Abstract
The high nutrients and organic matter (OM) content of sewage sludge make it an excellent fertilizer to enhance soil fertility and crop production. However, the presence of adsorbed and precipitated forms of heavy metals, especially cadmium (Cd), can be a major problem for such a utilization of sludge. This pot study aims at producing safe food with minimal Cd concentrations from sewage sludge amended soils. Two wheat cultivars (NARC-11 and Shafaq-06) were sown in soil amended with sewage sludge with rates 0, 15 and 30 g kg−1 soil. Application of sewage sludge resulted in enhancement of wheat grain yield while Cd concentrations in wheat grains of both cultivars remained within permissible limits (24.1 to 58.6 μg kg−1 dry weight). Fourier transform infrared (FTIR) spectroscopic analysis revealed more spectral changes in fulvic acids than in humic acids, which showed a higher humification degree, making them chemically and biologically more stable for Cd retention. Sequential extraction data of Cd after NARC-11 harvest exhibited a significant decrease in mobile fractions (exchangeable and reducible fractions were reduced by 3.6 and 5.2%, respectively) and increase in immobile fraction (the oxidizable and residual fractions increased by 7 and 1.8%, respectively). It is concluded that sewage sludge application could be useful for the improvement of wheat production due to formation of stable humate complexes and decrease in Cd availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the background of rapid industrialization and environmental risks, in the recent years, the attention of plant growers had shifted toward organic sources of nutrients such as sewage sludge rather than chemical fertilizers. High proportions of organic matter (OM), as well as macro and micro-nutrients in sewage sludge, make it an excellent soil conditioner that can boost soil microbial activity, improve soil physical conditions, and enhance crop yields (Ashraf et al. 2016). Land application of sewage sludge is increasing especially in European countries and the USA where more than 40% of the enhanced treated sewage sludge is used as fertilizer and would exceed 13 million t by 2020 (Lowman et al. 2013). Recent estimates showed that total solid sewage waste production in Pakistan is about 54,888 t per day. However, there are possible risks of soil contamination due to the presence of various toxic compounds, especially heavy metals and recalcitrant organic molecules, which can harm the soil-plant system (Zuberi et al. 2015).
It is essential to understand the processes governing the translocation and availability of heavy metals deriving from OM decomposition and uptake into plant (Lehmann and Kleber 2015). Transfer of heavy metals from sewage sludge to soil and later to plants depends on sewage sludge application rate, aging, metal interaction with soil colloids, and OM (Murtaza et al. 2012). Sewage sludge-derived OM has a major influence on redistribution and availability of heavy metals through the formation of humic and fulvic like acids fractions after humification (Chotzen et al. 2016). The bioavailability of metals from soil to plants also depends on soil properties, agronomic practices, plant growth, and genotype. Humic and fulvic acid-like fractions in sewage sludge have potential influences on the behavior of metals (Khan et al. 2006). Apparently, this character is due to the presence of a variety of aromatic moieties, aliphatic chains, and functional groups which make these substances a multi-ligand complex system.
Metal fractionation is a good indicator for assessing metal bioavailability. Sequential extraction procedures examine the partitioning of metals into various chemical fractions and help to estimate the potential toxicity of metals in an environment (Degryse et al. 2009; Fernandez-Ondono et al. 2017).
Fourier transform infrared (FTIR) spectroscopy is an important qualitative tool to characterize humic and fulvic acids transformations that OM undergoes during the humification process. In FTIR spectroscopy, maturity of OM is assessed through determining the relative intensity of spectral bands of various chemical (aromatic, aliphatic, alcoholic, amino, carboxylic and phenolic, etc.) groups (El Fels et al. 2015; Wu et al. 2016).
Wheat is the major staple food for more than half of the world’s population with 740 million t per year production (FAO 2017). Cadmium-contaminated wheat-derived products are the main source of Cd entering human body via food chain (Rehman et al. 2015; Waqas et al. 2014). Wheat is more susceptible to Cd toxicity as compared to other metals, because Cd is mostly found in soluble and exchangeable soil fractions due to its more labile adsorption on humate complexes than other metals, especially lead and copper (Rizwan et al. 2016; Ruyter-Hooley et al. 2017). Cadmium toxicity induces oxidative stress in wheat which may lead to reduction in growth, dry matter production, and yield. Furthermore, when Cd monthly intake exceeds critical thresholds levels (7.5 μg kg−1 body weight), its toxicity may damage liver and lungs and can cause cancer as well as other fatal disorders (Julin et al. 2012; Moynihan et al. 2017; Khan et al. 2017). However, wheat growth could influence OM transformations and the redistribution of different Cd fractions through root exudation and alterations in organic matter and nutrients cycling (Masciandaro et al. 2013). Therefore, safe wheat productions with minimal concentrations of Cd in grains is a challenge in agriculture, since the lack of natural resources is pushing toward the use of cheap recycled sources of nutrients like sewage sludge.
The present study aims at evaluating humic and fulvic acid-like fractions transformation on Cd availability and their effects on wheat growth, after sewage sludge application to soil at different rates.
Materials and methods
Soil and sewage sludge
The soil used in this study was collected from the farm area of the University of Agriculture of Faisalabad (UAF), Pakistan (N 31° 43′ 10″, E 73° 06′ 95″). Aerobically digested sewage sludge was collected from the I-9 sector wastewater treatment plant of Islamabad, Pakistan. Dry samples of sewage sludge and soil were ground to get a homogenous mixture. Total metal concentrations in soil and sewage sludge were determined after digestion with aqua regia (HNO3/HCl, 1:3; USEPA 2005) and analyzed using atomic absorption spectroscopy (AAS—model Thermo S-Series, USA). Selected initial properties of soil and sewage sludge used in the experiment are presented in Table 1.
Experimental design and raising of plants
All the experimental pots were placed in a protected glasshouse in UAF, with ambient temperature of 30/25 ± 3 °C (day/night) with relative humidity of 55–65% and bright sunlight prevailing during the experimental period. Soil was amended with sewage sludge at three rates (0, 15, and 30 g kg−1 soil, on dry weight basis), which is equivalent to 0, 15, and 30 Mg ha−1 soil (for the upper 10-cm soil layer). Six grains of each wheat cultivar viz. Shafaq-2006 and NARC-2011 were selected and were sown on December 15, 2015, in pots (which were thinned out to four plants per pot), each containing 5 kg of soil. Uprooted weeds and thinned out plants were cut into pieces and mixed in respective pots during crop growth. The pots were irrigated with tap water (ECw 0.69 dS m−1, SAR 1.24 and RSC 0.20 mmolc L−1) as per crop water demand on visual observations. The recommended dose of NPK @ 120:100:60 kg ha−1 was applied through fertilizer sources as urea, diammonium phosphate (DAP), and sulfate of potash (SOP), respectively, after subtracting the NPK amount already present in the sewage sludge. All the P and K were applied at sowing while half of N was applied at sowing, and the remaining N was applied in two equal splits at tillering and booting stages. Chemical control was carried out through application of imidacloprid (20 SL) to control the attack of aphids. Treatments were replicated thrice and arranged in a three-factor factorial design under completely randomized design (CRD).
Post-harvest soil and plant analysis
Wheat crop was harvested at maturity on April 20, 2016. Harvested plant samples were washed with deionized water and dried at 70 °C till constant weight. Root, straw, and grain yield per pot were recorded by weighing. Harvest index (HI) was measured by the following formula
Root, straw, and grain samples were digested in a diacid mixture (HClO4/HNO3 1:3 v/v) and analyzed for Cd by AAS.
Extraction of humic and fulvic acids
Thirty grams of the soil sample were treated three times with distilled water to extract non-humic substances. Each sample was then extracted with 300 mL of 0.1 M NaOH solution to separate humic substances (Helmke et al. 1996). Extraction was repeated until a clear supernatant was obtained after centrifugation at 4000 rpm for 15 min and filtered through Whatman filter paper No. 42 (125 mm). Humic acids were then precipitated out of the extracted solution with 1.5 M H2SO4 for 24 h at 4 °C. The precipitated humic acids were separated from fulvic acids by centrifugation (10,000 rpm for 20 min). The separated precipitates of humic acids were dissolved in 0.1 M NaOH. The separated samples of humic and fulvic acids were freeze dried and stored for FTIR analysis. The organic carbon content of humic and fulvic acids was determined by the KMnO4 oxidation method (Amir et al. 2004).
FTIR analysis
The instrument used for obtaining the infrared spectra was a PerkinElmer 1600 FTIR spectrophotometer covering a wavenumber range of 600–4000 cm−1 at the rate of 16 nm s−1 by using pellets containing 2 mg of the freeze-dried humic and fulvic acids fractions with 250 mg of the dry potassium bromide (Demyan et al. 2012).
Sequential extraction procedure
The Community Bureau of Reference (BCR) sequential extraction procedure (Ure et al. 1993), which is a modified experimental form of Tessier et al. (1979), was followed to determine the different fractions of Cd in soil. Each supernatant after each fractionation step was then analyzed by AAS. The adopted extractants, the experimental conditions, and the corresponding metal fractions were
-
1.
Exchangeable fraction (F1). Add 40 mL of (0.11 M) CH3COOH in 1 g sample, shake for 16 h, and centrifuge.
-
2.
Reducible fraction (F2). Add 40 mL of (0.5 M) NH2OH·HCl (pH 2) to the residual from 1., shake for 16 h, and centrifuge.
-
3.
Oxidizable fraction (F3). Add 10 mL of (8.8 M) H2O2 (pH 2–3) to the residual from 2., stay for 1 h, then add 10 mL of (8.8 M) H2O2 and digest at 85 °C for 2 h. Add 50 mL of (1 M) CH3COONH4 (pH adjusted at 2 with HNO3), shake for 16 h, and centrifuge.
-
4.
Residual fraction (F4). Add 2 mL of 65% HNO3 + 6 mL 37% HCl to the residual from 3., digest at 120 °C for 2 h.
Quality assurance
All the chemicals and solvents used were of analytical reagent grade and were purchased from Merck (Darmstadt, Germany). The reliability of all the analytical procedures was verified by including blanks within every set of digested samples.
Statistical analysis
The statistical significance of experimental treatments was analyzed by subjecting the data to analysis of variance (ANOVA) using Statistix 8.1® for Windows. The least significant difference (LSD) test (p ≤ 0.05) was used for comparison among treatment means.
Results and discussion
Cd uptake in wheat tissues
Results showed increased uptake of sewage sludge-derived Cd in wheat tissues (root, shoot, and grains) with the increase in sewage sludge rate (0, 15, and 30 g kg−1). Cd mainly accumulated in roots followed by shoots and grains, both in cultivar NARC-11 and in Shafaq-06 (Table 2). Significant values (p ≤ 0.05) for interactions of wheat cultivars and sewage sludge rate were found for Cd uptake in wheat tissues.
The higher concentrations of Cd sequestrated in roots indicated that wheat plants reduced Cd translocation to shoots and grains by Cd-exclusion mechanisms. Koo et al. (2013) reported that root exudates, organic acids, and microbial interactions in the rhizosphere may favor Cd uptake due to the dissolution of metal-humate complexes. They credited an increased uptake of Cd at higher sewage sludge rate due to an increase in metal solubility by root exudates in the rhizosphere.
Overall, NARC-11 showed less Cd uptake in different wheat tissues as compared to Shafaq-06. Differences among wheat cultivars with respect to Cd uptake and transport have been reported earlier by Jamali et al. (2009). The present study showed Cd concentrations in wheat grains of NARC-11 and Shafaq-06 cultivars grown in sewage sludge amended soils between 24.1–48.6 and 30.3–58.6 μg kg−1 dry weight, respectively. These values were found within safe limits (200 μg kg−1) as set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2011). Corguinha et al. (2015) attributed negligible Cd contamination in wheat grains due to short-term sewage sludge application. Liu et al. (2016) attributed the low concentrations of sludge-borne Cd contamination in food grains to a “plateau response” of added sewage sludge, but they stated that long-term application could seriously contaminate soil and plants. The plateau effect occurred due to both OM stabilization and Cd immobilization on humic and fulvic acids and amorphous Fe/Mn oxides (McBride 1995; Bolan et al. 2014).
Wheat growth and dry matter production
Results revealed an increase in dry matter production of different wheat tissues with the increase in sewage sludge application rates (0, 15, and 30 g kg−1) (Table 3). As regards physiological parameters, both cultivars (NARC-11 and Shafaq-06) showed a positive correlation with sludge application. In both cultivars, root and shoot length, root and shoot dry weight, grain weight, and harvest index significantly increased with sewage sludge application (Table 3) as compared to control (un-amended soil). As a whole, NARC-11 performed better than Shafaq-06 in terms of growth. Significant values (p ≤ 0.05) for interactions of wheat cultivars and sewage sludge rate were found for the abovementioned growth parameters.
Improved soil physical properties due to sewage sludge-derived organic acids and nutrients might have caused an increase in dry matter production of the different wheat tissues (Gonçalves et al. 2014). Liu et al. (2016) also found significant increase in root, shoot, and grain dry weight of wheat, when sewage sludge was applied at 40 t ha−1. They ascribed the beneficial effects of sewage sludge application to increased microbial activity, higher concentration of nutrients, and OM-derived organic acids which enhance nutrient translocation from soil to plant.
NARC-11 showed a better growth despite higher concentration of Cd added through sewage sludge. Similar findings were presented by Idrees et al. (2015) who found that NARC-11 better tolerated Cd stress than other wheat cultivars. This higher tolerance might be due to an increased production of antioxidant enzymes, phytochelatins, and plant growth regulators. In wheat plants, excess Cd stress upregulates proteins mainly involved in biochemical reactions for Cd detoxification (Poghosyan et al. 2014; Rizwan et al. 2016). Chen et al. (2010) also reported reduced Cd mobility and translocation in different tissues of Cd-tolerant wheat cultivars as compared to Cd-sensitive cultivars.
Cd fractionation
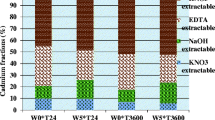
Cadmium fractionation results for the original soil and sewage sludge are presented in Table 4. Results after wheat harvest showed that the distribution of Cd extractable fractions changed significantly (p ≤ 0.05) in sewage sludge amended soil (Table 5). Overall, the concentrations of exchangeable, reducible, and residual Cd fractions decreased, while the concentration of oxidizable Cd fraction slightly increased after crop harvest. Both the soils sown with NARC-11 or Shafaq-06 showed a decrease in labile exchangeable (3.6 or 1.6%, respectively) and reducible (5.2 or 2.1%, respectively) Cd fractions and an increase in the immobile oxidizable (7 or 7.9%, respectively) and residual (1.8 or 1.5%, respectively) Cd fractions, when amended with sewage sludge at 30 g kg−1. Moreover in all Cd fractions, significant values (p ≤ 0.05) for interactions of wheat cultivars and sewage sludge rate were found, except for residual fraction.
The decrease in the exchangeable Cd fraction may be due to plant uptake, metal-humate complex formation, adsorption on the negative sites of organic acids (humic and fulvic-like acids), and Cd-phosphate or Cd-sulfide precipitation. The release of root exudates and the effect of CO2 partial pressure on the precipitation/dissolution of Cd as CdCO3 and Cd(OH)2 might have decreased the reducible Cd fraction (Murtaza et al. 2012). Liu et al. (2016) reported a decrease in exchangeable Cd fraction and increase in OM and residual bound Cd fractions after sewage sludge application. The observed increase in the oxidizable (OM bound) Cd fraction with aging may be due to the addition of OM which provide negative adsorptive sites for Cd ions. Krishnamurti et al. (1997) and Mimmo et al. (2014) stated that the amount of inorganic (e.g., protons) and organic (organic acids, siderophores, phenolics, and enzymes, etc.) compounds in rhizosphere exuded by plants increased after sewage sludge application. These organic acids may affect microbial activity, root morphology, and mobilization of metals (e.g., Cd) through soil acidification, chelation, precipitation, and oxidation-reduction reactions (Terzano et al. 2015). Changes in complex rhizosphere environment and distribution pattern of Cd fractions with sewage sludge application also depend on sewage sludge composition and its repeated application on agricultural soils (Koo et al. 2013).
The role of sewage sludge as metal immobilizing agent through chelation has been reported earlier by Keiluweit et al. (2015). They found that the composition of chelated complexes is directly related to OM decomposition through the action of soil microbes and root exudates (including organic acids). Organic matter decomposition may favor Cd release into soil solution and promote its translocation into plants (Billingham 2015). On the other hand, aging and humification of OM may cause increased metal retention by humic and fulvic acids through precipitation and chelation (Torri and Correa 2012).
Organic matter transformations
Fulvic acids
At sowing, FTIR spectra of fulvic acids obtained from sewage sludge amended and un-amended soils showed similar bands (Fig. 1). Four dominant regions of absorbance appeared: (a) a broad band around 3300–3400 cm−1 exhibiting the presence of H-bonded OH groups of alcohols, phenols, and H-bonded NH groups of amides and carboxylic acids; (b) a well-pronounced sharp peak at 1650 cm−1 showed the presence of aromatic and olefinic C=C, C=O in amide I, ketone, and quinone groups; (c) a small but sharp peak at 1250 cm−1confirmed the presence of amide III, aliphatic CH2 or alcohols, and C=O stretch of aryl ethers, as well as organo-sulfur compounds; and (d) a peak at 1040 cm−1 relates to the presence of polysaccharide and Si-O stretch of silicate impurities (Coates 2000; Wu et al. 2016).
Both the cultivars showed variations in fulvic acids spectra around 1000–1300 cm−1 spectral region in sewage sludge amended soils (Fig. 1a, b), while no obvious changes were observed in un-amended soil (Fig. 1c). These transformations in the OM of sewage sludge amended soil might be due to indigenous microbes which used labile aliphatic and carbohydrate molecules as a source of carbon and energy (Blume et al. 2016). El Fels et al. (2015) referred the disappearance of peaks at 1050–1300 cm−1 region due to polycondensation reactions of COOH groups. It might be also due to the increased OM addition through sewage sludge which favors the dissolution of soluble amides, aliphatic and carbohydrate groups and facilitate chelation, precipitation, and adsorption of metals on stable metal-humate complexes (Amir et al. 2010; Krishnamurti and Naidu 2008).
The influence of fulvic acids on metal speciation has been studied by Popovic et al. (2011), who reported that metal complexes with fulvic acids are non-available for plant uptake. Similar results were obtained by Nigam et al. (2001), who found an increased Cd-humate complex interactions in the soil-plant system and designated fulvic acid transformation as a key factor in minimizing Cd availability.
Humic acids
Spectral analysis of humic acids at sowing showed similar spectral bands as noted for fulvic acids, since both are characterized by the same functional groups in their chemical structure (Fig. 2). The absence of bands at 1250 and 1040 cm−1 in humic acids spectra showed its higher aromatic character (Zhang et al. 2015).
Slight variations in humic acids spectra with aging might have appeared due to the presence of recalcitrant compounds like lignin, minerals, and silicates (Fig. 2a, b). An increased stability in humic acids spectra after crop harvest was confirmed by the slight increase of the bands at 1650 cm−1. On the contrary, the band at 3400 cm−1 slightly decreased after crop harvest (El Fels et al. 2015). Sewage sludge addition and plant growth favor root exudates, microbial activity, and soluble metal-humate interaction, which decrease the hydrophilic character and increased the hydrophobic character of humic acids like fractions (Negrea et al. 2004). The increased hydrophobic character (aromatic) of humic acids led to a decreased Cd availability (decrease in the exchangeable Cd fraction and increase in OM and residual bound Cd fractions) through Cd chelation and adsorption on stable humate complexes.
The role of humic acids in reducing metal mobility in soil has been reported earlier by Harter and Naidu (2001), who attributed it to covalent bonding and the chelating capacity of the humic material. Billingham (2015) and Krishnamurti et al. (1997) reported that humic acids have the ability to decrease metal mobility with aging due to the presence of carboxyl and phenyl groups which favors the formation of stable complexes with metals, especially Cd. Humic acids like fractions could also act as a buffer for metal solubility by preventing rapid changes in soil pH. It might be due to enrichment of humic acids with aromatic compounds (sorption to soil particles and interactions with anions/cations) in sewage sludge amended soils (Pawar 2015).
Overall, the humification of OM after wheat growth decreased Cd availability by transferring available Cd fractions into less available Cd fractions and this is related to the formation of metal-humate complexes mainly from humic acids (Masciandaro et al. 2013). This short-term trial has shown that the uptake of many metals into crops decreased due to adsorptive behavior of sewage sludge, a protection governed by the added organic matter (McBride 1995). Stevenson (1994) also suggested that humification of organic matter leads to increased condensation and re-polymerization reactions of humic and fulvic acids like fractions with soil primary (mica and silicates, etc.) and secondary (clay minerals, calcite, iron, and aluminum oxides, etc.) minerals.
Conclusions
The cultivars, NARC-11 and Shafaq-06, showed better growth and dry matter production in sewage sludge amended soils. Cadmium uptake in different plant tissues (root, shoot, and grains) was found to be lower in NARC-11 than Shafaq-06 at different rates of sewage sludge application. The Cd concentrations remained within permissible limits in wheat grains of both cultivars grown in sewage sludge amended soil. Sewage sludge-derived Cd availability decreased due to increase in oxidizable Cd fraction. Fulvic acids showed more changes in FTIR spectra than humic acids which exhibited higher stability due to the presence of more aromatic groups. Nevertheless, regular monitoring is needed for metal buildup in soil and plants after continuous applications of sewage sludge.
References
Amir S, Hafidi M, Merlina G, Hamdi H, Revel JC (2004) Elemental analysis, FTIR, 13C-NMR of humic acids from sewage sludge composting. Agronomie 24:13–18
Amir S, Jouraiphy A, Meddich A, Gharous ME, Winterton P, Hafidi M (2010) Structural study of humic acids during composting of activated sludge-green waste: elemental analysis, FTIR and 13C NMR. J Hazard Mater 177:524–529
Ashraf I, Ahmad I, Nafees M, Yousaf MM, Ahmad B (2016) A review on organic farming for sustainable agricultural production. Pure Appl Biol 5:277–286
BillinghamK (2015)Humic products: potential or presumption for agriculture. Technol Engr NSW Agriculture
Blume HP, Brümmer GW, Fleige H, Horn R, Kandeler E, Kogel-Knabner I, Kretzschmar R, Stahr K, Wilke BM (2016) Soil organic matter. In: Scheffer/Schachtschabel soil science. Springer, Berlin Heidelberg, pp 55–86. https://doi.org/10.1007/978-3-642-30942-7-3.
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham K, Scheckel K (2014) Remediation of heavy metal (loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166
Chen CH, Zhou QX, Cai Z, Wang YY (2010) Effects of soil polycyclic musk and cadmium on pollutant uptake and biochemical responses of wheat (Triticum aestivum). Arch Environ Contam Toxicol 59:564–573
Chotzen RA, Polubesova T, Chefetz B, Mishael YG (2016) Adsorption of soil-derived humic acid by seven clay minerals: a systematic study. Clays Clay Min 64:628–638
CoatesJ (2000) Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry. Meyers RA (ed.) Copyright Ó John Wiley & Sons Ltd. doi: https://doi.org/10.1002/9780470027318.a5606
Corguinha AP, de Souza GA, Gonçalves VC, de Andrade CC, de Lima WE, Martins FA, Yamanaka CH, Francisco EA, Guilherme LR (2015) Assessing arsenic, cadmium, and lead contents in major crops in Brazil for food safety purposes. J Food Compos Anal 37:143–150
Degryse F, Smolders E, Parker DR (2009) Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications. A review. Eur J Soil Sci 60:590–612
Demyan MS, Rasche F, Schulz E, Breulmann M, Müller T, Cadisch G (2012) Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur J Soil Sci 63:189–199
El Fels L, Zamama M, Hafidi M (2015) Advantages and limitations of using FTIR spectroscopy for assessing the maturity of sewage sludge and olive oil waste co-composts. In: Chamy R, Rozenkranz F, Soler L (eds) Biodegradation and bioremediation of polluted systems—new advances and technologies. In Tech, pp 127–144
FAO - Food and Agriculture Organization of United Nations (2017) FAO cereal supply and demand brief. http:// wwwfaoorg / world food situation / csdb/en/ Release date: 04 May 2017
Fernandez-Ondoño E, Bacchetta G, Lallena AM, Navarro FB, Ortiz I, Jiménez MN (2017) Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J Geochem Explor 172:133–141
Gonçalves ICR, Araújo ASF, Nunes LAPL, Bezerra AAC, de Melo WJ (2014) Heavy metals and yield of cowpea cultivated under composted tannery sludge amendment. Acta Sci Agron 36:443–448
Harter RD, Naidu R (2001) An assessment of environmental and solution parameter impact on trace-metal sorption by soils. Soil Sci Soc Am J 65:597–612
HelmkePA, LoeppertRH, SoltanpourPN, TabatabaiMA, JohnsonCT, SummerME (1996) Methods of soil analysis, part 3: chemical methods. Soil Sci Soc Am Madison, pp1018–1020
Idrees S, Shabir S, Ilyas N, Batool N, Kanwal S (2015) Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium. Agrociencia 49:917–929
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Kandhroa GA, Shaha AQ, Baiga JA (2009) Heavy metal accumulation in different varieties of wheat (Triticum aestivum L.) grown in soil amended with domestic sewage sludge. J Hazard Mater 164:1386–1391
JECFA - Joint FAO/WHO Expert Committee on Food Additives (2011) Safety evaluation of certain food additives and contaminants. WHO Food Additives Series 64. World Health Organization, Geneva
Julin B, Wolk A, Bergkvist L, Bottai M, Akesson A (2012) Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res 72:1459–1466
Keiluweit M, Bougoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5:588–595
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601:1591–1605
Khan S, Cao Q, Chen BD, Zhu YG (2006) Humic acids increase the phytoavailability of Cd and Pb to wheat plants cultivated in freshly spiked, contaminated soil. J Soils Sediments 6:236–242
Koo BJ, Chang AC, Crowley DE, Page AL, Taylor A (2013) Availability and plant uptake of biosolid-borne metals. Appl Environ Soil Sci 2013:1–10
Krishnamurti GSR, Cieslinski G, Huang PM, Van RKCJ (1997) Kinetics of cadmium release from soils as influenced by organic acids: implication in cadmium availability. J Environ Qual 26:271–277
KrishnamurtiGSR, NaiduR (2008) Chemical speciation and bioavailability of trace metals. In: Wiley Inter Sci New York. pp 419–466
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68
Liu Z, Yang Y, Bai Y, Huang Y, Nan Z, Zhao C, Ma J, Wang H (2016) The effect of municipal sludge compost on the mobility and bioavailability of Cd in a sierozem-wheat system in an arid region northwest of China. Environ Sci Pollut Res 23:20232–20242
Lowman A, McDonald M, Wing A, Muhammad SN (2013) Land application of treated sewage sludge: community health and environmental justice. Environ Health Perspect 121:537–542
Masciandaro G, Macci C, Peruzzi E, Ceccanti B, Doni S (2013) Organic matter-microorganism-plant in soil bioremediation: a synergic approach. Rev Environ Sci Biotechnol 12:399–419
McBride MB (1995) Toxic metal accumulation from agricultural use of sludge: are USEPA regulations protective? J Environ Qual 24:5–18
Mimmo T, Del Buono D, Terzano R, Tomasi N, Vigani G, Crecchio C, Pinton R, Zocchi G, Cesco S (2014) Rhizospheric organic compounds in the soil–microorganism–plant system: their role in iron availability. Eur J Soil Sci 65:629–642
Moynihan M, Peterson KE, Cantoral A, Song PX, Jones A, Solano-González M, Meeker JD, Basu N, Téllez-Rojo MM (2017) Dietary predictors of urinary cadmium among pregnant women and children. Sci Total Environ 575:1255–1262
Murtaza G, Haynes RJ, Kim KR, Zia MH, Naidu R, Belyaeva O (2012) Effect of ageing biosolids with soils of contrasting pH on subsequent concentrations of Cu and Zn in pore water and on their plant uptake. Environ Sci Pollut Res 19:636–645
Negrea M, Leone P, Trichet J, Defarge C, Boero V, Gennari M (2004) Characterization of model soil colloids by cryo-scanning electron microscopy. Geoderma 121:1–16
Nigam R, Srivastava S, Prakash S, Srivastava MM (2001) Cadmium mobilisation and plant availability—the impact of organic acids commonly exuded from roots. Plant Soil 230:107–113
Pawar RM (2015) The effect of soil pH on bioremediation of polycyclic aromatic hydrocarbons (PAHS). J Bioremd Biodeg 6:291. https://doi.org/10.4172/2155-6199.1000291
Poghosyan GH, Mukhaelyan ZH, Vardevanyan PH (2014) Influence of cadmium ions on growth and antioxidant system activity of wheat (Triticum aestivum L.) seedlings. Int J Sci Res Environ Sci 2:371–378
Popovic O, Almas AR, Manojlovic M, Muratovic S, Singh BR (2011) Chemical speciation and bioavailability of Cd, Cu, Pb and Zn in Western Balkan soils. Acta Agric Scand Sect B Soil Plant Sci 61:730–738
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rizwan M, Ali S, Abbas T, Rehman MZ, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Ruyter-Hooley M, Johnson BB, Morton DW, Angove MJ (2017) The adsorption of myo-inositol hexaphosphate onto kaolinite and its effect on cadmium retention. App Clay Sci 135:405–413
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, 2nd edn. John Wiley & Sons, New Jersey
Terzano R, Cesco S, Mimmo T (2015) Dynamics, thermodynamics and kinetics of exudates: crucial issues in understanding rhizosphere processes. Plant Soil 386:399–406
Tessier A, Campbell PGC, Bission M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–858
TorriSI, CorreaRS (2012) A review article: downward movement of potentially toxic elements in biosolids amended soils. Appl Environ Soil Sci 1–7.
Ure AM, Quevauviller PH, Griepink H (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of European Communities. Int J Environ Anal Chem 51:135–151
USEPA (2005) Test methods for evaluating solid waste, physical/chemical methods, SW-846. U S Government Printing Office, Washington, DC
Waqas M, Khan S, Qing S, Brian RJ, Cai C (2014) The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 105:53–61
Wu M, Song M, Liu M, Jiang C, Li Z (2016) Fungicidal activities of soil humic/fulvic acids as related to their chemical structures in greenhouse vegetable fields with cultivation chronosequence. Sci Rep 6:32858. https://doi.org/10.1038/srep32858
Zhang J, Baoyi L, Meiyan X, Jian Y (2015) Tracking the composition and transformation of humic and fulvic acids during vermicomposting of sewage sludge by elemental analysis and fluorescence excitation-emission matrix. Waste Manag 39:111–118
Zuberi M, Jibran S, Ali SF (2015) Greenhouse effect reduction by recovering energy from waste landfills in Pakistan. Renew Sust Energ Rev 44:117–131
Acknowledgements
The principal author is thankful to Higher Education Commission (HEC), Pakistan, for granting PhD Indigenous scholarship to support financially this research work. Special thanks to Saffron Pharmaceutical Pvt. (Ltd.) Khurianwala, Faisalabad, for providing the facility of Fourier transform infrared (FTIR) spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roberto Terzano
Rights and permissions
About this article
Cite this article
Rashid, I., Murtaza, G., Zahir, Z.A. et al. Effect of humic and fulvic acid transformation on cadmium availability to wheat cultivars in sewage sludge amended soil. Environ Sci Pollut Res 25, 16071–16079 (2018). https://doi.org/10.1007/s11356-018-1821-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1821-9