone oxidation using ozone: energy consumption analysis. Clean Technol Environ 17:2431–2439
Kordkandi SA, Forouzesh M (2014) Application of full factorial design for methylene blue dye removal using heat-activated persulfate oxidation. J Taiwan Inst Chem Eng 45:2597–2604
Kordkandi SA, Motlagh AM (2017) Optimization of peroxone reaction rate using metaheuristic approach in the dearomatization and discoloration process. Environ Prog Sustain Energy 37
Latifoglu A, Gurol MD (2003) The effect of humic acids on nitrobenzene oxidation by ozonation and O3/UV processes. Water Res 37:1879–1889
Li YM, Li XH, Wang Y, Chen YY, Ji JB, Yu YL, Xu ZC (2014) Distillation in a counterflow concentric-ring rotating bed. Ind Eng Chem Res 53:4821–4837
Lin SH, Wang CH (2003) Industrial wastewater treatment in a new gas-induced ozone reactor. J Hazard Mater 98:295–309
Lin CC, Chen BC, Chen YS, Hsu SK (2008) Feasibility of a cross-flow rotating packed bed in removing carbon dioxide from gaseous streams. Sep Purif Technol 62:507–512
Lin WW, Li PP, Zhang H, Shi R, Tong SP (2010) Degradation of acetic acid by Ti(IV)-catalyzed H2O2/O3. Chin J Chem. Ind Eng 61:1790–1795
Liu Y, He X, Fu YS, Dionysiou DD (2016) Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem Eng J 284:1317–1327
Luo Y, Chu GW, Zou HK, Xiang Y, Shao L, Chen JF (2012) Characteristics of a two-stage counter-current rotating packed bed for continuous distillation. Chem Eng Process 52:55–62
Ma J, Graham NJD (2000) Degradation of atrazine by manganese-catalysed ozonation-influence of radical scavengers. Water Res 34:3822–3828
Mandal BP, Biswas AK, Bandyopadhyay SS (2004) Selective absorption of H2S from gas streams containing H2S and CO2, into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Sep Purif Technol 35:191–202
Martins RC, Quinta-Ferreira RM (2011) Remediation of phenolic wastewaters by advanced oxidation processes (AOPs) at ambient conditions: comparative studies. Chem Eng Sci 66:3243–3250
Mondal A, Pramanik A, Bhowal A, Datta S (2012) Distillation studies in rotating packed bed with split packing. Chem Eng Res Des 90:453–457
Pera-Titus M, GarciA-Molina V, Barios MA, Giménez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B Environ 47:219–256
Rosenfeldt EJ, Linden KG, Canonica S, Gunten UV (2006) Comparison of the efficiency of ·OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res 40:3695–3704
Stefan MI, John M, James RB (2015) Degradation pathways during the treatment of methyl-tert-butyl ether by the UV/H2O2 process. Environ Sci Technol 34:650–658
Tong SP, Li WW, Zhao SQ, Ma CA (2011) Titanium(IV)-improved H2O2/O3 process for acetic acid degradation under acid conditions. Ozone Sci Eng 33:441–448
Wang H, Liu YZ, Meng XL, Liu HX, Jiao WZ (2008) Mass transfer model of ozone oxidation treatment of trinitrotoluene alkaline wastewater in a rotating packed bed. Chem Eng 12:6–9 (in chinese)
Xie J, Qu CT, Wang XQ (2005) Researches on degradation of nitrobenzene in aqueous solution using ultrasonic technology. Chem Ind Times 11:3–5 (in chinese)
Yang Y, Ma J, Qin Q, Zhai X (2007) Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J Mol Catal A Chem 267:41–48
Yang B, Zuo J, Li P, Wang K, Yu X, Zhang M (2016) Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem Eng J 287:30–37
Yao Y, Wang L, Sun L, Zhu S, Huang Z, Mao Y, Lu WY, Chen WX (2013) Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem Eng Sci 101:424–431
Yi F, Zou HK, Chu GW, Shao L, Chen JF (2009) Modeling and experimental studies on absorption of CO2 by benfield solution in rotating packed bed. Chem Eng J 145:377–384
Yuan MH, Chen YH, Tsai JY, Chang CY (2016) Ammonia removal from ammonia-rich wastewater by air stripping using a rotating packed bed. Process Saf Environ Prot 102:777–785
Zeng ZQ, Zou HK, Li X, Sun BC, Chen JF, Shao L (2012) Ozonation of phenol with O3/Fe(II) in acidic environment in a rotating packed bed. Ind Eng Chem Res 51:10509–10516
Zeng ZQ, Zou HK, Li X, Arowo M, Sun BC, Chen JF, Chu GW, Shao L (2013) Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed. Chem Eng J 229:404–401
Zhang YL, Zhang K, Dai CM, Zhou XF (2014a) Performance and mechanism of pyrite for nitrobenzene removal in aqueous solution. Chem Eng Sci 111:135–141
Zhang YL, Zhang K, Dai CM, Zhou XF, Si H (2014b) An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem Eng J 244:438–445
Funding
This work was supported by the Natural Science Foundations of China (U1610106) and Shanxi excellent talent science and technology innovation project (201705D211011), Specialized Research Fund for Sanjin Scholars Program of Shanxi Province (201707), and North University of China Fund for Distinguished Young Scholars (201701).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Highlights
• Ti(IV)/H2O2/O3 process coupled with RPB was developed to degrade acidic nitrobenzene.
• The degradation efficiency reached 94.64% with the nitrobenzene concentration of 1.22 mmol/L in 30 min.
• Direct reaction and indirect reaction coexisting in the oxidation system were confirmed.
• Indirect ozonation accounted for 84.31% in the whole oxidation process.
• The possible degradation pathways were proposed.
Rights and permissions
About this article
Cite this article
Yang, P., Luo, S., Liu, Y. et al. Degradation of nitrobenzene wastewater in an acidic environment by Ti(IV)/H2O2/O3 in a rotating packed bed. Environ Sci Pollut Res 25, 25060–25070 (2018). https://doi.org/10.1007/s11356-018-2551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2551-8